Abstract
Background
Selenium (Se) is an essential trace element mainly obtained from seafood, meat, and cereals. Se deficiency has been identified as a major contributing factor in the pathogenesis of certain congestive heart failure (CHF) syndromes. Since there is controversy over the prevalence of Se deficiency among patient with CHF, the aim of this study was to assess the serum Se concentrations in patients with CHF and compared them with the Se status of healthy controls.
Methods:
The study included 77 patients (age, 68.4 ± 10.4 years old; 40.3% female) and 73 healthy volunteers (64.9 ± 4.7 years old; 35.6% female). A complete medical/drug history and physical examination were performed for all patients and healthy volunteers. All patients had symptoms and signs of CHF and had a left ventricular ejection fraction (EF) of < 40% obtained by echocardiography. The Se concentration was assessed by atomic absorption spectrometer with the Graphite Tube Atomizer. The limit of measurement was 5 μg/L.
Results:
The Se concentrations in CHF patients did not show a significant difference from those of healthy controls (185.9 ± 781.2 μg/L vs. 123.3 ± 115.5 μg/L, respectively; p value = 0.499). There was no correlation between serum Se concentrations and EF in both the normal group and the patients with heart failure (p value = 0.96 and 0.99; r = 0.006 and 0.002 for patients and healthy volunteers, respectively).
Conclusion:
In this study, serum Se levels in CHF patients were similar to those of controls and the Se concentrations did not correlate with the degree of left ventricular dysfunction.
Keywords: Selenium, Heart failure, Atrial fibrillation
Introduction
Chronic heart failure (CHF) is the common end-result of many cardiovascular diseases. Despite the improvements in the methods of prevention and the new strategies in the treatment of patients, the incidence of CHF is increasing in many countries.1, 2 In recent years, the focus of research for the treatment of CHF patients has been on drug therapies and devices, while other factors may affect the rise in the number of CHF patients. For example, oxidative stress may contribute to the pathogenesis of CHF;3, 4 and some clinical and experimental studies have shown that in CHF patients, free radical formation is increased and antioxidant defenses are reduced.3, 5, 6 Also, dietary trials that assessed the impact of high intakes of natural antioxidants showed that the incidence of CHF was reduced.7, 8
Selenium (Se), an essential trace mineral, is mainly obtained from seafood, meat, and cereals. Se deficiency has been identified as a major contributing factor in the pathogenesis of certain CHF syndromes. For instance, in areas with low soil Se contents such as eastern China and western Africa, Se deficiency is associated with cardiomyopathy.9, 10 Moreover, there are cases of cardiomyopathy associated with low Se levels in malnourished HIV-infected patients in western countries11 and in patients on chronic parenteral nutrition.12 It has also been reported that serum levels of Se were lower in idiopathic dilated and also ischemic cardiomyopathy patients compared with levels in healthy controls.13 On the other hand, in another report, the mean serum Se levels in 30 patients with idiopathic cardiomyopathy did not show any difference from those of healthy individuals.14 It has been suggested that Se may be involved in the deconditioning of skeletal and cardiac muscles and in CHF symptoms such as fatigue and low exercise tolerance, rather than in ventricular dysfunction.15, 16
Regarding the controversy over the prevalence of Se deficiency among CHF patients, the present study aimed at assessing serum Se levels in a relatively large population of advanced CHF patients and comparing them with those of healthy controls.
Methods
This study was performed at Mazandaran Heart Center, Sari, Iran, during an 11-month period beginning in March 2010. We enrolled 77 patients and 73 healthy volunteers as the control group. The patients were selected from those who were followed at the hospital clinic. All the patients had CHF symptoms and a left ventricular ejection fraction (LVEF) of < 0.40. In the CHF group, 39 patients had chronic atrial fibrillation (AF) and 38 patients were in sinus rhythm.
