Abstract
Atherosclerosis is a chronic and progressive inflammatory disease. Novel anti-inflammatory therapies may have promise as treatment strategies for cardiovascular risk reduction. Rosemary (Rosemarinus officinalis L.) has been used in folk medicine to treat headaches, epilepsy, poor circulation, and many other ailments. It was found that rosemary could act as a stimulant and mild analgesic and could reduce inflammation. However, the mechanisms underlying the anti-inflammatory and antiatherosclerotic effects of rosemary need more study. This study investigated effects of the rosemary components, carnosic acid (CA), and carnosol (CAR), on cell migration. Monocyte chemoattractant protein-1 (MCP-1) and matrix metalloproteinase-9 (MMP-9) were determined by Western blot and gelatin zymography, respectively, in RAW 264.7 macrophages and vascular smooth muscle cells (VSMCs). VSMC migration was assessed by a Matrigel migration assay. Active compounds of rosemary extracts were also analyzed using a reversed-phase high-performance liquid chromatography. MMP-9 and MCP-1 activities were markedly diminished with methanol extract (RM), n-hexane fraction (RH), and CA in RAW 264.7 cells. RM, RH, CA, and CAR suppressed tumor necrosis factor-alpha–induced VSMC migration by inhibiting MMP-9 expression. Chromatograms of RM- and RH-containing CA and CAR revealed higher CA contents of RM (9.4%, 93.85 μg/mg dry wt.) and, especially, RH (18.4%, 184.00 μg/mg dry wt.), which were appreciably elevated compared with the similar CAR content in RM and RH (3.7%, 37.30 μg/mg dry wt.; and 2.5%, 25.05 μg/mg dry wt., respectively). Rosemary, especially its CA component, has potential antiatherosclerosis effects related to cell migration.
Key Words: Rosemarinus officinalis L., rosemary, matrix metalloproteinase-9 (MMP-9), monocyte chemoattractant protein-1 (MCP-1), migration, RAW 264.7 macrophages, vascular smooth muscle cell
Introduction
Atherosclerosis is a chronic and progressive inflammatory disease that leads to advanced cardiovascular diseases, such as coronary thrombosis, myocardial infarction, and stroke.1,2 Vascular inflammation occurs in response to shear stress, infection, or oxidative stress initiated, for instance, by the oxidized lipid moiety of oxidized low-density lipoprotein (OxLDL). OxLDL, in turn, induces an immune response within the arterial wall.3
A key initial step in this process involves the binding of monocytes to the arterial endothelium. Activated endothelial cells express a number of adhesion molecules, including vascular cell adhesion molecule-1 and intracellular adhesion molecule-1, which bind the monocytes to the surface of endothelial cells.1 Recruitment to the subendothelial space is promoted by the expression of chemoattractants, such as the monocyte chemoattractant protein-1 (MCP-1) chemokine within the tissue.4 Monocytes that have entered the artery wall are different from macrophages and take up modified LDL via scavenger receptors to form foam cells, the hallmark of atherosclerosis. Foam cell accumulation is characterized by fatty streaks and deposition of fibrous tissue defines more advanced atherosclerotic lesions.5
Extravasation of circulating leukocytes, particularly macrophages and T cells, requires the secretion of matrix-degrading enzymes and matrix metalloproteinases (MMPs), which allow leukocytes to penetrate the vessel wall into the extracellular matrix. It has been suggested that MMP-9 is involved in the disruption of basement membrane, which is required for the process of monocyte transmigration.6,7
The migration of vascular smooth muscle cells (VSMCs) from the tunica media to the subendothelial region is crucial in the development and progression of many vascular diseases, including atherosclerosis and postangioplasty restenosis.8 Activation of MMP may contribute to the pathogenesis of atherosclerosis by facilitating migration of VSMCs.9 Analysis of human atherosclerotic lesions and advanced plaques has demonstrated increased expression of MMP, especially the MMP-9 gelatinase, in VSMCs.10 The plaque cap could form by migration of typical, contractile VSMCs from the media, which would account for the frequent medial wasting observed at the base of atherosclerotic plaques.11
Rosemarinus officinalis L., commonly called rosemary, has a long-standing reputation for improving memory and has been used as a symbol of remembrance in Europe.12 R. officinalis leaves possess a variety of bioactivities, including antioxidant,13 antitumor, 14 anti-inflammatory,15,16 and anti-human immunodeficiency virus.15 The large number of relevant main constituents include volatile oils, terpenoids, flavonoids, and polyphenolics, including carnosic acid (CA), carnosol (CAR), rosmarinic acid, and ursolic acid.13,17–20 Among the antioxidant compounds, about 90% of the antioxidant activity can be attributed to CA and CAR, which are the major phenolic diterpene constituents in rosemary.21 CA and CAR have a typical O-diphenol structure.22,23 CA and CAR also exhibit anti-inflammatory, anticancer, and scavenging activities.24
The antiatherosclerotic mechanism(s) of rosemary are ill-understood. The present study investigated the inhibitory effects of CA and CAR from rosemary on MMP-9, MCP-1 levels, and cell migraion.
