Abstract
Dengue, caused by the four serotypes of dengue virus (DENV), represents an expanding global health challenge. The potential for serotype-cross-reactive antibodies to exacerbate disease during a secondary infection with a heterologous DENV serotype has driven efforts to study human DENV-specific antibodies. Most DENV-specific antibodies generated in humans are serotype-cross-reactive, weakly neutralizing, and directed against the immature pre-membrane (prM), envelope (E), and nonstructural 1 (NS1) proteins. To broaden the characterization of human DENV-specific antibodies, we assessed B-cell responses by ELISpot assays and isolated B cells from the peripheral blood of a human subject with previous DENV infection. Forty-eight human IgG monoclonal antibodies (hMAbs) were initially characterized by their potential to bind to an inactivated lysate of DENV-infected cells. Subsequently, most DENV-specific hMAbs were found to bind soluble, recombinant E protein (rE). Two hMAbs were unable to bind rE, despite strongly binding to the DENV-infected cell lysate. Further analyses showed that these two hMAbs bound conformation-dependent, reduction-sensitive epitopes on E protein. These data shed light on the breadth of DENV-specific hMAbs generated within a single immune donor.
Introduction
Dengue viruses (DENV) comprise a family of four antigenically-related positive-strand RNA viruses transmitted to humans by mosquitoes. Most DENV infections are asymptomatic. Clinical disease ranges from an acute febrile illness lasting 4–7 d (classic dengue fever), to a more severe form, dengue hemorrhagic fever (DHF), characterized by fever, hemorrhagic manifestations, and increased vascular permeability with leakage into interstitial spaces (21,28).
A primary infection with one serotype of DENV induces lifelong immunity to that serotype. The strong association of severe dengue illness, DHF, with a heterologous secondary infection and high cytokine levels has led to the prevailing view that DHF is immunologically mediated (28). Antibody-dependent enhancement (ADE) of infection, whereby anti-DENV antibodies acquired from a previous heterologous infection, or passively acquired by an infant from the mother, is thought to be an important trigger of the immunological cascade responsible for DHF (21).
Initial studies of antibody responses to DENV were performed in mice. The majority of flavivirus-neutralizing murine antibodies recognize the structural envelope (E) protein, although some also bind to the immature pre-membrane (prM), or mature membrane (M), protein (5,10,12,27,33,34). Serotype-specific epitopes elicit murine antibodies with the strongest neutralizing activities, and protection in animals by antibodies correlates with neutralizing activity in vitro. Based on epitope-mapping studies, many DENV serotype-specific neutralizing antibodies were localized to domain III (DIII) of the E protein, whereas neutralizing monoclonal antibodies (MAbs) that cross-reacted with other flaviviruses localized primarily to domain II (DII), near the fusion sequence (23,30,33).
Until recently, the human antibody response was evaluated by analyzing the quality of antibodies in the sera of DENV-immune individuals (4,14,31,39,40). In the last couple years, several groups have generated and characterized human (h)MAbs by stimulating memory B cells obtained from DENV-immune donors in vitro (1,6,8,29,32). Antibodies specific for the E protein with poor, moderate, or potent neutralizing activity, and antibodies specific for the prM protein that were poorly neutralizing but highly cross-reactive, have been isolated. In contrast to the findings in mice, antibodies against DIII are a minority in human immune sera and among isolated hMAbs (1,8,29). B-cell ELISpot assays represent an alternative approach to analyze the human B-cell response to DENV. We recently reported on the use of ELISpot assays to compare responses to homologous and heterologous DENV serotypes in primary and secondary DENV infections (19). ELISpot assays allow more accurate quantitation of cell frequencies than isolation of MAbs, but definition of serotype cross-reactivity at the clonal level is more difficult.
The goal of the present study was to expand and isolate B cells that secrete antibodies specific for DENV from the peripheral blood of individuals following DENV infection. We define the major antigens and epitopes recognized by a panel of antibodies secreted by memory B cells from a single DENV-immune subject, and characterize the phenotype of B cells that continued to secrete DENV-specific antibodies long-term.
Materials and Methods
Samples
Samples were obtained from five DENV-immune subjects (Table 1). Peripheral blood mononuclear cells (PBMCs) were purified, resuspended at 107 cells/mL in RPMI 1640 medium (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO), and 10% dimethyl sulfoxide (DMSO), and cryopreserved until use.
Table 1.
Donor Information
| Donor no. | Exposure | Serotype | Infection | Time pointa | Country of origin |
|---|---|---|---|---|---|
| 1 | Vaccine | DENV-4 | Primary | 6 months | USA |
| 2 | Natural | DENV-3b | Primaryb | ∼3 years | USAc |
| 3 | Natural | Unknown | Primaryb | ∼3 months | USA |
| 4 | Natural | Unknown | Unknown | ∼15 years | Philippines |
| 5 | Natural | Unknown | Unknown | Unknown | Thailand |
Time point of PBMC collection post-illness.