The patients were on medications, including angiotensin converting enzyme (ACE) inhibitors, diuretics, digitalis, beta blockers, and angiotensin receptor blockers (ARBs). The calculation of the LVEF was according to Simpson’s rule.17
The healthy controls were recruited from the family members of the patients at the hospital or those undergoing cardiovascular screening at the clinic. For the control group, in addition to echocardiography, we took a complete medical and drug history and performed a complete physical examination. None of the control group members had chronic diseases such as diabetes mellitus, hypertension, and hyperlipidemia. Nearly all the patients and controls were permanent residents in the northern part of Iran at the time of study. Electrocardiograms (ECG) and echocardiography were performed for all the subjects. All the volunteers had normal ECG and echocardiographic findings. The volunteers did not have a history of consuming ACE inhibitors, ARBs, digitalis, and diuretics and did not receive any Se-containing supplements within the prior three weeks.
A 10 ml blood sample was taken from the patients and the control group, which was then heated in special tubes of water bath (37 °C) for 1 h. The samples were centrifuged (1500 rpm) and frozen at −20 °C after serum isolation. Varian AA240FS atomic absorption spectrometer with the Graphite Tube Atomizer (Mulgrave Victoria, Australia) was used to determine Se concentrations. Five hundred micro liters of the sample were diluted with 2 ml of the Trinon-ascorbic acid reagent. A 50 ppb standard was prepared by diluting the 1000 ppm standard, with distilled water. The sample concentrations were calculated on the basis of computer-determined calibration graphs. The limit of measurement was 5 μg/L.18
Statistical analysis was performed using the SPSS 16 software. An independent samples t-test was used for the comparison of the quantitative variables between the patient and control groups. The chi-Square test was utilized for the nominal variables. The relationship between Se concentration and LVEF was assessed using Pearson’s Correlation test. A p value < 0.05 was considered significant, and the power of study was 10% (Z1−β = − 1.28).
Results
The demographic, echocardiographic data, and Se levels of “heart failure” and “control” groups are shown in Table 1. The CHF patients were more frequently diabetic. The average age of the control group was near that of the patients. The mean values of left atrial areas (LAA) and left ventricular systolic and diastolic diameters were higher in patients than in the controls, which was statistically significant.
Table 1.
Basic demographic, clinical, and echocardiographic data as well as selenium levels of heart failure and control groups*
| Heart Failure (n=77) | Control (n=73) | P value** | |
|---|---|---|---|
| Age (y) | 68.4±10.4 | 64.9±4.7 | 0.02 |
| Female | 31 (40.3) | 26 (35.6) | 0.55 |
| Weight | 67.1±13.6 | 66.5±5.0 | 0.77 |
| Height | 165.2±9.2 | 166.7±7.5 | 0.32 |
| BMI | 24.4±3.7 | 24.0±2.2 | 0.46 |
| HLP | 18 (25.4) | - | < 0.01 |
| DM | 15 (18.3) | - | < 0.01 |
| SBP | 136.4±29.1 | 129.2±7.5 | 0.04 |
| DBP | 81.3±15.4 | 72.4±6.6 | < 0.01 |
| HR | 92.0±21.4 | 72.1±4.7 | < 0.01 |
| LAA | 28.5±8.2 | 19.5±1.1 | < 0.01 |
| LVDD | 61.9±8.9 | 49.8±1.5 | < 0.01 |
| LVSD | 52.4±8.7 | 31.4±2.3 | < 0.01 |
| LVEF | 25.2±6.3 | 54.3±2.6 | < 0.01 |
| Selenium | 185.9±781.2 | 123.3±115.5 | 0.49 |
Data are presented as mean±SD or n (%)
P value (independent samples t-test, and Chi-Square for quantitative and qualitative variables, respectively)
BMI, Body mass index; HLP, Hyperlipidemia; DM, Diabetes mellitus; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; HR, Heart rate; LAA, Left atrium area; LVDD, Left ventricle diastolic diameter; LVSD, Left ventricle systolic diameter; LVEF, Left ventricle ejection fraction
Also, the average heart rate and systolic and diastolic blood pressures of the patients were higher than the values in the control group and it was statistically significant.
