Abstract
Background
Obesity is a growing public health problem among reproductive-aged women, with consequences for chronic disease risk and reproductive and obstetric morbidities. Evidence also suggests that body shape (i.e., regional fat distribution) may be independently associated with risk, yet it is not known if women adequately perceive their shape. This study aimed to assess the validity of self-reported body size and shape figure drawings when compared to anthropometric measures among reproductive-aged women.
Methods
Self-reported body size was ascertained using the Stunkard nine-level figures and self-reported body shape using stylized pear, hourglass, rectangle, and apple figures. Anthropometry was performed by trained researchers. Body size and body mass index (BMI) were compared using Spearman's correlation coefficient. Fat distribution indicators were compared across body shapes for nonobese and obese women using analysis of variance (ANOVA) and Fisher's exact test. Percent agreement and kappa statistics were computed for apple and pear body shapes.
Results
The 131 women studied were primarily Caucasian (81%), aged 32 years, with a mean BMI of 27.1 kg/m2 (range 16.6–52.8 kg/m2). The correlation between body size and BMI was 0.85 (p<0.001). Among nonobese women, waist-to-hip ratios (WHR) were 0.75, 0.75, 0.80, and 0.82 for pear, hourglass, rectangle, and apple, respectively (p<0.001). Comparing apples and pears, the percent agreement (kappa) for WHR≥0.80 was 83% (0.55).
Conclusions
Self-reported size and shape were consistent with anthropometric measures commonly used to assess obesity and fat distribution, respectively. Self-reported body shape may be a useful proxy measure in addition to body size in large-scale surveys.
Introduction
The prevalence of obesity has more than doubled over the past 30 years,1 with almost a quarter of reproductive-aged women currently estimated as obese.2 The long-term health impact of obesity is considerable, with consequences for all-cause mortality and chronic disease risks, including cardiovascular disease (CVD), type 2 diabetes, asthma, and various cancers.3 In addition to chronic disease risk, the growing rate of obesity in reproductive-aged women has consequences for reproductive and perinatal health outcomes, including subfertility, pregnancy complications, and neonatal morbidities.4,5 Evidence suggests that regional adiposity is an important predictor of chronic disease risk independent of total adiposity,6–8 particularly among nonobese and normal weight women.9 What remain unclear from the literature are the independent contributions of total and regional adiposity on health outcomes for women across the life course. This uncertainty may be partially attributable to limitations in methods for assessing regional adiposity on a large scale.
Most epidemiologic studies use anthropometric measurements of body size and shape as proxies for general and regional fat distribution, respectively. Body mass index (BMI, kg/m2) is the most widely used indicator of body size10 but cannot account for differences in body fat distribution or its corresponding health risks. The importance of regional differences in fat distribution for assessing health risk was first proposed by Vague11 who differentiated women anthropometrically into three body shapes: android (upper body fat, including visceral deposits at the level of the waist and subcutaneous deposits on the back), intermediate, and gynoid (lower body fat, including on the thighs and buttocks). More recent anthropometric indicators of body shape that have shown the greatest use in clinical and epidemiologic studies as predictive of disease risk are primarily assessing measures of central adiposity.12 These include waist circumference (WC),13 waist-to-hip ratio (WHR),14 and waist-to-height ratio (WHtR).15 However, anthropometric evaluation to assess body size and shape may not always be feasible in large epidemiologic studies where resources are limited or where historical information is of interest for life course studies.
Alternative data collection approaches for assessment of body size and shape are needed, especially approaches that can be based on self-report and attained though administration of questionnaires. A commonly used measure of self-reported whole body size in population-based studies is the Stunkard nine-level figure drawings,16 which have been demonstrated as a valid indicator of both current and past body weight.17–21 On the other hand, self-reported measures of body shape are limited. Some studies have looked at pant22 and bra cup size23,24 as proxies for abdominal or extra-abdominal upper body fat, although they cannot distinguish overall differences in upper, lower, and central body fat distributions. To date, there are no comparable self-reported indicators of whole body fat distribution patterns. The aim of this study was to assess whether reproductive-aged women adequately perceive and report their shape for incorporation into women's health and life course studies. Using the Stunkard nine-level figure drawings16 and stylized whole-body shape figure drawings adapted from Connell et al.,25 we compared self-reported body size and shape to anthropometric indicators of general and regional fat distribution, respectively.
