Abstract
Background:
Differences in the quantity and distribution of coronary veins between patients with ischemic and nonischemic cardiomyopathy might affect the potential for the left ventricular (LV) lead targeting in patients undergoing cardiac resynchronization therapy (CRT). In the current study, we assessed and compared the suitability of the coronary venous system for the LV lead placement in ischemic and dilated cardiomyopathy.
Methods:
This single-centre study, performed at our hospital, retrospectively studied 173 patients with the New York Heart Association class III or IV who underwent CRT. The study population was comprised of 74 patients with an ischemic underlying etiology and 99 patients with a non-ischemic etiology. The distribution of the veins as well as the final lead positions was recorded.
Results:
There was no significant difference between the two groups in terms of the position of the available suitable vein with the exception of the posterior position, where the ischemic group had slightly more suitable veins than did the dilated group (48.4% versus 32.1%, p value = 0.049). There was also no significant difference with respect to the final vein, through which the LV lead was inserted. Comparative analysis showed that the patients with previous coronary artery bypass grafting surgery (CABG) had significantly fewer suitable veins in the posterolateral position than did the non-CABG group (16.3% versus 38.7%, p value = 0.029). There was, however, no significant difference between the two subgroups regarding the final vein position in which the leads were inserted.
Conclusion:
The final coronary vein position suitable and selected for the LV lead insertion was not different between the cases with cardiomyopathy with different etiologies, and nor was it different between the ischemic cases with and without a history of CABG. Patients with a history of procedures around the coronary vessel may have an intact or recovered venous system and may, therefore, benefit from transvenous LV lead placement for CRT.
Keywords: Coronary vessels, Cardiac resynchronization therapy, Cardiomyopathies, Ischemia
Introduction
Cardiac resynchronization therapy (CRT) has been identified as a well-established treatment protocol for selected patients with progressive heart failure. Responders to this procedure frequently experience reversal of left ventricular (LV) remodeling and thus enhanced systolic function, which contributes to a reduction in hospital admission and improvement in survival.1–5 On the other hand, non-responders may represent a consistently high proportion of all recipients of biventricular stimulation (estimated at 25 to 30%), which can be in consequence of inappropriate patient selection or implementation deficiency.6 Along with lack of LV dyssynchrony, extensive myocardial scarring, posterolateral transmural infarction, and inappropriate device programming, suboptimal LV lead position is implicated in non-response.6–8 It has been recently demonstrated that the correlation between the pacing site and the maximal dyssynchrony site translates into an improved clinical outcome, as well as reduced mortality and heart failure hospitalizations.9
One of the main restricting factors of the LV lead placement is the variability in the coronary venous anatomy. Some previous invasive studies have shown the variation of the coronary anatomy between individual patients, especially in those with a history of ischemic events like myocardial infarction or previous coronary artery bypass grafting surgery (CABG).10–14 However, only a few studies have thus far been published on the impact of these pathological differences on the potential for the LV lead targeting. Therefore, based on a hypothesis that the suggested differences in the quantity and distribution of coronary veins between patients with ischemic and non-ischemic cardiomyopathy might affect the potential for the LV lead targeting in patients undergoing CRT, we reviewed the retrograde coronary sinus venograms at the time of CRT implantation to assess the potential for targeted LV lead placement. In other words, we assessed the suitability of the coronary venous system for the LV lead placement in ischemic and dilated cardiomyopathy.
Methods
This single-centre study, conducted at Tehran Heart Center, retrospectively reviewed the retrograde contrast venograms of 173 patients (aged 18–78 years) who underwent CRT device implantation with documented ischemic or dilated cardiomyopathy between March 2003 and March 2010. Past medical history, clinical data, and paraclinical findings, comprising suitability of coronary venouses alongside final LV lead implantation, were assessed. Selection for device implantation was based on the following criteria: New York Heart Association (NYHA) class III or IV symptoms and impaired LV systolic function with a mean ejection fraction less than 35%. In all the study subjects, the devices were implanted in the electrophysiology laboratory under local anesthesia. The study was approved by the Review Board of Tehran University of Medical Sciences.
Retrograde radio-opaque contrast injection was performed to delineate the coronary venous anatomy following coronary sinus intubation. If coronary venography was deemed to have been performed incompletely, an occlusive balloon catheter was inserted into the coronary sinus to maximize coronary venous opacification. The retrograde contrast venograms were prospectively evaluated, and the quantity and distribution of the coronary veins as well as the final lead position were evaluated. The coronary veins were documented for their presence as well as their deemed suitability to receive an LV pacing lead by an experienced cardiologist based upon the vessel caliber, course, and tortuosity.