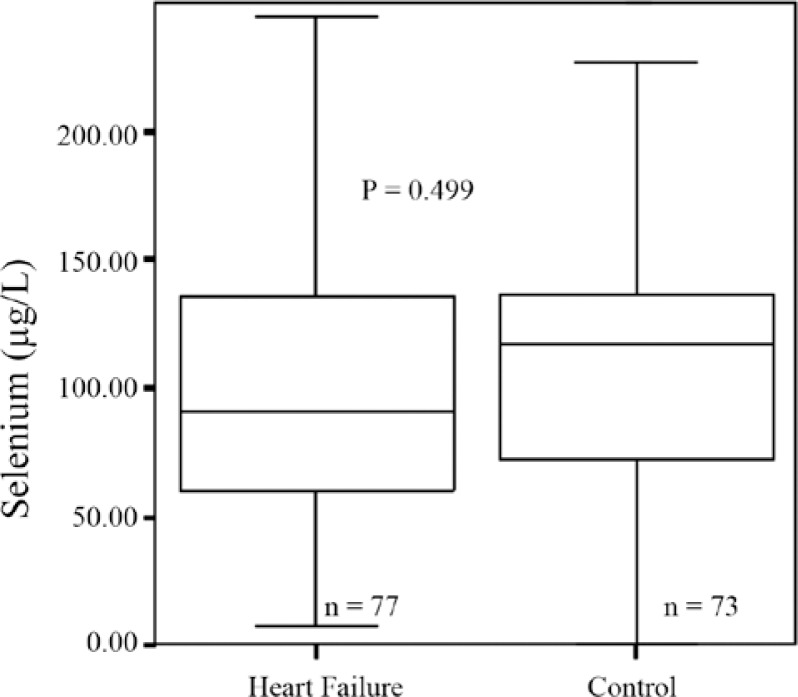
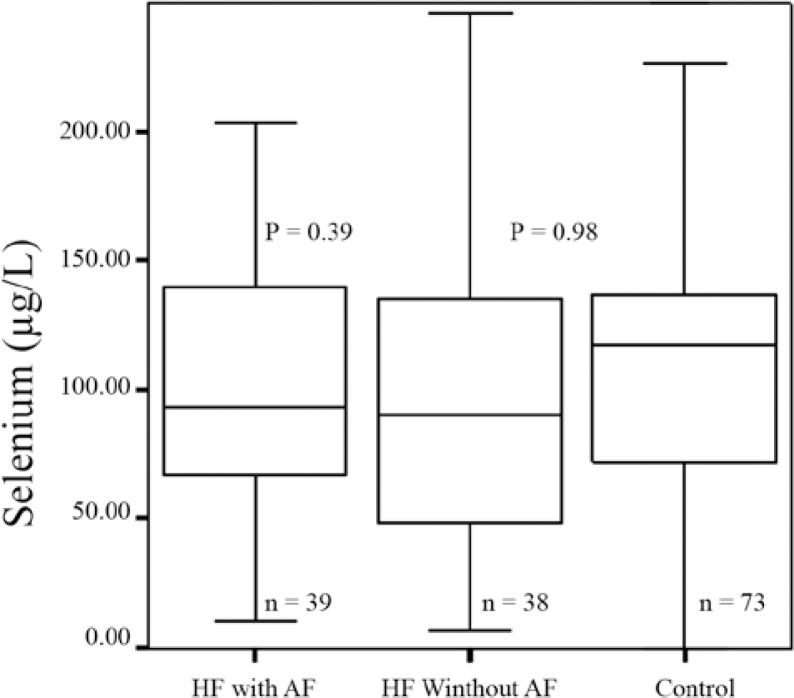
As is presented in Figure 1, the median Se concentrations in the CHF patients were not significantly different from those of the control group. Also, we did not find any statistically significant difference between the Se concentrations in the CHF patients with atrial fibrillation and those who had sinus rhythm (p value = 0.39). We also made separate comparisons between the Se concentrations in patients with and without AF and those of the control group, with the Se concentrations showing no statistically significant difference between the groups (Figure 2).