Materials and Methods
Study design and population
Menstruating women between the ages of 18 and 44 years were enrolled in the Endometriosis, Natural History, Diagnosis and Outcomes (ENDO) Study at five participating hospital sites in Utah and nine participating clinical centers in California between 2007 and 2009. A matched exposure (operative) cohort design was used in which participants were considered to be exposed if they were scheduled to undergo a diagnostic or therapeutic laparoscopy or laparotomy irrespective of clinical indication. An unexposed (population) cohort of menstruating women, aged 18–44 years, was matched for age and residence within the geographic catchment area of participating sites. Women in both cohorts were excluded if they could not communicate in either English or Spanish or if they had a history of surgically confirmed endometriosis (prevalent disease). Enrollment in the population cohort required a willingness to undergo pelvic magnetic resonance imaging (MRI) to identify possible endometriosis; 217 women in the population were screened, and 131 enrolled in the study. Fifty-five women were excluded based on age, language, or prior history of endometriosis, and 31 refused to participate. For this article, we analyzed data from the population cohort of 131 currently menstruating women, aged 18–44 years, to assure representativeness of body size and shape distributions from a general population sample. All women participating provided informed consent. This study was conducted with the approval of Institutional Review Boards (IRB) at participating clinical centers. Further details of this study have been described previously.26
Data collection and variable descriptions
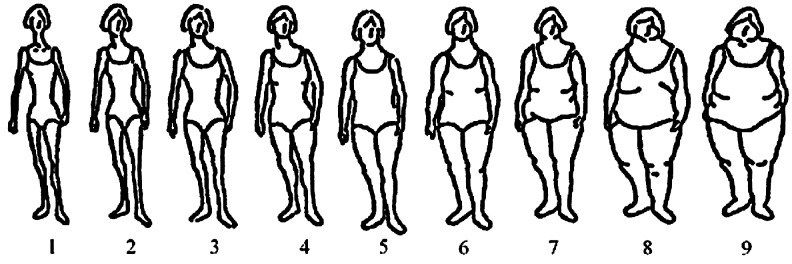
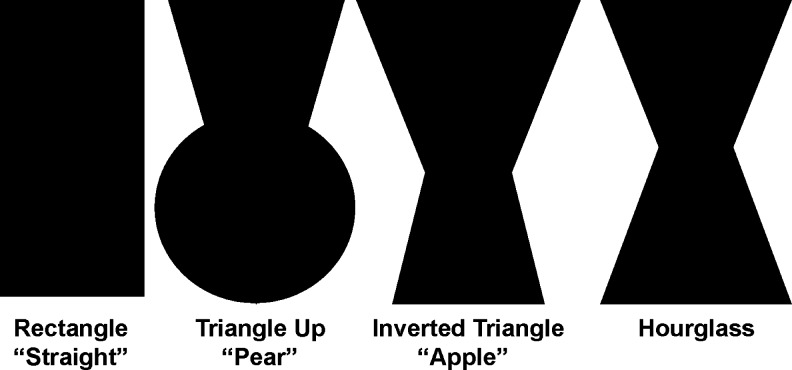
Trained research assistants collected descriptive information, including demographic factors, reproductive history, physical characteristics, and lifestyle factors, at baseline using computer-assisted technology with visual prompts. Specifically, women were asked to recall their body size according to the Stunkard nine-figure scale (Fig. 1)16 and shape (Fig. 2) according to stylized figures at 5-year age intervals (15–19, 20–24, 25–29, 30–34, 35–39, and 40–44) up to their current age. The four body shape figures (hourglass, pear, rectangle, and apple [inverted triangle]) used in this study were based on shapes identified in the Body Shape Assessment Scale (BSAS©) developed by Connell et al.25 The BSAS was developed among a representative sample of adult women using three-dimensional (3D) scanning methods originally designed to address sizing and fit problems in women's apparel in the United States. Reliability (average κ=0.46, average γ=0.80) of this scale was demonstrated when compared to evaluation from experts in the garment and textile industries.25 In addition to pictorial figures, we added medically relevant descriptions for each shape and asked women to think about how and where they tended to gain weight and about the relative proportions of their chest, waist, and hips.
FIG. 1.
Stunkard nine-level figure scale (used with permission).16
FIG. 2.
- Rectangle (straight): The circumferences of your chest and hips are about the same and you have little to no waist; when you gain weight, it distributes evenly, although with excess, your stomach may protrude.
- Triangle upward (pear or spoon): Your hip circumference is greater than your chest, and your waist is not prominent; when you gain weight, it tends to be disproportionately in your hips, rear, and thighs.
- Inverted triangle (apple): Your chest circumference is greater than your hips, and your waist is not prominent; when you gain weight, it tends to be disproportionately in your upper arms, shoulders (back), and chest (not necessarily the breasts).
- Hourglass: The circumferences of your chest and hips are about the same but you have a pronounced waist; when you gain weight, it is distributed on your shoulders, chest, hips, and rear before affecting your waist and stomach. Adapted from Connell et al.25
Following in-person interviews, anthropometric assessments were conducted by trained female nurses or research assistants according to procedures described by Lohman et al.27 Anthropometric measurements included height using a portable stadiometer, body weight using calibrated electronic scales, circumferences (waist, hip, and midupper arm), and skinfold thickness (triceps, subscapular, and suprailiac). WC measurements were taken at the natural waist location (i.e., the narrowest part of the torso), and hip circumference was taken at the level of maximum extension of the buttocks.27 For most women (95%–97%), circumference and skinfold thickness measurements were taken twice, and discrepancies >0.1–0.5 cm for various measurements were resolved by a third measurement (four height, seven waist circumference, and four hip circumference measures). The final value used was the average of the two closest measurements. During the anthropometric session, researchers recorded participants' current bra size. In addition, women were asked to report if they had a breast augmentation or reduction and their presurgery bra size.