The results are reported as mean ± standard deviation (SD) for the quantitative variables and percentages for the categorical variables. The groups were compared using the Student t-test for the continuous variables and the chi-square test (or Fisher exact test if required) for the categorical variables. P values ≤ 0.05 were considered statistically significant. All the statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 173 patients were assessed. The study population included 74 patients with a history of ischemic heart disease, defined as coronary stenoses > 50% in at least one epicardial artery. Of this group, 43 (58.1%) patients had previously undergone CABG. The baseline characteristics of all the patients according to the underlying etiology are depicted in Table 1. Except for the male-to-female ratio and mean age, which were higher in the ischemic group, the other baseline characteristics were similar between the two study groups.
Table 1.
Baseline characteristics of all patients*
| Characteristics | Ischemic group (n=74) | Non-ischemic group (n=99) | P value |
|---|---|---|---|
| Male gender | 63 (85.1) | 55 (55.6) | < 0.001 |
| Age (yr) | 60.7±7.7 | 53.1±14.7 | < 0.001 |
| Diabetes mellitus | 49 (66.2) | 74 (74.7) | 0.221 |
| Hypertension | 60 (81.1) | 80 (80.8) | 0.964 |
| NYHA score | 0.409 | ||
| III | 53 (71.6) | 64 (64.6) | |
| IV | 21 (28.4) | 35 (35.4) | |
| Valvular\heart disease | 8 (10.8) | 13 (13.1) | 0.644 |
| QRS (ms) | 148.2±34.9 | 144.8±23.9 | 0.455 |
| EF (%) | 21.9±8.7 | 20.7±5.4 | 0.318 |
| LVESV (mL) | 169.2±77.5 | 154.9±81.7 | 0.320 |
| LVEDV (mL) | 212.4±81.7 | 200.3±96.1 | 0.450 |
| Medication | |||
| Digoxin | 56 (75.7) | 82 (82.8) | 0.247 |
| Carvedilol | 44 (59.5) | 52 (52.5) | 0.364 |
| Nitrate | 51 (68.9) | 35 (35.4) | < 0.001 |
| Beta-blocker | 7 (9.5) | 13 (13.1) | 0.455 |
| Anti-arrhythmia | 23 (31.1) | 31 (31.3) | 0.974 |
| Warfarin | 5 (6.8) | 10 (10.1) | 0.439 |
| Calcium-blocker | 3 (4.1) | 4 (4.0) | 0.999 |
| Statin | 45 (60.8) | 32 (32.3) | < 0.001 |
| Diuretic | 55 (74.3) | 76 (76.8) | 0.711 |
| ACE-inhibitor | 46 (62.2) | 53 (53.5) | 0.257 |
| Spironolactone | 35 (47.3) | 63 (63.6) | 0.032 |
Data are presented as mean±SD or number (%)
NYHA, New York Heart Association; EF, Ejection fraction; LVESV, Left ventricular end systolic volume; LVEDV, Left ventricular end diastolic volume; ACE, Angiotensin converting enzyme
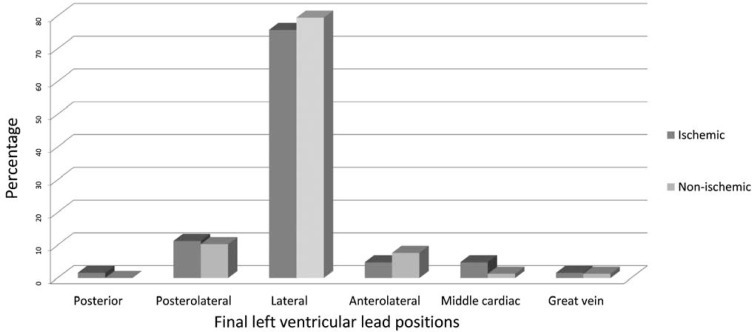
Regarding the drugs used, nitrates, statins, and spironolacton were administered more in the ischemic group than in the non-ischemic group. The LV lead positions in the ischemic and non-ischemic patients were as follows: posterior vein (1.6% versus 0.0%); posterolateral vein (11.3% versus 10.3%); lateral vein (75.8% versus 79.5%); anterolateral vein (4.8% versus 7.7%); middle vein (4.8% versus 1.3%); and anterior (1.6% versus 1.3%). There were no significant differences in terms of the distribution of the final LV lead positions between the two groups (Figure 1).
Figure 1.
Final left ventricular lead positions (p = 0.647)
Tables 2 and 3 illustrate the quantity and distribution of the coronary veins identified and the proportion of the veins deemed suitable targets for potential LV lead placement. Twenty-three (31.1%) patients in the ischemic group and 24 (24.2%) in the dilated group had only one suitable vein. The most available suitable vein in both groups was the lateralvein, followed by the posterior vein in the ischemic and the posterolateral vein in the dilated group. There was no significant difference between the two groups with respect to the position of the available suitable vein with the exception of the posterior position, in which the ischemic group had slightly more suitable veins than did the dilated group. Nevertheless, there was no significant difference as regards the final vein through which the LV lead was inserted.