Figure 1.
Selenium concentrations in congestive heart failure patients versus control (P value: independent samples t-test; patients versus control)
Figure 2.
Comparison between selenium concentrations in congestive heart failure (HF) patients with/without atrial fibrillation (AF) and those of the control (P value, independent samples t-test; patients of each group versus control)
There was no correlation between serum Se concentrations and EF in both the normal group and CHF patients (p value = 0.96 and 0.99; r = 0.006 and 0.002 for patients and healthy volunteers, respectively).
Discussion
This is the first study in the northern part of Iran to compare Se concentrations in a relatively large number of CHF patients with those of a control group. Nonetheless, we did not find any significant difference between the two groups. It had previously been shown that in both CHF and healthy groups there was a similar relationship between dietary and blood Se levels.14 The northern part of Iran has a Mediterranean climate and diet. The Mediterranean diet is a Se-rich diet including seafood and vegetables, and it is considered a cardioprotective diet.8 The relatively wider dispersion of Se concentrations in CHF patients may partially be related to the consumption of a wider range of Se-containing food. However, we do not have any definite explanation as to the cause.
It is now widely accepted that diet-derived antioxidants may play a role in the development, progress, and prevention of CHF. For instance, some clinical studies have suggested that CHF may be associated with increased free radical formation and reduced antioxidant defenses.15, 19, 20 Still, it is important to know whether any specific micronutrient deficiency might have a causal relationship with CHF and/ or whether it can aggravate CHF symptoms. For example, hypozincemia in CHF patients may be an effect of diuretic drugs, but there are no definite data on the clinical effect of zinc supplementation in these patients. Nevertheless, there are some CHF syndromes in which Se deficiency has been identified as a contributing factor in the etiology. For example, in China an endemic cardiomyopathy called Keshan disease has been illustrated to be the result of Se deficiency.10, 21 But, in Keshan disease Se levels correlate with the clinical severity of CHF, rather than the degree of left ventricular dysfunction. Interestingly though, Se supplementation resulted in a reduced mortality rate.12, 21, 22 In endemic areas, however, after raising Se levels in residents, clinically latent cases were still found. Thus, although Se deficiency can also influence the clinical severity of Keshan disease, it is not the specific etiologic factor for the occurrence of it. Also, in Western countries, cases of congestive cardiomyopathy associated with low serum vitamins and trace elements have been reported in malnourished HIV-infected patients and in subjects on chronic parenteral nutrition.15
In a relatively small number of CHF patients, de Lorgeril et al. found lower dietary intake and blood levels of Se compared with those of healthy controls.15 Also, blood levels of Se were lower in CHF patients. There was a positive correlation between Se intake and blood levels, and the low dietary intake accounted for the low blood Se levels. In our study, we did not find any statistically significant difference regarding the blood Se levels between the CHF and healthy controls. Also, the blood Se levels in both CHF and healthy controls in our study were higher than those in the study by Lorgeril et al. Thus, we think that the Mediterranean diet in the northern part of Iran, which includes seafood and vegetables, has resulted in normal, rather than deficient, blood Se levels in this area. This confirms the finding by de Lorgeril et al., who did not find a causative relationship between Se deficiency and CHF. In addition, in our study, Se concentrations did not show any association with the severity of ventricular dysfunction assessed by echocardiography. On the other hand, blood Se was strongly related to maximum oxygen consumption and exercise tolerance in CHF patients,15 and also in Keshan disease even a mild deficiency in Se could influence the severity of the disease. Be that as it may, the mechanism by which Se deficiency results in some CHF symptoms is not well defined and it has been suggested that Se may be involved in skeletal and cardiac muscle deconditioning rather than in left ventricular dysfunction.16, 21
Se is an essential trace element. Its primary role is that of an antioxidant in the enzyme glutathione peroxidase (GP), the main intracellular antioxidant. Depletion of Se leads to a decrease in GP activity. Selenoprotein P is another selenoprotein and its primary role is as an extracellular antioxidant.23 Also the Se-dependent thioredoxin reductase system may be involved in ascorbate regeneration.24 Consequently, in addition to their role in vascular endothelial function,25 selenoproteins act as antioxidants. Considering the fact that CHF is associated with peripheral vasoconstriction and impaired skeletal muscle metabolism,26 Se-dependent systems are very important defense mechanisms in humans.