BMI was calculated from height and weight measurements and divided into normal or underweight (BMI<25), overweight (25≤BMI<30), and obese (BMI>30). Chest circumference (cm) was determined by summing frame size (cm) and cup size (cm), similar to methods proposed by Wright.28 First, frame size was calculated by subtracting 5 inches from the bra size (e.g., 34 or 36) and multiplying the difference by 2.54 to convert to cm. Second, cup size was converted to cm by assigning values of 0.6, 1.9, 4.4, 7.6, 10.2, 13.3, 16.5, and 19.1 cm for cup sizes AA, A, B, C, D, DD (E), DDD (F), and G, respectively, and an additional 2.6 cm for each larger size. None of the women in this sample had a cup size of AA or larger than a size F. For women reporting breast surgery or liposuction, their current frame and cup size was used to determine chest circumference, which more accurately reflects their current perception of body shape. Using chest circumference and anthropometric measures, relative proportions of chest-to-waist (CWR), chest-to-hip (CHR), WHR, and WtHR were calculated. Binary indicators of central adiposity were determined using predefined cutoffs for WC (≥88cm), WHR (≥0.80), and WHtR (≥0.50) in women, as described in the literature.15,29–31 Using skinfolds, we also determined the arm fat index (AFI):
 |
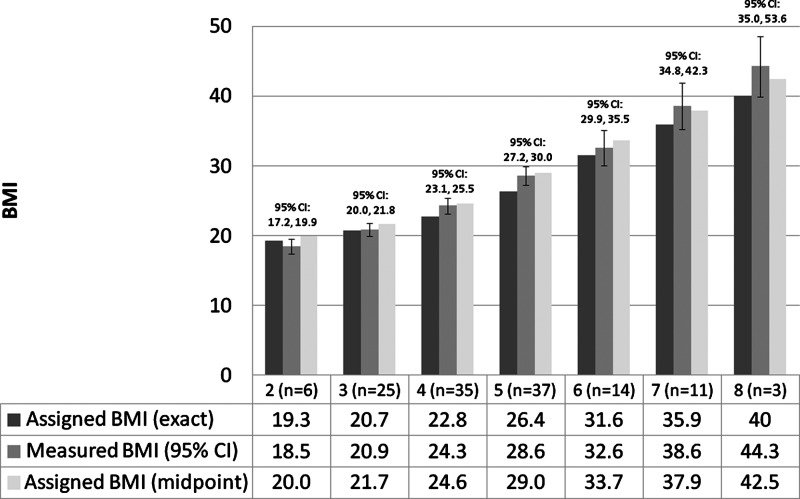
Self-reported current body size and shape were determined for each woman based on her current age and reported size and shape for that age group. For each body size figure, we assigned a BMI centile using a ranking procedure developed for girls at menarche18 and applied subsequently to adult women.32 The assigned centiles corresponding to figures one to nine were the 5th, 10th, 25th, 50th, 75th, 90th, 95th, 97th, and 99th, respectively. In this sample, we did not observe any women with self-reported figure sizes of one or nine. Given that the mean age of the study population was 32.6 years and the majority (81%) of women were Caucasian, each centile cutoff was assigned a BMI value in two ways using the distribution for white women aged 30–35 years, and the 97th and 99th percentiles were extrapolated based on BMI percentile charts.33 The first method assigned the exact BMI value for each centile cutoff, which corresponded to 19.3, 20.7, 22.8, 26.4, 31.6, 35.9, and 40.0 kg/m2 for figures two to eight, respectively. The second method assigned the midpoint of BMI values between two consecutive figures, which correspond to 20.0, 21.7, 24.6, 29.0, 33.7, 37.9, and 42.5 kg/m2 for body size figures two to eight, respectively, hypothesizing that women would self-report the lower figure if they perceived their size to be intermediate between two consecutive figures.
Statistical analysis
Descriptive characteristics of the population cohort were examined for all women and stratified by obesity status using t tests for continuous variables and Fisher's exact test for categorical variables. The correlation between body size figures and measured BMI was compared using Spearman's rank correlation coefficient, which allows for comparison of the relative ranking of women by the two values. Assigned BMI values (exact or midpoint) for each self-reported figure level were compared to the participants' measured BMI by anthropometry using a paired t test.
Anthropometric variables were compared across body shape categories for all women and by obesity status using analysis of variance (ANOVA) for continuous variables and Fisher's exact test for categorical variables. The normality of anthropometric indicators of obesity and fat distribution on the original scale and transformed scale was assessed visually using a subset of the ladder of powers34 and a modified skewness-kurtosis test of normality.35 If distributions were skewed, transformations were applied to achieve normality and back-transformed to compare values on the original scale. Based on the skewness-kurtosis test of normality, an inverse transformation was applied to all circumference measurements (chest, hip, and waist) and BMI, a natural log transformation was applied to the CWR, and a negative inverse of the square root transformation was applied to weight measurements. Because body shape is more distinguishable anthropometrically in nonobese women, the final analysis was limited to nonobese women. Percent agreement and kappa statistics were calculated to determine the extent to which self-reported apple and pear shapes agreed with anthropometric indicators of central adiposity. A two-sided p value of 0.05 was considered statistically significant. All analyses were performed in Stata 11.1 SE (StataCorp, College Station, TX).
Results
The study population was primarily non-Hispanic white (81%) or Hispanic white (11%), mean age of 32 years, married or living as married (60.3%), and nonsmokers (73.3%), with a mean BMI of 27.1 kg/m2 (range 16.6–52.8) (Table 1). Fourteen (11.0%) women were diagnosed with endometriosis by MRI, four (3.1%) reported they had been diagnosed with polycystic ovary syndrome (PCOS) by a physician, and eight (6.2%) reported breast surgery or liposuction. Obese and nonobese women differed significantly by self-reported figure sizes, mean age, age at menarche, and self-reported health status. No woman reported being the smallest or largest figure based on the Stunkard nine-level figure drawings. Measured BMI was highly correlated with self-reported figure (ρ=0.85, p<0.001) (Fig. 3). The mean (standard deviation [SD]) difference between measured BMI and BMI assigned from the nine-level figure drawings was 1.46 (3.84) (p<0.001) and −0.43 (3.75) (p=0.19) for BMI values assigned at the centile cutoff for each figure or at the midpoint between two consecutive figures, respectively.