Materials and Methods
Chemicals and reagents
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated. Cell culture reagents were purchased from Gibco BRL (Rockville, MD, USA). Fetal bovine serum (FBS) and bovine calf serum (BCS) were purchased from Hyclone (Logan, UT, USA). Recombinant human tumor necrosis factor-alpha (TNF-α) was obtained from R&D Systems (Minneapolis, MN, USA). Polyclonal MCP-1 and secondary antibodies (horseradish peroxidase-linked anti-rabbit, and anti-mouse IgGs) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The polyclonal MMP-9 antibody was obtained from Abcam (Cambridge, United Kingdom). The enhanced chemiluminescence Western blotting detection system was from Amersham (Arlington Heights, IL, USA). Transwell was purchased from Corning Incorporated (Corning, NY, USA). CAR was purchased from Cayman Chemical Company (Ann Arbor, MI, USA).
Preparation of methanolic extracts and their fractions from rosemary
Rosemary leaves were extracted with a 10-fold volume of 80% methanol at room temperature for 24 h. The extraction was repeated three times. The extracts were evaporated in a rotary evaporator at 55°C. The resulting methanol extracts (RM) were suspended in water and successively extracted with equal volumes of n-hexane fraction (RH), chloroform, ethyl acetate, and n-butanol. The fractions were evaporated on a rotary evaporator under reduced pressure. Each concentrated fraction was freeze-dried to yield a solid fraction.
Cell culture
Rat VSMCs were obtained from the American Type Culture Collection (Rockville, MD, USA) and RAW 264.7 mouse macrophage cells were purchased from Korean Cell Line Bank (Seoul, Korea). Both cell types were maintained in the Dulbecco's modified Eagle's medium supplemented with 10% FBS and BCS containing 100 U/mL of penicillin and 100 μg/mL of streptomycin and 5% CO2 at 37°C. For all experiments, early passage cells were grown to 80–90% confluence.
Gelatin zymography assay
RAW 264.7 cells and VSMCs were plated in wells of six-well plates at a cell density 2×106/well and 8×105/well, respectively, and treated with various concentrations of sample in the absence or presence of 100 ng/mL lipopolysaccharide (LPS) and TNF-α, respectively. The culture supernatant was electrophoresed in a polyacrylamide gel containing 0.1% (w/v) gelatin. The gel was then washed at room temperature for 30 min with 2.5% Triton X-100 and subsequently incubated at 37°C for 24 h in a buffer containing 10 mM CaCl2, 0.01% NaN3, and 50 mM Tris-HCl (pH 7.5). The gel was stained with 0.2% Coomassie brilliant blue and photographed on a light box. Proteolysis was detected as a white zone in a dark blue field.
Western blot analysis
RAW 264.7 cells and VSMCs were plated in wells of six-well plates as described above and incubated with or without 100 ng/mL LPS and TNF-α, respectively, in the absence or presence of various concentrations of sample for 24 h. Cells were harvested by scraping the cells from cultured dishes using a cell scraper. Cellular lysates were prepared in a lysis buffer containing 50 mM Tris-HCl (pH 7.5), 2 mM EDTA, 150 mM NaCl, 0.5% deoxycholate, 0.1% sodium dodecylsulfate (SDS), 1 mM NaF, 1 mM Na3VO4, 1 mM phenyl methyl sulfonyl fluoride, 1 mM dithiothreitol, 1 μg/mL leupeptin, and 1% NP-40. The cells were disrupted and extracted at 4°C for 30 min. After centrifugation at 13,000 g for 15 min, the supernatant was obtained as the cell lysate. Protein concentrations were measured using a protein kit (BioRad, Hercules, CA, USA). Cellular proteins (10 μg/lane) were resolved in aliquots subjected to 10% SDS-polyacylamide gel electrophoresis. The resolved proteins were transferred to an Immobilon-P-membrane (Millipore, Billerica, MA, USA) and allowed to react with a specific antibody. The detection of specific proteins was carried out by enhanced chemiluminescence following the manufacturer's instructions. Loading differences were normalized using the polyclonal anti-β-actin antibody.