However, there are some limitations to the present study. For example, the relationship between dietary and blood Se levels in CHF and healthy groups has previously been shown. In our study, we did not have a definite dietary comparison between patients and controls; CHF patients may have been advised to take more vegetables and seafood after developing the disease.
The power of the present study is 10%. We do realize that this amount of the power is really low; it is worthy of note, however, that choosing the number of cases was based on the previous similar studies (study of Sahin et al.,13 Heart failure: n = 54, Healthy Volunteers: n = 30; study of de Lorgeril et al.,15 Heart failure: n = 21, Healthy Volunteers: n = 18). The number of cases included in our study was 77 patients and 73 healthy volunteers, which was higher than those in the aforementioned studies. The low power of our study can be explained by the great SD of the Se levels, especially in heart failure patients.
Conclusion
In conclusion, although we did not find a causal relationship between Se and CHF in the northern part of Iran, we recommend that monitoring dietary determinants can be helpful in the treatment and management of CHF patients in all areas.
Acknowledgments
This study was approved and supported by a grant from the Research and Technology Deputy of Mazandaran University of Medical Sciences.
References
- 1.Cowie MR, Mosterd A, Wood DA, Deckers JW, Poole-Wilson PA, Sutton GC, Grobbee DE. The epidemiology of heart failure. Eur Heart J. 1997;18:208–225. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 3.Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 4.Givertz MM, Colucci WS. New targets for heart failure therapy: endothelin, inflammatory cytokines and oxidative stress. Lancet. 1998;352:34–38. doi: 10.1016/s0140-6736(98)90017-4. [DOI] [PubMed] [Google Scholar]
- 5.Bauersachs J, Bouloumi′e A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation. 1999;100:292–298. doi: 10.1161/01.cir.100.3.292. [DOI] [PubMed] [Google Scholar]
- 6.Dhalla AK, Hill M, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol. 1996;28:506–514. doi: 10.1016/0735-1097(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 7.Singh RB, Rastogi SS, Verma R, Laxmi B, Singh R, Ghosh S, Niaz MA. Randomized controlled trial of cardioprotective diet in patients with recent acute myocardial infarction: results of one year follow-up. Br Med J. 1992;304:1015–1019. doi: 10.1136/bmj.304.6833.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors and the rate of cardiovascular complications after myocardial infarction. Final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 9.Neve J. Selenium as a risk factor for cardiovascular diseases. J Cardiovasc Risk. 1996;3:42–47. [PubMed] [Google Scholar]
- 10.Ge K, Yang G. The epidemiology of Se deficiency in the etiological study of endemic diseases in China. Am J Clin Nutr. 1993;57:259S–263S. doi: 10.1093/ajcn/57.2.259S. [DOI] [PubMed] [Google Scholar]
- 11.Chariot P, Perchet H, Monnet I. Dilated cardiomyopathy in HIV-infected patients. N Engl J Med. 1999;340:732–735. doi: 10.1056/NEJM199903043400911. [DOI] [PubMed] [Google Scholar]