Table 1.
Descriptive Characteristics of Population Cohort for All Women and by Obesity Status
| Characteristic | All | Nonobese BMI<30 | Obese BMI (30+) | p valuea |
|---|---|---|---|---|
| Total | 131 | 94 | 37 | |
| Current body size and shape | ||||
| BMI (kg/m2) | ||||
| Normal/underweight (BMI<25) | 60 (45.8) | - | - | - |
| Overweight (BMI 25–29.9) | 34 (26.0) | - | - | |
| Obese (BMI≥30) | 37 (28.2) | - | - | |
| Mean (SD) | 27.1 (7.0) | 23.6 (3.5) | 36.1 (5.4) | <0.001 |
| Self-reported body size, mean (SD) | ||||
| Assigned BMI (exact)b | 25.7 (5.1) | 23.5 (3.1) | 31.3 (5.0) | <0.001 |
| Assigned BMI (midpoint)b | 27.6 (5.5) | 25.2 (3.7) | 33.5 (4.9) | <0.001 |
| Self-reported body shape | ||||
| Pear | 41 (31.3) | 31 (33.0) | 10 (27.0) | 0.90 |
| Hourglass | 40 (30.5) | 28 (29.8) | 12 (32.4) | |
| Rectangle | 35 (26.7) | 25 (26.6) | 10 (27.0) | |
| Apple | 15 (11.5) | 10 (10.6) | 5 (13.5) | |
| Demographics | ||||
| Age (years) | ||||
| 15–19 | 4 (3.1) | 4 (4.3) | 0 (0) | 0.26 |
| 20–24 | 25 (19.1) | 21 (22.3) | 4 (10.8) | |
| 25–29 | 23 (17.6) | 15 (16.0) | 8 (21.6) | |
| 30–34 | 21 (16.0) | 16 (17.0) | 5 (13.5) | |
| 35–39 | 27 (20.6) | 20 (21.3) | 7 (18.9) | |
| 40–44 | 31 (23.7) | 18 (19.2) | 13 (35.1) | |
| Mean (SD) | 32.3 (7.8) | 31.5 (7.7) | 34.6 (7.5) | 0.04 |
| Race/ethnicity | ||||
| Non-Hispanic white | 106 (80.9) | 78 (83.0) | 28 (75.7) | 0.34 |
| Otherc | 25 (19.1) | 16 (17.0) | 9 (24.3) | |
| Education | ||||
| High school diploma or less | 13 (9.9) | 8 (8.5) | 5 (13.5) | 0.56 |
| Some college or technical school | 58 (44.3) | 41 (43.6) | 17 (46.0) | |
| College degree or greater | 60 (45.8) | 45 (47.9) | 15 (40.5) | |
| Household Incomed | ||||
| Below poverty line | 16 (12.3) | 10 (10.8) | 6 (16.2) | 0.50 |
| Within 180% of poverty line | 17 (13.1) | 14 (15.1) | 3 (8.1) | |
| Above 180% of poverty line | 97 (74.6) | 69 (74.2) | 28 (75.7) | |
| Marital status | ||||
| Married or living as married | 79 (60.3) | 55 (58.5) | 24 (64.9) | 0.56 |
| Single, divorced, or widowed | 52 (39.7) | 39 (41.5) | 13 (35.1) | |
| Reproductive | ||||
| Age at menarche categories | ||||
| ≤11 | 23 (17.6) | 11 (11.7) | 12 (32.4) | 0.006 |
| 12–13 | 71 (54.2) | 51 (54.3) | 20 (54.1) | |
| ≥14 or older | 37 (28.2) | 32 (34.0) | 5 (13.5) | |
| Mean (SD) | 12.7 (1.5) | 12.9 (0.15) | 12.1 (0.26) | 0.006 |
| Parity (no. of live births) | ||||
| Nulliparous | 64 (48.9) | 49 (52.1) | 15 (40.5) | 0.25 |
| Parous | 67 (51.1) | 45 (47.9) | 22 (59.5) | |
| Mean (SD) | 1.3 (1.6) | 1.3 (1.6) | 1.5 (1.5) | 0.39 |
| MRI-confirmed endometriosis | ||||
| No | 113 (89.0) | 81 (88.0) | 32 (91.4) | 0.76 |
| Yes | 14 (11.0) | 11 (12.0) | 3 (8.6) | |
| Lifestyle and health | ||||
| Smoking | ||||
| Never | 96 (73.3) | 70 (74.5) | 26 (70.3) | 0.86 |
| Former | 21 (16.0) | 14 (14.9) | 7 (18.9) | |
| Current | 14 (10.7) | 10 (10.6) | 4 (10.8) | |
| Alcohol (drinks per week) | ||||
| None | 100 (76.3) | 70 (74.5) | 30 (81.1) | 0.50 |
| ≥1 | 31 (23.7) | 24 (25.5) | 7 (18.9) | |
| Health status | ||||
| Excellent | 31 (23.7) | 28 (29.8) | 3 (8.1) | 0.02 |
| Very good | 57 (43.5) | 40 (42.6) | 17 (46.0) | |
| Good | 35 (26.7) | 22 (23.4) | 13 (35.1) | |
| Fair | 8 (6.1) | 4 (4.3) | 4 (10.8) | |
Data presented as n (%) unless otherwise specified.
p value comparing obese and nonobese women by t test (continuous variables) or Fisher's exact test (categorical variables).