Cell migration assay
Matrigel-coated filter inserts (8 μm pore size) that fit into 24-well invasion chambers were obtained from Becton-Dickinson (Piscataway, NJ, USA). VSMCs were detached from the tissue culture plates, washed, and resuspended in a conditioned medium (4×104 cells/well), and then added to the upper compartment of the invasion chamber with or without the presence of a sample. The conditioned medium (500 μL) was added to the lower compartment of the invasion chamber. The Matrigel invasion chambers were incubated at 37°C for 12 h in an atmosphere of 5% CO2. After incubation, the filter inserts were removed from the wells and the cells on the upper side of the filter were removed using cotton swabs. The filters were fixed, stained, and mounted according to the manufacturer's instructions (Becton-Dickinson). The cells that migrated through the Matrigel to the underside of the filter were counted. Three-to-five invasion chambers were used per condition. The values were obtained by averaging the total number of cells from three filters.
High-performance liquid chromatography analysis
The extracted sample was filtered through a 0.45-μm poly(tetrofluoroethylene) syringe-tip filter. Using a 20-μL sample loop, the sample was analyzed using a reversed-phase high-performance liquid chromatography (HPLC) system (Waters 2690; Milford, MA, USA), a quaternary pump, and a vacuum degasser. For HPLC analyses of CA contents in the RM and RH fractions, a C18 reversed-phase Xbridge analytical column (Waters Xbridge C18, 5 μm×150 mm×4.6 mm) was used. Two mobile phases were used: solvent A was comprised of water and solvent B consisted of 0.1% trifluoroacetic acid in acetonitrile. The gradient for HPLC analysis was linearly changed as follows (total 40 min): 30% B at 0 min, 40% B at 3 min, 50% B at 5 min, 53% B at 7 min, 58% B at 10 min, 62% B at 14 min, 68% B at 19 min, 75% B at 24 min, and 100% B at 30 min. For HPLC analyses of CAR contents in the RM and RH fractions, a C18 reversed-phase Symmetry analytical column (Waters Symmetry C18, 5 μm×150 mm×4.6 mm) was used. Two mobile phases were used; solvent A and B as described above. The gradient for HPLC analysis was linearly changed (total 40 min): 30% B at 0 min, 40% B at 3 min, 50% B at 5 min, 53% B at 7 min, 58% B at 10 min, 68% B at 14 min, 80% B at 19 min, 95% B at 24 min, and 100% B at 30 min. The flow rate was set to 1.0 mL/min at constant room temperature (25°C). The detector was set at 254 nm. Individual CA and CAR in the sample were tentatively identified by comparison of their ultraviolet-visible spectra and retention times with spiked CA and CAR standards.
Statistical analysis
The results are expressed as mean±standard deviation. Significance of differences between the means of the two groups was determined by the unpaired Student's t-test using Sigma plot (8.0) software (SigmaStat, San Diego, CA, USA). The minimum significance level was set at P value ≤.05 for all analysis.
Results
Effect of RM and RH on LPS-induced MMP-9 secretion and MCP-1 expression in RAW 264.7 cells
MMPs are a family of zinc-containing enzymes that are involved in the degradation of different components of the extracellular matrix. Considerable evidence indicates that individual MMPs have important roles in tumor invasion and subsequent spread. MMPs are divided into four subgroups according to their substrate specificity and structural homology, and each subgroup can degrade at least one component of the extracellular matrix.25 Especially, MMP-9 (gelatinase B, 92 kDa type IV collagenase) has been suggested to play an important role in cell migration by facilitating the destruction of type IV collagen-containing basement membrane that separates the epithelial and stromal compartments.26
To identify the active compounds responsible for inhibitory effect on MMP-9 and MCP-1 of rosemary, the activities of MMP-9 secreted from VSMCs were measured with RM and its fractions (10 μg/mL). RM and RH significantly decreased MMP-9 secretion compared to the other fractions, as ascertained using a gelatin zymogaphy assay (data not shown).
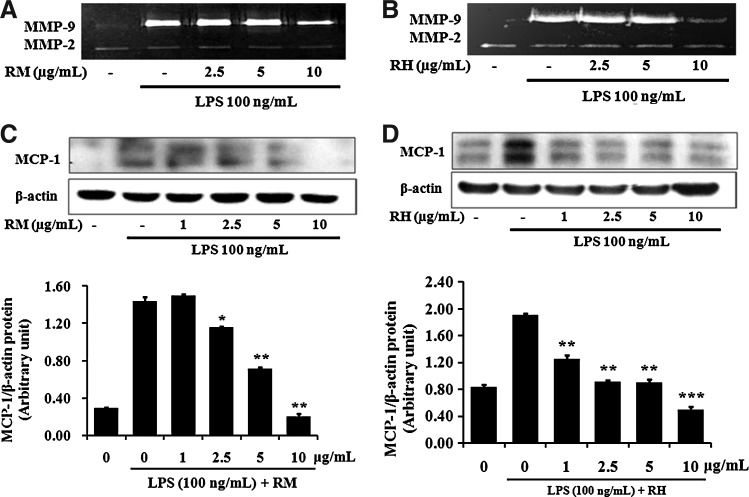
The supernatant obtained from cultured RAW 264.7 cells displayed very weak proteolytic activity at 92 kDa, corresponding to MMP-9. In contrast, treatment with LPS (100 ng/mL) induced the expression of the target gene, resulting in the amplification of the band with proteolytic MMP-9 activity. The expression of MMP-9 (type IV collagenase) by LPS was markedly inhibited by both RM and RH at a concentration of 10 μg/mL (Fig. 1A, B), while the activity of MMP-2 was not affected by LPS or the sample.