- 12.Huttunen JK. Selenium and cardiovascular diseases. An update. Biomed Environ Sci. 1997;10:220–226. [PubMed] [Google Scholar]
- 13.Sahin I, Taşkapan C. Trace element status (Se, Zn, Cu) in heart failure. Anadolu Kardiyol Derg. 2006;6:216–220. [PubMed] [Google Scholar]
- 14.da Cunha S, Albanesi Filho FM, da Cunha Bastos VL, Antelo DS, Souza MM. Thiamin, Se, and copper levels in patients with idiopathic dilated cardiomyopathy taking diuretics. Arq Bras Cardiol. 2002;79:454–465. doi: 10.1590/s0066-782x2002001400003. [DOI] [PubMed] [Google Scholar]
- 15.de Lorgeril M, Salen P, Accominotti M, Cadau M, Steghens JP, Boucher F, de Leiris J. Dietary and blood antioxidants in patients with chronic heart failure. Insights into the potential importance of Se in heart failure. Eur J Heart Fail. 2001;3:661–669. doi: 10.1016/s1388-9842(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 16.de Lorgeril M, Salen P. Selenium and antioxidant defenses as major mediators in the development of chronic heart failure. Heart Fail Rev. 2006;11:13–17. doi: 10.1007/s10741-006-9188-2. [DOI] [PubMed] [Google Scholar]
- 17.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 18.Navarro M, Lopez H, Ruiz ML, Gonzalez S, Perez V, Lopez MC. Determination of selenium in serum by hydride generation atomic absorption spectrometry for calculation of daily dietary intake. Sci Total Environ. 1995;175:245–252. doi: 10.1016/0048-9697(95)04859-6. [DOI] [PubMed] [Google Scholar]
- 19.Shokrzadeh M, Ghaemian A, Salehifar E, Aliakbari S, Saravi SS, Ebrahimi P. Serum zinc and copper levels in ischemic cardiomyopathy. Biol Trace Elem Res. 2009;127:116–123. doi: 10.1007/s12011-008-8237-1. [DOI] [PubMed] [Google Scholar]
- 20.Salehifar E, Shokrzadeh M, Ghaemian A, Aliakbari S, Saeedi Saravi SS. The study of Cu and Zn serum levels in idiopathic dilated cardiomyopathy (IDCMP) patients and its comparison with healthy volunteers. Biol Trace Elem Res. 2008;125:97–108. doi: 10.1007/s12011-008-8151-6. [DOI] [PubMed] [Google Scholar]
- 21.de Lorgeril M, Salen P, Defaye P. Importance of nutrition in chronic heart failure patients. Eur Heart J. 2005;26:2215–2217. doi: 10.1093/eurheartj/ehi490. [DOI] [PubMed] [Google Scholar]
- 22.Xu GL, Wang SC, Gu BQ, Yang YX, Song HB, Xue WL, Liang WS, Zhang PY. Further investigation on the role of Se deficiency in the etiology and pathogenesis of Keshan disease. Biomed Environ Sci. 1997;10:316–326. [PubMed] [Google Scholar]
- 23.Burk RF, Hill KE, Selenoprotein P. A selenium-rich extracellular glycoprotein. J Nutr. 1994;124:1891–1897. doi: 10.1093/jn/124.10.1891. [DOI] [PubMed] [Google Scholar]
- 24.May JM, Mendiratta S, Hill KE, Burk RF. Reduction of dehydroascorbate to ascorbate by the selenoenzyme thioredoxin reductase. J Biol Chem. 1997;272:22607–22610. doi: 10.1074/jbc.272.36.22607. [DOI] [PubMed] [Google Scholar]
- 25.Drexler H. Endothelium as a therapeutic target in heart failure. Circulation. 1998;98:2652–2655. doi: 10.1161/01.cir.98.24.2652. [DOI] [PubMed] [Google Scholar]
- 26.Okita K, Yonezawa K, Nishijima H, Hanada A, Ohtsubo M, Kohya T, Murakami T, Kitabatake A. Skeletal muscle metabolism limits exercise capacity in patients with chronic heart failure. Circulation. 1998;98:1886–1891. doi: 10.1161/01.cir.98.18.1886. [DOI] [PubMed] [Google Scholar]