Self-reported figure numbers ranged from two to eight. Assigned body mass index (BMI) values were 19.3, 20.7, 22.8, 26.4, 31.6, 35.9, and 40.0 and 20.0, 21.7, 24.6, 29.0, 33.7, 37.9, and 42.5 for exact and midpoint cutoffs, respectively, based on the distribution for white women aged 30–35 years and extrapolation.30
14 (11% of total) Hispanic white, 2 (1.5% of total) Black, 5 (3.8% of total) Asian, and 4 (3.1% of total) other and multiracial.
Based on the 2007 HHS Poverty Guidelines accounting for the numbers of persons in the household for the 48 contiguous states and District of Columbia.
MRI, magnetic resonance imaging; SD, standard deviation.
FIG. 3.
Body mass index (BMI) measured by anthropometry compared to BMI assigned based on centiles of the distribution for white women between ages 30 and 3536 by self-reported figure sizes 2 through 8 (Spearman's ρ=0.85, p<0.001). Mean difference (Δ), standard deviation (SD), and p value based on paired t test comparing measured BMI and assigned BMI based on exact centiles (Δ=1.46, SD 3.84, p<0.001) or midpoint of centiles (Δ=−0.43, SD 3.75, p=0.19). CI, confidence interval.
There were significant differences in mean ( ) height, CHR, and WHR, but not in AFI or CFR, across body shapes among nonobese women (Table 2). Mean WHR was highest in rectangles (
) height, CHR, and WHR, but not in AFI or CFR, across body shapes among nonobese women (Table 2). Mean WHR was highest in rectangles (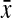 =0.80, 95% confidence interval [CI] 0.79-0.82) and apples (
=0.80, 95% confidence interval [CI] 0.79-0.82) and apples (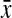 =0.82, 95% CI 0.75-0.89) compared with hourglass (
=0.82, 95% CI 0.75-0.89) compared with hourglass (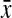 =0.75, 95% CI 0.74-0.77) and pears (
=0.75, 95% CI 0.74-0.77) and pears (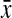 =0.75, 95% CI 0.73-0.76) in nonobese women. Similarly, mean CHR was higher in rectangles (
=0.75, 95% CI 0.73-0.76) in nonobese women. Similarly, mean CHR was higher in rectangles (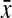 =0.83, 95% CI 0.80-0.85) and apples (
=0.83, 95% CI 0.80-0.85) and apples (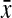 =0.85, 95% CI 0.82-0.88) compared to hourglass (
=0.85, 95% CI 0.82-0.88) compared to hourglass (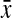 =0.82, 95% CI 0.80-0.84) and pears (
=0.82, 95% CI 0.80-0.84) and pears (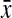 =0.80, 95% CI 0.78-0.81). There was borderline statistical significance in CWR and WC measures (p=0.08 and 0.09, respectively). Among indicators of central adiposity, apples had a higher proportion of WC≥88 cm (55.6%) and WHR≥0.80 (70.0%) compared with other shapes (p=0.03 and 0.001, respectively). No significant differences were found with WHtR≥0.5 (p=0.24). Comparing apples and pears, the percent agreement (kappa) for WHR≥0.80, WC≥88 cm, and WHtR≥0.5 was 82.9% (κ=0.55), 82.9% (κ=0.48), and 68.3% (κ=0.26), respectively. Among obese women, circumference measures, ratios, and central adiposity indicators (WC≥88, WHR≥0.80, and WHtR≥0.5) were not statistically significantly different across body shapes (Table S1, supplementary data available online at www.liebertpub.com).
=0.80, 95% CI 0.78-0.81). There was borderline statistical significance in CWR and WC measures (p=0.08 and 0.09, respectively). Among indicators of central adiposity, apples had a higher proportion of WC≥88 cm (55.6%) and WHR≥0.80 (70.0%) compared with other shapes (p=0.03 and 0.001, respectively). No significant differences were found with WHtR≥0.5 (p=0.24). Comparing apples and pears, the percent agreement (kappa) for WHR≥0.80, WC≥88 cm, and WHtR≥0.5 was 82.9% (κ=0.55), 82.9% (κ=0.48), and 68.3% (κ=0.26), respectively. Among obese women, circumference measures, ratios, and central adiposity indicators (WC≥88, WHR≥0.80, and WHtR≥0.5) were not statistically significantly different across body shapes (Table S1, supplementary data available online at www.liebertpub.com).
Table 2.