FIG. 1.
Effect of RM and RH on LPS-induced MMP-9 secretion and MCP-1 expression in RAW 264.7 cells. Cells were pretreated with various concentrations of RM or RH for 1 h and induced by LPS (100 ng/mL) for an additional 23 h. The supernatants of cell culture were used for gelatin zymography (A, B). Total cell lysates were examined for MCP-1 protein expression (C, D) by Western blot. β-actin was used as an internal control. Data represent the mean±SEM of three independent experiments (n=3). *P<.05, **P<.01, ***P<.001 when compared with the groups treated with LPS (100 ng/mL) alone. CA, carnosic acid; LPS, lipopolysaccharide; MCP, monocyte chemotactic protein; MMP, matrix metalloproteinase; RH, n-hexane fraction; RM, methanol extract; SEM, standard error of the mean.
MCP-1 is a CC-chemokine consisting of 76 amino acids, which has been recently suggested to play a key role in atherogenesis and tissue injury, since it is involved in the recruitment of monocytes into the arterial wall.27,28 As shown in Figure 1C and D, treatment of RAW 264.7 cells with LPS (100 ng/mL) induced the expression of MCP-1 protein. This expression was markedly inhibited by RM and RH. RM and RH significantly decreased the levels of expressed MCP-1 protein at concentrations of 2.5, 5, and 10 μg/mL, compared to LPS treatment.
Effect of RM and RH on TNF-α–induced MMP-9 expression and migration of VSMCs
MMP-9 synthesis can be stimulated by a variety of stimuli, including cytokines, during various pathological processes, such as atherosclerosis, inflammation, tumor invasion, and rheumatoid arthritis, while MMP-2 is usually expressed constitutively.29,30
We investigated the inhibitory effect of RM and RH from rosemary on TNF-α–induced MMP-9 secretion in VSMCs. At RM and RH concentrations of 1, 2.5, 5, and 10 μg/mL, MMP-9 secretion decreased in a dose-dependent manner (Fig. 2A, B). On the other hand, MMP-2 was strongly secreted without induction by TNF-α in cultured VSMCs. Activity of MMP-2 was not affected by RM or RH. Also, MMP-9 protein expression was decreased by treatment with RM and RH, as shown by Western blotting. Since the upregulation of MMP-9 expression contributes to VSMC migration in vivo and in vitro,31,32 we examined whether TNF-α–induced VSMC migration was decreased by RM and RH, which previously shown to inhibit TNF-α–induced MMP-9 expression. As shown in Figure 2C, VSMC migration was increased by treatment with TNF-α compared with the migration of TNF-α–untreated control cells in a Matrigel migration assay. RM and RH inhibited the migration of TNF-α–induced VSMCs to levels comparable to TNF-α–untreated control cells at a concentration of 10 μg/mL. Cell viability was not affected by TNF-α or sample (data not shown).
FIG. 2.
Effect of RM and RH on TNF-α-induced cell migration by inhibiting the MMP-9 activity in VSMC. Cells were treated with the indicated concentrations of RM and RH with the TNF-α (100 ng/mL) for 24 h. The supernatant of VSMC culture was prepared and used for gelatin zymography (black background). The pellets were used for Western blot (white background). β-actin was used as an internal control (A, B). A Matrigel migration assay was carried out with RM and RH with the TNF-α (100 ng/mL). After 12 h incubation, cells on the bottom side of the filter were fixed, stained, and counted (400×) (C). Data represent the mean±SEM of three independent experiments (n=3). *P<.05, **P<.01 when compared with the groups treated with LPS (100 ng/mL) alone. TNF, tumor necrosis factor; VSMC, vascular smooth muscle cell.
These results indicate that RM and RH suppress TNF-α–induced VSMC migration by inhibiting MMP-9 secretion, and further indicate the antiatherosclerotic potential of RM and RH. Accordingly, we next investigated the MMP-9 and MCP-1 activity of CA and CAR from rosemary leaves.
