Abstract
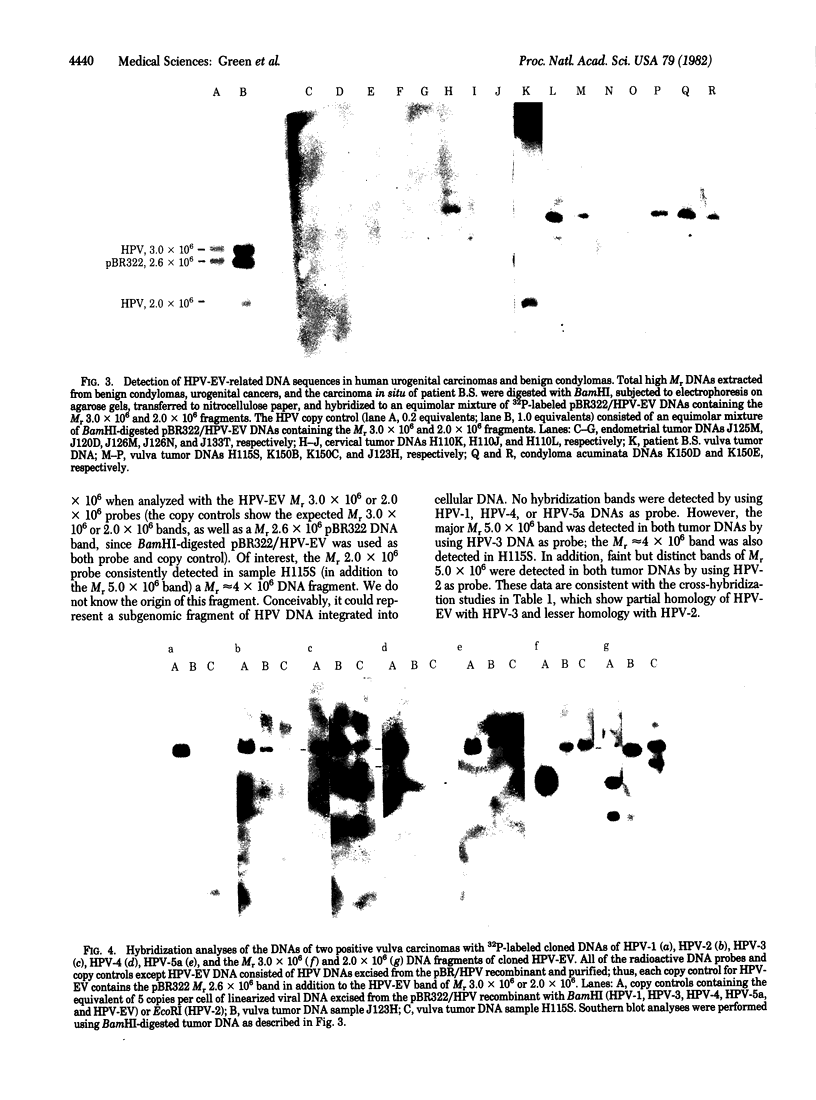
The DNA genome of a human papillomavirus (HPV), tentatively designated HPV-EV, was molecularly cloned from hand to leg lesions of a patient with epidermodysplasia verruciformis, a chronic skin disease associated with a 30% risk of developing cancer. Using stringent hybridization conditions, we observed less than 5% homology between HPV-EV and the cloned genomes of HPV-1, HPV-4, HPV-5, and HPV-5a. HPV-EV DNA showed approximately 6% homology with HPV-2 and 36% homology with HPV-3. These data suggest that HPV-EV is partially related to HPV-3. Using 32P-labeled cloned HPV-EV as probe in Southern blot hybridization experiments, we detected HPV-EV-related DNA in the carcinoma in situ (Bowenoid lesion) of the vulva of the patient from which HPV-EV was isolated. HPV-EV-related DNA was detected in 2 of 10 vulva carcinomas and in 2 of 31 cervical carcinomas. Related DNA sequences were found in papillomas from each of two patients with condyloma acuminata (anogenital warts), which is of interest considering that condylomas have been reported to convert occasionally to carcinomas. The positive vulva DNAs were also probed with other cloned HPV DNAs: HPV-1, HPV-4, and HPV-5a-related sequences were not detected; HPV-3 and HPV-2 DNA probes detected strong and weak DNA bands, respectively, of the same size as found with HPV-EV. The HPV DNA sequences were present in the positive tumors mainly as free viral DNA molecules; no evidence for integration into cellular DNA was found. The emerging biological picture with papillomaviruses is that cells transformed by these viruses are maintained in a transformed state by free episomal genomes. Thus, our findings are consistent with the idea, but by no means establish, that HPVs play a role in human cancer by a similar mechanism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Green M., Orth G., Wold W. S., Sanders P. R., Mackey J. K., Favre M., Croissant O. Analysis of human cancers, normal tissues, and verruce plantares for DNA sequences of human papillomavirus types 1 and 2. Virology. 1981 Apr 15;110(1):176–184. doi: 10.1016/0042-6822(81)90019-2. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S., Mackey J. K., Rigden P. Analysis of human tonsil and cancer DNAs and RNAs for DNA sequences of group C (serotypes 1, 2, 5, and 6) human adenoviruses. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6606–6610. doi: 10.1073/pnas.76.12.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman C. A., Law M. F., Israel M. A., Howley P. M. Cloning of human papilloma virus genomic DNAs and analysis of homologous polynucleotide sequences. J Virol. 1980 Nov;36(2):395–407. doi: 10.1128/jvi.36.2.395-407.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett W. F., McNeil P. E., Grimshaw W. T., Selman I. E., McIntyre W. I. High incidence area of cattle cancer with a possible interaction between an environmental carcinogen and a papilloma virus. Nature. 1978 Jul 20;274(5668):215–217. doi: 10.1038/274215a0. [DOI] [PubMed] [Google Scholar]