Anthropometric Indicators of Body Size and Fat Distribution by Self-Reported Body Shape Among Nonobese Women
| Pear | Hourglass | Rectangle | Apple | p valuea | |
|---|---|---|---|---|---|
| Total | 31 | 28 | 25 | 10 | |
| Body size | |||||
| Height (cm) | 163.1 (160.9-165.2) | 164.6 (161.9-167.2) | 167.8 (165.2-170.5) | 165.0 (160.6-169.4) | 0.05 |
| Weight (kg) | 64.0 (60.1-68.3) | 61.0 (57.4-65.0) | 65.0 (61.0-68.3) | 63.0 (54.9-74.3) | 0.67 |
| BMI (kg/m2) | 23.9 (22.6-25.3) | 22.6 (21.5-23.8) | 22.7 (21.3-24.4) | 23.3 (20.4-27.0) | 0.54 |
| BMI groups, n (%) | |||||
| Normal/underweight | 18 (58.1) | 21 (75.0) | 16 (64.0) | 5 (50.0) | 0.42 |
| Overweight | 13 (41.9) | 7 (25.0) | 9 (36.0) | 5 (50.0) | |
| Body shape | |||||
| Chest circumference (cm)b | 82.0 (80.0-84.0) | 81.3 (78.7-84.0) | 82.6 (79.3-85.5) | 84.0 (78.7-90.1) | 0.66 |
| Hip circumference (cm) | 102.0 (100.0-105.3) | 99.0 (96.2-103.1) | 100.0 (98.0-103.1) | 100.0 (90.9-105.3) | 0.48 |
| Waist circumference (cm) | 76.3 (73.5-79.4) | 74.6 (71.4-78.1) | 80.0 (76.3-83.3) | 80.6 (71.9-90.9) | 0.09 |
| Chest-to-waist ratiob | 1.06 (1.03-1.09) | 1.09 (1.06-1.12) | 1.03 (0.99-1.06) | 1.04 (0.96-1.13) | 0.08 |
| Chest-to-hip ratiob | 0.80 (0.78-0.81) | 0.82 (0.80-0.84) | 0.83 (0.80-0.85) | 0.85 (0.82-0.88) | 0.04 |
| Waist-to-hip ratio | 0.75 (0.73-0.76) | 0.75 (0.74-0.77) | 0.80 (0.79-0.82) | 0.82 (0.75-0.89) | 0.001 |
| Waist-to-height ratio | 0.47 (0.46-0.49) | 0.46 (0.44-0.48) | 0.48 (0.46-0.51) | 0.50 (0.45-0.55) | 0.15 |
| Central adiposity indicators | |||||
| Waist circumference, n (%) | |||||
| <88 cm | 18 (90.0) | 20 (83.3) | 12 (63.2) | 4 (44.4) | 0.03 |
| ≥88 cm | 2 (10.0) | 4 (16.7) | 7 (36.8) | 5 (55.6) | |
| Waist-to-hip ratio, n (%) | |||||
| <0.80 | 27 (87.1) | 22 (78.6) | 13 (52.0) | 3 (30.0) | 0.001 |
| ≥0.80 | 4 (12.9) | 6 (21.4) | 12 (48.0) | 7 (70.0) | |
| Waist-to-height ratio, n (%) | |||||
| <0.50 | 22 (71.0) | 21 (75.0) | 17 (68.0) | 4 (40.0) | 0.24 |
| ≥0.50 | 9 (29.0) | 7 (25.0) | 8 (32.0) | 6 (60.0) | |
Data presented as means (95% confidence interval [CI]) on original scale unless specified otherwise.
p value based on ANOVA for continuous variables or Fisher's exact test for categorical variables.
n=30, 27, 25, and 10 for pear, hourglass, rectangle, and apple shapes, respectively.
Discussion
Simple methods for ascertaining general and regional fat distribution are needed in epidemiologic studies where direct measures are not always practical. In this study, self-reported figure size and shape were consistent with anthropometric measures commonly used to assess obesity and central adiposity, respectively. The correlation between self-reported body size figure and measured BMI was high. There was a nonsignificant difference between measured and assigned BMI when BMI was assigned at the midpoint value between two consecutive figures. This method of assigning BMI from the self-reported body figure performed better than assigning a BMI based on the exact centile cutoff, which tended to significantly underestimate measured BMI. Consequently, the former method of assigning BMI should be preferred in future studies. Anthropometric measurements for identifying upper, as opposed to lower, body fat patterning were associated with body shape, and self-identified apples and pears tended to distinguish between these patterns with moderate agreement.
Our results are consistent with other validation studies comparing self-reported body size figures to measured BMI, which showed a high correlation between the two measures and the ability to distinguish obese and nonobese women.20,21 In contrast to other studies,18,32 we evaluated two methods for assigning BMI values to body size figures and showed that assignment of BMI values at the midpoint of two consecutive figures corresponded better to measured BMI than assignment at the exact centile. Other studies have compared self-measured indices of body fat distribution in women, in particular waist and hip circumferences, and found self-reported measures either overestimated36 or underestimated37–39 body circumferences. Self-measurement is not always feasible, however, and may contribute to additional study costs and limit the rate of response. In this study, we used stylized body shape figures from the Connell et al. scale25 augmented with descriptions about weight distribution and weight gain to obtain self-reported measures of whole body shape. These shapes are consistent with figure descriptions used by the clothing industry and found in popular media and, as a result, are likely to be familiar to many women in the United States.40 We showed that the relationships are consistent with indices of regional fat distribution and are able to distinguish upper (apple) and lower (pear) fat distribution patterns with reasonable concordance, with the exception of WHtR. Significant differences were mainly found for ratios (i.e., relative proportions) of the circumference measures, as opposed to absolute circumference or general adiposity measures. This is consistent with the survey question on perceived body shape, which asks women to assess the relative proportions of their chest, waist, and hips (Fig. 1) when reporting their shape.
We are not aware of other studies that have evaluated the ability of self-reported body shape figures for describing fat distribution patterns, especially among nonobese women. This provides a potentially new, cost-effective method for assessment of fat distribution in large surveys. In addition, this method has the advantage of distinguishing whole-body fat distribution patterns, in addition to central adiposity, which may have further utility in identifying risk and understanding the etiology of disease. As with the body size figures,32,41 retrospective reporting of body shape figures may be used to assess the life history of body shape changes with age and parity or other health outcomes. Of interest for future studies are the independent contributions of body size and shape on chronic and reproductive health conditions across a woman's life course. Further population-based data are needed to clarify these issues.