Table 2.
Characteristics of suitable veins in all patients and subgroups according to underlying etiology*
| Vein | Total | Ischemic group | Non-ischemic group | P value |
|---|---|---|---|---|
| Lateral | 117 (83.6) | 50 (80.6) | 67 (85.9) | 0.405 |
| Posterior | 55 (39.3) | 30 (48.4) | 25 (32.1) | 0.049 |
| Posterolateral | 47 (33.6) | 18 (29.0) | 29 (37.2) | 0.311 |
| Anterolateral | 42 (30.0) | 17 (27.4) | 25 (32.1) | 0.552 |
| Middle | 14 (10.0) | 7 (11.3) | 7 (9.0) | 0.650 |
Data are presented as number (%)
Table 3.
Number of suitable veins in all patients and subgroups according to underlying etiology*
| Vein | Total | Ischemic group | Non-ischemic group | P value |
|---|---|---|---|---|
| None | 25 (14.5) | 7 (9.5) | 18 (18.2) | 0.160 |
| One | 47 (27.2) | 23 (31.1) | 24 (24.2) | 0.451 |
| Two | 73 (42.2) | 32 (43.2) | 41 (41.4) | 0.878 |
| Three | 22 (12.7) | 8 (10.8) | 14 (14.1) | 0.566 |
| Four | 6 (3.5) | 4 (5.4) | 2 (2.0) | 0.246 |
Data are presented as number (%)
The results were also considered in the two subgroups of ischemic patients with and without a history of CABG (Table 4). Comparative analysis showed that the patients with pre-CABG had significantly fewer suitable veins in the posterolateral position than did the others; there was, however, no significant difference between the two subgroups regarding the final vein position through which the leads were inserted.
Table 4.
Characteristic of suitable veins in ischemic group according to the history of coronary artery bypass surgery (CABG)*
| Vein | CABG group | Non-CABG group | P value |
|---|---|---|---|
| Lateral | 32 (74.4) | 19 (61.3) | 0.229 |
| Posterior | 20 (46.5) | 13 (41.9) | 0.696 |
| Posterolateral | 7 (16.3) | 12 (38.7) | 0.029 |
| Anterolateral | 7 (16.3) | 10 (32.3) | 0.107 |
| Middle | 4 (9.3) | 3 (9.7) | 0.957 |
Data are presented as number (%)
Discussion
The present study is a report on the quantity and distribution of the coronary venous anatomy for the LV lead targeting in patients undergoing CRT. We hypothesized that the main etiology of heart failure might affect the quality of the lead targeting and showed that the underlying ischemic or nonischemic etiologies of heart failure did not appear to make significant differences in the potential for the ventricular lead targeting.
Newly designed pacemakers along with developments in lead implantation techniques such as trans-coronary venous lead insertion have thoroughly revolutionized CRT and management of cardiomyopathy diseases. The LV lead placement is restricted by the variability in the coronary venous anatomy. In addition, there may be issues related to the lead instability, inadequate pacing and sensing thresholds, and diaphragmatic pacing. In this context, the LV lead position has emerged as an important determinant of response. Even small changes in the LV lead position may be associated with exquisite changes in acute myocardial performance.15
On the basis of our findings and the abovementioned points, the general approach at present is to place the LV lead in the lateral region, which has been previously reported.16 In a similar study by Zaman Khan et al.,6 the underlying etiology could not affect the quantity and distribution of the coronary veins available for the ventricular lead placement.
With respect to the influence of the ischemic etiology of heart failure, we observed no significant differences between the two subgroups with and without a history of CABG regarding the final vein position through which the leads were inserted. In addition, in our research, the patients with pre-CABG had significantly fewer suitable veins in only the posterolateral position. However, differences between groups of patients have been suggested by some authors who reported a paucity of lateral veins in subgroups of patients with either a history of myocardial infarction or previous CABG.17, 18 In light of the fact that some previous studies using MDCT have shown that variations in the coronary venous anatomy are consistent with a prior history of MI or CABG, our finding is slightly unexpected.
Conclusion
The results of our study demonstrated that the final coronary vein position suitable and selected for the LV lead insertion was not different between the cases with cardiomyopathy with different etiologies, and nor was it different between the ischemic cases with and without a history of CABG. In other words, patients with a history of procedures around the coronary vessel may have an intact or recovered venous system and may, as a result, benefit from the transvenous LV lead placement for CRT.
Acknowledgments
This study was supported by Tehran University of Medical Sciences and Tehran Heart Center. We thank the University authorities, who offered critical administrative support and managerial services in carrying out the study, and also all the researchers for their help and support.