Effect of CA and CAR on MMP-9 secretion, MCP-1 expression, and cell migration
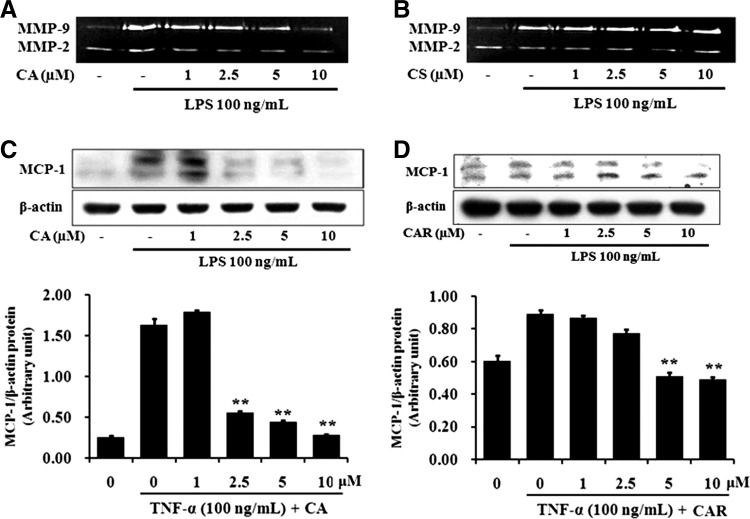
CA and CAR (both at 10 μM) did not reduce cell viability appreciably (<10%) when compared to nontreated control (data not shown). LPS-induced MMP-9 secretion in RAW 264.7 cells was markedly reduced by 10 μM CA, but not by the same concentration of CAR (Fig. 3A, B). Also, as shown in Figure 3C and D, CA and CAR significantly decreased the levels of MCP-1 protein in LPS-stimulated RAW 264.7 cells in a concentration-dependent manner. CA and CAR at concentrations of 1–10 μM had no effect on VSMC viability (data not shown).
FIG. 3.
Effect of CA and CAR on LPS-induced MMP-9 secretion and MCP-1 expression in RAW 264.7 cells. Cells were pretreated with various concentrations of RM or RH for 1 h and induced by LPS (100 ng/mL) for an additional 23 h. The supernatants of cell culture were used for gelatin zymography (A, B). Total cell lysates were examined for MCP-1 protein expression (C, D) by Western blot. β-actin was used as an internal control. Data represent the mean±SEM of three independent experiments (n=3). **P<.01 when compared with the groups treated with LPS (100 ng/mL) alone. CAR, carnosol.
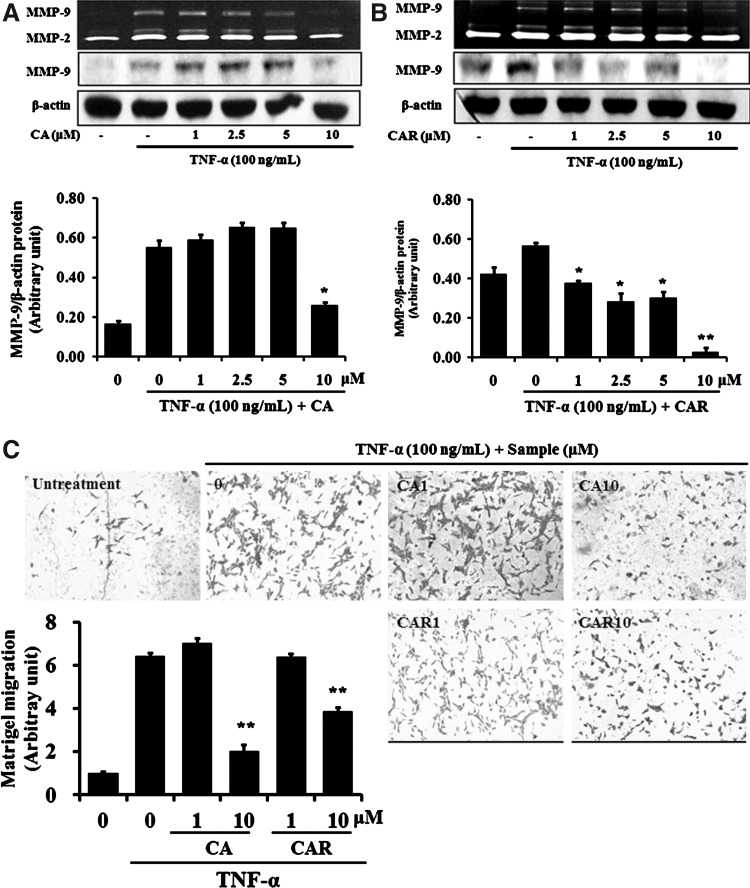
Inhibition of MMP-9 expression and activity by 1–10 μM CA and CAR was tested. The TNF-α–induced MMP-9 expression levels were decreased by both CA and CAR in dose-dependent manners (Fig. 4A, B).
FIG. 4.