There are limitations to this study, and further validation studies in other populations are needed. Our study population was largely non-Hispanic white, and we could not assess differences in reporting based on race or ethnicity, which may influence perceptions of body size and shape.42 We did not have information on other psychosocial factors, such as eating disorders, that may also affect perceptions of body size and shape.43 Extremes in reported body size (highest or lowest figure) were not observed in this sample, which is expected, given the sample size and distribution of extremely thin or obese women in the population. Women at both extremes may have more skewed perceptions of their body size, and inclusion of these women may affect the overall validity; however, our results are consistent with results of larger studies that observed women across the full spectrum of body size figures. In addition, although some shapes are very distinct, body shape can be thought of as a continuum that varies with the extent of fat accumulation at a given point in time. To guide women in their assessment, we included descriptions of overall proportions and their tendency to gain weight in particular areas. Additional modifications to the shape descriptions (Fig. 2) might include further clarification of a pronounced or prominent waist to mean a small or defined indentation at the waistline. Finally, we did not have a direct measure of chest circumference, which was approximated based on bra size and may be imprecise because of incorrect bra fit.28 As discussed previously, anthropometric measurement of body circumferences and perception of body shape in obese women is complex and should be a consideration in designing future studies.
In summary, body shape may be a useful proxy measure of fat distribution patterns in addition to self-reported body size for use in large population-based studies. Validation in a larger, representative sample is needed, as well as validation of historical reporting of shape, as has been done for body size figures. Future studies may also need to consider routine collection of self-reported presurgical shape to appropriately identify the biologic patterns of fat distribution that are associated with health risks. As technology becomes more accessible, it may also be of interest to validate self-reported shapes using 3D scans and classification by experts, similar to methods applied by Connell et al.25
Supplementary Material
Acknowledgments
This work was funded by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contracts NO1-DK-6-3428, NO1-DK-6-3427, 10001406-02). Results from this study were presented at the Annual Meeting of the American Society for Reproductive Medicine in October 2011.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.World Health Organization. Obesity and overweight. 2011. www.who.int/mediacentre/factsheets/fs311/en/index.html. [Aug;2011 ]. www.who.int/mediacentre/factsheets/fs311/en/index.html
- 2.Vahratian A. Prevalence of overweight and obesity among women of childbearing age: Results from the 2002 National Survey of Family Growth. Matern Child Health J. 2009;13:268–273. doi: 10.1007/s10995-008-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316:104–108. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Wise LA. Rothman KJ. Mikkelsen EM. Sorensen HT. Riis A. Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25:253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leddy MA. Power ML. Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- 6.Huxley R. Mendis S. Zheleznyakov E. Reddy S. Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—A review of the literature. Eur J Clin Nutr. 2010;64:16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 7.Qiao Q. Nyamdorj R. Is the association of type II diabetes with waist circumference or waist-to-hip ratio stronger than that with body mass index? Eur J Clin Nutr. 2010;64:30–34. doi: 10.1038/ejcn.2009.93. [DOI] [PubMed] [Google Scholar]
- 8.Seidell JC. Cigolini M. Charzewska J. Ellsinger BM. di BG. Fat distribution in European women: A comparison of anthropometric measurements in relation to cardiovascular risk factors. Int J Epidemiol. 1990;19:303–308. doi: 10.1093/ije/19.2.303. [DOI] [PubMed] [Google Scholar]
- 9.Ruderman NB. Schneider SH. Berchtold P. The “metabolically-obese,” normal-weight individual. Am J Clin Nutr. 1981;34:1617–1621. doi: 10.1093/ajcn/34.8.1617. [DOI] [PubMed] [Google Scholar]
- 10.Obesity: Preventing and managing the global epidemic. Geneva, Switzerland: World Health Organization; 2000. WHO technical report series No. 894. [PubMed] [Google Scholar]
- 11.Vague J. The degree of masculine differentiation of obesities: A factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 12.Mueller WH. Wear ML. Hanis CL, et al. Which measure of body fat distribution is best for epidemiologic research? Am J Epidemiol. 1991;133:858–869. doi: 10.1093/oxfordjournals.aje.a115966. [DOI] [PubMed] [Google Scholar]
- 13.Seidell JC. Waist circumference and waist/hip ratio in relation to all-cause mortality, cancer and sleep apnea. Eur J Clin Nutr. 2010;64:35–41. doi: 10.1038/ejcn.2009.71. [DOI] [PubMed] [Google Scholar]
- 14.Hartz AJ. Rupley DC. Rimm AA. The association of girth measurements with disease in 32,856 women. Am J Epidemiol. 1984;119:71–80. doi: 10.1093/oxfordjournals.aje.a113727. [DOI] [PubMed] [Google Scholar]
- 15.Ashwell M. Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56:303–307. doi: 10.1080/09637480500195066. [DOI] [PubMed] [Google Scholar]