- KAZAL H. L., LONG J. P. Squamous cell papillomas of the uterine cervix; a report of 20 cases. Cancer. 1958 Sep-Oct;11(5):1049–1059. doi: 10.1002/1097-0142(195809/10)11:5<1049::aid-cncr2820110527>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Watts S. L., Anderson D. L., Faras A. J., Pass F. Anogenital warts contain several distinct species of human papillomavirus. J Virol. 1980 Oct;36(1):236–244. doi: 10.1128/jvi.36.1.236-244.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C., Meinke W. Bovine papilloma virus: presence of virus-specific DNA sequences in naturally occurring equine tumors. Proc Natl Acad Sci U S A. 1977 Feb;74(2):524–528. doi: 10.1073/pnas.74.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moar M. H., Campo M. S., Laird H. M., Jarrett W. F. Unintegrated viral DNA sequences in a hamster tumor induced by bovine papilloma virus. J Virol. 1981 Sep;39(3):945–949. doi: 10.1128/jvi.39.3.945-949.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moar M. H., Campo M. S., Laird H., Jarrett W. F. Persistence of non-integrated viral DNA in bovine cells transformed in vitro by bovine papillomavirus type 2. Nature. 1981 Oct 29;293(5835):749–751. doi: 10.1038/293749a0. [DOI] [PubMed] [Google Scholar]
- Orth G., Favre M., Jablonska S., Brylak K., Croissant O. Viral sequences related to a human skin papillomavirus in genital warts. Nature. 1978 Sep 28;275(5678):334–336. doi: 10.1038/275334a0. [DOI] [PubMed] [Google Scholar]
- Ostrow R. S., Bender M., Niimura M., Seki T., Kawashima M., Pass F., Faras A. J. Human papillomavirus DNA in cutaneous primary and metastasized squamous cell carcinomas from patients with epidermodysplasia verruciformis. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1634–1638. doi: 10.1073/pnas.79.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow R. S., Krzyzek R., Pass F., Faras A. J. Identification of a novel human papilloma virus in cutaneous warts of meathandlers. Virology. 1981 Jan 15;108(1):21–27. doi: 10.1016/0042-6822(81)90524-9. [DOI] [PubMed] [Google Scholar]
- Pfister H., Fink B., Thomas C. Extrachromosomal bovine papillomavirus type 1 DNA in hamster fibromas and fibrosarcomas. Virology. 1981 Dec;115(2):414–418. doi: 10.1016/0042-6822(81)90125-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Underwood P. B., Jr, Hester L. L., Jr Diagnosis and treatment of premalignant lesions of the vulva: a review. Am J Obstet Gynecol. 1971 Jul 15;110(6):849–857. doi: 10.1016/0002-9378(71)90582-5. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Condylomata acuminata and human genital cancer. Cancer Res. 1976 Feb;36(2 Pt 2):794–794. [PubMed] [Google Scholar]