Effect of CA and CAR on TNF-α-induced cell migration by inhibiting the MMP-9 activity in VSMC. Cells were treated with the indicated concentrations of RM and RH with the TNF-α (100 ng/mL) for 24 h. The supernatant of VSMC culture was prepared and used for gelatin zymography (black background). The pellets were used for Western blot (white background). β-actin was used as an internal control (A, B). A Matrigel migration assay was carried out with RM and RH with the TNF-α (100 ng/mL). After 12 h incubation, cells on the bottom side of the filter were fixed, stained, and counted (400×) (C). Data represent the mean±SEM of three independent experiments (n=3). *P<.05, **P<.01 when compared with the groups treated with LPS (100 ng/mL) alone.
Also, as shown in Figure 4C, VSMC migration was increased by TNF-α compared with TNF-α–untreated control cells. CA and CAR at 10 μM inhibited the migration of TNF-α–induced VSMCs, reaching the levels of TNF-α–untreated control cells.
These results show that CA and CAR treatments can lead to a decrease in the migration potential of VSMC in vitro, and suggest that TNF-α–induced migration of VSMCs can be decreased by CA and CAR, which both selectively inhibit TNF-α–induced MMP-9 expression. It has been reported that activation of MMP may contribute to the migration of VSMCs.10 Thus, the increased expression of MMP in VSMCs is closely related to the human atherosclerotic lesions.
In conclusion, these results indicate that CA and CAR have potential as antiatherosclerosis agents.
Contents of CA and CAR
CA and CAR, which are the major phenolic constituents in rosemary (R. officinalis), have a typical O-diphenol structure.22,23 As shown in Table 1, the CA contents in RM and RH were higher compared with CAR. The content of CA in RH (18.4%, 184.00 μg/mg dry wt.) was almost two times higher than those in RM (9.4%, 93.85 μg/mg dry wt.), whereas the contents of CAR in RM (3.7%, 37.30 μg/mg dry wt.) and RH (2.5%, 25.05 μg/mg dry wt.) were similar.
Table 1.
Carnosic Acid and Carnosol Contents in Methanol Extract and n-Hexane
| RT (min) | Contents (μg/mg) | |
|---|---|---|
| Carnosic acid | 16.052 | — |
| RM | 16.060 | 93.85 |
| RH | 15.979 | 184.00 |
| Carnosol | 14.142 | — |
| RM | 14.236 | 37.30 |
| RH | 14.149 | 25.05 |
RM, methanol extract; RH, n-hexane fraction; RT, retention time.
These results indicate that the major active compounds of rosemary for antiatherosclerosis efficacy are the phenolic diterpenes CA and CAR.
Discussion
Despite improved pharmacological agents and changes in lifestyle, atherosclerosis is still a leading cause of mortality and morbidity in industrialized countries33. It is a chronic immune-inflammatory disease that leads to advanced cardiovascular diseases.1,2 Thus, inflammation plays a critical role in atherosclerosis.
R. officinalis L. (family Lamiaceae), commonly called rosemary, is a culinary as well as medicinal herb with biological activities.23 It is native to the Mediterranean region and is now widely distributed in European countries.15 It has an ancient reputation for improving memory, and has been used as a symbol for remembrance in Europe.12 Rosemary extracts have been incorporated into drugs and cosmetics, and are used to enhance the flavor and fragrance of foods.15 Also, it is extremely high in iron, calcium, and vitamin B6.12 Notably, rosemary possesses a potential anti-inflammatory activity.15,16
In the present study, we investigated the antiatherosclerotic effect of rosmary and its active components. RM and RH from rosemary showed significant inhibition of LPS-induced expression of MMP-9 and MCP-1 expression in RAW 264.7 cells (Fig. 1A–D).
MMP-9 is controlled by the inflammatory cytokine nitric oxide.34 Decreasing nitric oxide may be effective for treating inflammation due to decreased MMP-9 activation. Also, the present results demonstrated the inhibition of TNF-α–induced MMP-9 expression and VSMC migration. The migration of VSMCs from the tunica media to the subendothelial region is a key event in the development and progression of atherosclerosis.8 Activity of MMP species, including MMP-2 and MMP-9, may contribute to the pathogenesis of atherosclerosis by facilitating the migration of VSMCs.9 These results reveal aspects of the mechanism of the antiatherosclerosis effect of rosemary. To determine the active compounds of RM and RH, the inhibitory effects on MMP-9 of camphene, camphor, bonyl acetate, the main compounds in essential oil and rosmarinic acid, CA, and CAR were tested. Only CA and CAR were effective (data not shown).
CA is quite unstable and, usually, is converted to CAR upon heating. CAR can be degraded further to produce other compounds, such as rosmanol, epirosmanol, and metoxyeporosmanol, which still possess antioxidative activity.19 CA is ∼10× as effective as CAR.35 CA and CAR are the principal compounds responsible for the anti-inflammatory effects of fresh rosemary leaves.36 The LPS-induced MMP-9 levels were dramatically reduced with CA at a concentration of 10 μM, but not by CAR.