- 16.Stunkard AJ. Sorensen T. Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. In: Kety SS, editor; Rowland LP, editor; Sidman SW, editor; Mathysee SW, editor. The genetics of neurological and psychiatric disorders. New York: Raven Press; 1983. pp. 115–120. [PubMed] [Google Scholar]
- 17.Must A. Willett WC. Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138:56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 18.Must A. Phillips SM. Stunkard AJ. Naumova EN. Expert opinion on body mass index percentiles for figure drawings at menarche. Int J Obes Relat Metab Disord. 2002;26:876–879. doi: 10.1038/sj.ijo.0801986. [DOI] [PubMed] [Google Scholar]
- 19.Koprowski C. Coates RJ. Bernstein L. Ability of young women to recall past body size and age at menarche. Obes Res. 2001;9:478–485. doi: 10.1038/oby.2001.62. [DOI] [PubMed] [Google Scholar]
- 20.Tehard B. van Liere MJ. Com NC. Clavel-Chapelon F. Anthropometric measurements and body silhouette of women: Validity and perception. J Am Diet Assoc. 2002;102:1779–1784. doi: 10.1016/s0002-8223(02)90381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keshtkar AA. Semnani S. Pourshams A, et al. Pictogram use was validated for estimating individual's body mass index. J Clin Epidemiol. 2010;63:655–659. doi: 10.1016/j.jclinepi.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Han TS. Gates E. Truscott E. Lean ME. Clothing size as an indicator of adiposity, ischaemic heart disease and cardiovascular risks. J Hum Nutr Diet. 2005;18:423–430. doi: 10.1111/j.1365-277X.2005.00646.x. [DOI] [PubMed] [Google Scholar]
- 23.Ray JG. Mohllajee AP. van Dam RM. Michels KB. Breast size and risk of type 2 diabetes mellitus. Can Med Assoc J. 2008;178:289–295. doi: 10.1503/cmaj.071086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusano AS. Trichopoulos D. Terry KL. Chen WY. Willett WC. Michels KB. A prospective study of breast size and premenopausal breast cancer incidence. Int J Cancer. 2006;118:2031–2034. doi: 10.1002/ijc.21588. [DOI] [PubMed] [Google Scholar]
- 25.Connell LJ. Ulrich PV. Brannon EL. Alexander M. Presley AB. Body Shape Assessment Scale: Instrument development for analyzing female figures. Cloth Text Res J. 2006;24:80–95. [Google Scholar]
- 26.Buck Louis GM. Hediger ML. Peterson CM, et al. Incidence of endometriosis by study population and diagnostic method: The ENDO study. Fertil Steril. 2011;96:360–365. doi: 10.1016/j.fertnstert.2011.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohman TG, editor; Roche AF, editor; Martorell R, editor. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 28.Wright MCM. Graphical analysis of bra size calculation procedures. Int J Cloth Sci Technol. 2002;14:41–45. [Google Scholar]
- 29.Executive summary of the clinical guidelines on the identification, evaluation, treatment of overweight, obesity in adults. Arch Intern Med. 1998;158:1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 30.Bjorntorp P. Regional patterns of fat distribution. Ann Intern Med. 1985;103:994–995. doi: 10.7326/0003-4819-103-6-994. [DOI] [PubMed] [Google Scholar]
- 31.Dietary guidelines for Americans. Washington, DC: U.S. Department of Agriculture; 1990. Report No. 261-495/20124. [Google Scholar]
- 32.Hediger ML. Hartnett HJ. Louis GM. Association of endometriosis with body size and figure. Fertil Steril. 2005;84:1366–1374. doi: 10.1016/j.fertnstert.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frisancho AR. Anthropometric standards for the assessment of growth and nutritional status. Ann Arbor: University of Michigan Press; 1990. [Google Scholar]
- 34.Tukey JW. Exploratory data analysis. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- 35.Royston P. sg3.5: Comment on sg3.4 and an improved D'Agostino test. Stata Technical Bull. 1991;3:23–24. [Google Scholar]
- 36.Bigaard J. Spanggaard I. Thomsen BL. Overvad K. Tjonneland A. Self-reported and technician-measured waist circumferences differ in middle-aged men and women. J Nutr. 2005;135:2263–2270. doi: 10.1093/jn/135.9.2263. [DOI] [PubMed] [Google Scholar]
- 37.Rimm EB. Stampfer MJ. Colditz GA. Chute CG. Litin LB. Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Kushi LH. Kaye SA. Folsom AR. Soler JT. Prineas RJ. Accuracy and reliability of self-measurement of body girths. Am J Epidemiol. 1988;128:740–748. doi: 10.1093/oxfordjournals.aje.a115027. [DOI] [PubMed] [Google Scholar]
- 39.Freudenheim JL. Darrow SL. Accuracy of self-measurement of body fat distribution by waist, hip, and thigh circumferences. Nutr Cancer. 1991;15:179–186. doi: 10.1080/01635589109514125. [DOI] [PubMed] [Google Scholar]
- 40.Play up your shape. People Style Watch. 2011:126–138. [Google Scholar]
- 41.Vitonis AF. Baer HJ. Hankinson SE. Laufer MR. Missmer SA. A prospective study of body size during childhood and early adulthood and the incidence of endometriosis. Hum Reprod. 2010;25:1325–1334. doi: 10.1093/humrep/deq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padgett J. Biro FM. Different shapes in different cultures: Body dissatisfaction, overweight, and obesity in African-American and Caucasian females. J Pediatr Adolesc Gynecol. 2003;16:349–354. doi: 10.1016/j.jpag.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Stice E. Shaw HE. Role of body dissatisfaction in the onset and maintenance of eating pathology: A synthesis of research findings. J Psychosom Res. 2002;53:985–993. doi: 10.1016/s0022-3999(02)00488-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.