References
- 1.Kazemi Saeid A, Bozorgi A, Davoodi G, Sadeghian S, Yaminisharif A, Moezi A, Alenabi T, Khoshnevis M. Comparison of benefits from cardiac resynchronization therapy between patients with ischemic cardiomyopathy and patients with idiopathic dilated cardiomyopathy. J Teh Univ Heart Ctr. 2009;2:115–118. [Google Scholar]
- 2.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure. Eur Heart J. 2006;27:1928–1932. doi: 10.1093/eurheartj/ehl099. [DOI] [PubMed] [Google Scholar]
- 3.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC, Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 4.Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J, MIRACLE Study Group Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 5.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K, Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 6.Khan FZ, Virdee MS, Gopalan D, Rudd J, Watson T, Fynn SP, Dutka DP. Characterization of the suitability of coronary venous anatomy for targeting left ventricular lead placement in patients undergoing cardiac resynchronization therapy. Europace. 2009;11:1491–1495. doi: 10.1093/europace/eup292. [DOI] [PubMed] [Google Scholar]
- 7.Ypenburg C, Roes SD, Bleeker GB, Kaandorp TA, de Roos A, Schalij MJ, van der Wall EE, Bax JJ. Effect of total scar burden on contrast-enhanced magnetic resonance imaging on response to cardiac resynchronization therapy. Am J Cardiol. 2007;99:657–660. doi: 10.1016/j.amjcard.2006.09.115. [DOI] [PubMed] [Google Scholar]
- 8.Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. 2007;153:105–112. doi: 10.1016/j.ahj.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Ypenburg C, van Bommel RJ, Delgado V, Mollema SA, Bleeker GB, Boersma E, Schalij MJ, Bax JJ. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol. 2008;52:1402–1409. doi: 10.1016/j.jacc.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 10.Van de Veire NR, Schuijf JD, De Sutter J, Devos D, Bleeker GB, de Roos A, van der Wall EE, Schalij MJ, Bax JJ. Non-invasive visualization of the cardiac venous system in coronary artery disease patients using 64-slice computed tomography. J Am Coll Cardiol. 2006;48:1832–1838. doi: 10.1016/j.jacc.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 11.Christiaens L, Ardilouze P, Ragot S, Mergy J, Allal J. Prospective evaluation of the anatomy of the coronary venous system using multidetector row computed tomography. Int J Cardiol. 2008;126:204–208. doi: 10.1016/j.ijcard.2007.03.128. [DOI] [PubMed] [Google Scholar]
- 12.Meisel E, Pfeiffer D, Engelmann L, Tebbenjohanns J, Schubert B, Hahn S, Fleck E, Butter C. Investigation of coronary venous anatomy by retrograde venography in patients with malignant ventricular tachycardia. Circulation. 2001;104:442–447. doi: 10.1161/hc2901.093145. [DOI] [PubMed] [Google Scholar]
- 13.Blendea D, Mansour M, Shah RV, Chung J, Nandigam V, Heist EK, Mela T, Reddy VY, Manzke R, McPherson CA, Ruskin JN, Singh JP. Usefulness of high-speed rotational coronary venous angiography during cardiac resynchronization therapy. Am J Cardiol. 2007;100:1561–1565. doi: 10.1016/j.amjcard.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 14.Blendea D, Shah RV, Auricchio A, Nandigam V, Orencole M, Heist EK, Reddy VY, McPherson CA, Ruskin JN, Singh JP. Variability of coronary venous anatomy in patients undergoing cardiac resynchronization therapy: a high-speed rotational venography study. Heart Rhythm. 2007;4:1155–1162. doi: 10.1016/j.hrthm.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Dekker AL, Phelps B, Dijkman B, van der Nagel T, van der Veen FH, Geskes GG. Epicardial left ventricular lead placement for cardiac resynchronization therapy: optimal pace site selection with pressure-volume loops. J Thorac Cardiovasc Surg. 2004;127:1641–1647. doi: 10.1016/j.jtcvs.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Purerfellner H, Nesser HJ, Winter S. Transvenous left ventricular lead implantation with the EASYTRAK lead system: the European experience. Am J Cardiol. 2000;86:157–164. doi: 10.1016/s0002-9149(00)01299-6. [DOI] [PubMed] [Google Scholar]
- 17.Van de Veire NR, Schuijf JD, De Sutter J, Devos D, Bleeker GB, de Roos A. Non-invasive visualization of the cardiac venous system in coronary artery disease patients using 64-slice computed tomography. J Am Coll Cardiol. 2006;48:1832–1838. doi: 10.1016/j.jacc.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 18.Christiaens L, Ardilouze P, Ragot S, Mergy J, Allal J. Prospective evaluation of the anatomy of the coronary venous system using multidetector row computed tomography. Int J Cardiol. 2008;126:204–208. doi: 10.1016/j.ijcard.2007.03.128. [DOI] [PubMed] [Google Scholar]