CA and CAR significantly decreased MCP-1 protein in LPS-stimulated RAW 264.7 cells in a concentration-dependent manner. TNF-α–induced MMP-9 levels in enzyme activity and protein were decreased with CA and CAR in a dose-dependent manner in VSMCs. CA and CAR at 10 μM inhibited the migration of TNF-α–induced VSMCs to levels comparable to TNF-α–untreated control cells. Thus, the analytical HPLC of RM and RH containing CA and CAR were measured. The CA contents in RM and RH were higher compared with CAR. The content of CA in RH (18.4%) was almost 2× higher than those in RM (9.4%), whereas the contents of CAR in RM (3.7%) and RH (2.5%) were similar. CA is a phenolic diterpene compound found in sage (2–5 mg CA/g sage) and rosemary (12–15 mg CA/g rosemary).22,23 The major active compounds of rosemary for control of cell migration can be considered as the phenolic diterpenes CA and CAR.
The contents of the active compounds, RM and RH, differed when we checked the similar effect from RM and RH at identical concentrations, despite fractionation. The findings indicate RM has other active compounds besides CA and CAR. The same may be true of RH. To assess these speculations, separation and purification of the active compound of RH is in progress in our laboratory.
These findings suggest that supression of cell migration was related to the decrease of MMP-9 and MCP-1 levels by rosemary. The major active compounds of rosemary for the effect can be considered as the phenolic diterpenes CA and CAR.
Further studies are necessary to detect other potential mechanisms. It is acticipated that rosemary has the potential to be developed as an antiatherosclerosis medicine or functional food.
Acknowledgments
This work was supported by the Settlement research grant of Keimyung University in 2011 and the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. 353-2009-1-F00018).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mestas J. Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan MJ. Management of cardiovascular disease risk in chronic inflammatory disorders. Nat Rev Rheum. 2009;5:208–217. doi: 10.1038/nrrheum.2009.29. [DOI] [PubMed] [Google Scholar]
- 3.Jara LJ. Medina G. Vera-Lastra O. Amigo MC. Accelerated atherosclerosis, immune response and autoimmune rheumatic diseases. Autoimmun Rev. 2006;5:195–201. doi: 10.1016/j.autrev.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan GW. Sarembock IJ. Linden J. The role of inflammation in vascular diseases. J Leukocyte Biol. 2000;67:591–602. doi: 10.1002/jlb.67.5.591. [DOI] [PubMed] [Google Scholar]
- 5.Raines EW. Ferri N. Thematic review series: the immune system and atherogenesis, cytokines affecting endothelial and smooth muscle cells in vascular disease. J Lipid Res. 2005;46:1081–1092. doi: 10.1194/jlr.R500004-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Galis ZS. Sukhova GK. Lark MW. Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagaoka I. Hirota S. Increased expression of matrix metalloproteinase-9 in neutrophils in glycogen-induced peritoneal inflammation of guinea pigs. Inflamm Res. 2000;49:55–62. doi: 10.1007/s000110050559. [DOI] [PubMed] [Google Scholar]
- 8.Maeda K. Kuzuya M. Cheng XW. Asai T. Kanda S. Tamayamori N. Sasaki T. Shibata T. Iguchi A. Green tea catechins inhibit the cultured smooth muscle cell invasion through the basement barrier. Atherosclerosis. 2003;166:23–30. doi: 10.1016/s0021-9150(02)00302-7. [DOI] [PubMed] [Google Scholar]
- 9.Jones CB. Sane DC. Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59:812–823. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 10.Galis ZS. Johnson C. Godin D. Magid R. Shipley JM. Senior RM. Ivan E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91:852–859. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 11.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 12.Mehdi JH. Rudabeh SM. Hassan S. Analysis of Iranian rosemary essential oil: application of gas chromatography–mass spectrometry combined with chemometrics. J Chromatogr A. 2011;1218:2569–2576. doi: 10.1016/j.chroma.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 13.Richheimer SL. Bernart MW. King GA. Kent MC. Bailey DT. Antioxidant activity of lipid soluble phenolic diterpenes from rosemary. J Am Oil Chem Soc. 1996;73:507–514. [Google Scholar]
- 14.Singletary K. MacDonald C. Wallig M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)–induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996;104:43–48. doi: 10.1016/0304-3835(96)04227-9. [DOI] [PubMed] [Google Scholar]
- 15.Aruoma OI. Spencer JP. Rossi R. Aeschbach R. Khan A. Mahmood N. Munoz A. Murcia A. Butler J. Halliwell B. An evaluation of the antioxidant and antiviral action of extracts of rosemary and Provençal herbs. Food Chem Toxicol. 1996;34:449–456. doi: 10.1016/0278-6915(96)00004-x. [DOI] [PubMed] [Google Scholar]
- 16.Altinier G. Sosa S. Aquino RP. Mencherini T. Della Loggia R. Tubaro A. Characterization of topical antiinflammatory compounds in Rosmarinus officinalis L. J Agric Food Chem. 2007;55:1718–1723. doi: 10.1021/jf062610+. [DOI] [PubMed] [Google Scholar]
- 17.Okamura N. Fujimoto Y. Kuwabara S. Yagi A. High performance liquid chromatographic determination of carnosic acid and carnosol in Rosmarinus officinalis and Salvia officinalis. J Chromatogr A. 1993;679:381–386. [Google Scholar]
- 18.Senorans FJ. Ibanez E. Cavero S. Tabera J. Reglero G. Liquid chromatographic-mass spectrometric analysis of supercritical-fluid extracts of rosemary plants. J Chromatogr A. 2000;870:491–499. doi: 10.1016/s0021-9673(99)00941-3. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez P. Garcia-Risco MR. Santoyo S. Senorans FJ. Ibanez E. Reglero G. Isolation of functional ingredients from rosemary by preparative-supercritical fluid chromatography (Prep-SFC) J Pharm Biomed Anal. 2006;41:1606–1613. doi: 10.1016/j.jpba.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Almela L. Sanchez-Munoz B. Fernandez-Lopez JA. Roca MJ. Rabe V. Liquid chromatograpic-mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J Chromatogr A. 2006;1120:221–229. doi: 10.1016/j.chroma.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 21.Huang MT. Ho CT. Wang ZY. Ferraro T. Lou YR. Stauber K. Ma W. Georgiadis C. Laskin JD. Conney AH. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54:701–708. [PubMed] [Google Scholar]
- 22.Luis JC. Martin Perez R. Valdes Gonzalez F. UV-B radiation effects on foliar concentrations of rosmarinic and carnosic acids in rosemary plants. Food Chem. 2007;101:1211–1215. [Google Scholar]
- 23.Baskan S. Oztekin N. Erim B. Determination of carnosic acid and rosmarinic acid in sage by capillary electrophoresis. Food Chem. 2007;101:1748–1752. [Google Scholar]
- 24.Sotelo-Félix JI. Martinez-Fong D. Muriel de la Torre P. Protective effect of carnosol on CCl(4)-induced acute liver damage in rats. Eur J Gastroenterol Hepatol. 2002;14:1001–1006. doi: 10.1097/00042737-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Parsons SL. Watson SA. Brown PD. Collins HM. Steele RJ. Matrix metalloproteinases. Br J Surg. 1997;84:160–166. [PubMed] [Google Scholar]
- 26.McMillan JI. Weeks R. West JW. Busten S. Rice GC. Lovett DH. Pharmacological inhibition of gelatinase B induction and tumor cell invasion. Int J Cancer. 1996;67:523–531. doi: 10.1002/(SICI)1097-0215(19960807)67:4<523::AID-IJC11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura T. Takeya M. Takahashi K. Kuratsu JI. Leonard EJ. Production and characterization of mouse monoclonal antibodies against human monocyte chemoattractant protein-1. J Immunol. 1991;147:2229–2233. [PubMed] [Google Scholar]
- 28.Gu L. Okada Y. Clinton SK. Gerard C. Sukhova GK. Libby P. Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 29.Chambers AF. Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 30.Nabeshima K. Inoue T. Shimao Y. Sameshima T. Matrix metalloproteinases in tumor invasion: Role for cell migration. Pathol Int. 2002;52:255–264. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 31.Stetler-Stevenson WG. Hewitt R. Corcoran M. Matrix metalloproteinases and tumor invasion: from correlation and causality to the clinic, Semin. Cancer Biol. 1996;7:147–154. doi: 10.1006/scbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- 32.Mason DP. Kenagy RD. Hasenstab D. Bowen-Pope DF. Seifert Ra. Aoats S. Hawkins SM. Clowes AW. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ Res. 1999;85:1179–1185. doi: 10.1161/01.res.85.12.1179. [DOI] [PubMed] [Google Scholar]
- 33.Braunwald E. Shattuck lecture-cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto T. Akaike T. Sawa T. Miyamoto Y. van der Vliet A. Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 35.Aruoma OI. Haliwell B. Aeschbach R. Loligers J. Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica. 1992;22:257–268. doi: 10.3109/00498259209046624. [DOI] [PubMed] [Google Scholar]
- 36.Bai N. He K. Roller M. Lai CS. Shao X. Pan MH. Ho CT. Flavonoids and phenolic compounds from Rosemarinus officinalis. J Agric Food Chem. 2010;58:5363–5367. doi: 10.1021/jf100332w. [DOI] [PubMed] [Google Scholar]