Based on history provided by the donor.
Donor 2 traveled to Thailand, where the infection was thought to occur.
DENV, dengue virus; PBMC, peripheral blood mononuclear cell.
B-cell bulk cultures and isolation of hMAbs
Cryopreserved PBMCs were thawed and washed twice. Cells were counted and diluted to 2×106/mL in RPMI 1640 (Gibco) with 10% FBS (Gibco), 100 U/mL penicillin/streptomycin (Gibco), and 200 mM L-glutamine (Gibco). PBMCs were stimulated with 2.5 μg/mL R848 (InvivoGen, San Diego, CA) and 1000 U/mL recombinant human (rh) IL-2 (Peprotech, Rocky Hill, NJ). These cells were then added to a 24-well plate. After 7 d at 37°C and 5% CO2, ELISpot assays were performed. Supernatants from stimulated PBMCs were collected and assessed for antibody secretion. To isolate hMAbs, CD22+ memory B cells were isolated by magnetic sorting (MACS kit MS; Miltenyi Biotec, Bergisch Gladbach, Germany), and immortalized with Epstein-Barr virus (EBV) in the presence of 2.5 μg/mL CpG (Operon Technologies, Alameda, CA), 1000 U/mL rhIL-2, and 30 μg/mL holo-transferrin (Sigma-Aldrich), as previously described (36). Cells were plated at 100 cells/well in 96-well plates and maintained with bi-weekly stimulation with CpG, rhIL-2, and transferrin. Supernatants were regularly collected and tested for antibody secretion.
ELISpot assay
ELISpot assays were performed as previously described (19). Briefly, wells of Millipore ELISpot plates (Millipore, Billerica, MA) were coated with 100 μL DENV E or NS1 protein (15 μg/mL; Hawaii Biotech, Aiea, HI). The strains used to generate the four DENV-E proteins were DENV-1 strain 258848, DENV-2 strain PR159/S1, DENV-3 strain CH53489, and DENV-4 strain H241. To detect total IgG, the wells were coated with 100 μL anti-human IgG (15 μg/mL, MT91/145; Mabtech, Nacka Strand, Sweden). The plates were stored at 4°C overnight. B-cell bulk cultures were counted, and ≤2.5×104 cells in 100 μL were added to each well in duplicate. The plates were incubated at 37°C overnight. After washing, 100 μL biotinylated MAb directed against human IgG (1 μg/mL in PBS/0.5% FBS; MT78/145; Mabtech) was added. The plates were incubated for 2 h at room temperature, washed, and developed with horseradish peroxidase (HRP)-conjugated streptavidin (100 μL of 1:1000 dilution in PBS/0.5% FBS; BD Pharmingen, San Diego, CA). The plates were analyzed using a CTL ImmunoSpot reader (CTL, Shaker Heights, OH).
Detection of DENV-specific antibody responses
DENV-1-4 E and DENV-2 NS1-specific antibodies in the supernatants of B-cell cultures were detected using an enzyme-linked immunosorbent assay (ELISA). For standard assays, 384-well microplates were coated overnight with 20 ng/well DENV-1-4 E or DENV-2 NS1 protein (Hawaii Biotech), a 1:40 dilution of DENV-1-4-infected or uninfected (control) Vero cell lysate, or infectious or immature DENV virions. The recombinant proteins were expressed in Drosophila sp. S2 cells, which are known to bind to native conformation-dependent MAbs (20). Furthermore, x-ray crystallography studies have demonstrated that these proteins retain a native-like structure (3). The DENV-1-4 antigens were prepared from infected Vero cell monolayers, as previously described (24). Briefly, Vero cell monolayers were infected at a multiplicity of infection (MOI) of 1.0 plaque-forming units (PFU)/cell, and incubated at 37°C in minimal essential medium (MEM) supplemented with 25 mM N-2-hydroxyethylpiperazine-N-9-2-ethanesulfonic acid (HEPES), 2 mM glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 2% FBS (Gibco), until 50% of the cells displayed cytopathology. Cells were then harvested, washed, fixed in 0.025% glutaraldehyde in PBS for 15 min on ice, washed again, and resuspended in RPMI 1640. The suspension of fixed cells was then sonicated on ice in a sonic dismembrator (Fisher Chemical, Loughborough, U.K.) and centrifuged at 1500 g for 10 min at 4°C. The supernatant was collected, aliquotted, and frozen at −80°C as viral antigen. Control antigen was prepared similarly from uninfected Vero cell monolayers. Immature DENV particles were produced in human adenocarcinoma LoVo cells (ATCC CCL-229), as described previously (26). Briefly, LoVo cells were infected with DENV in serum-free Ham's F12K medium (Life Technologies, Grand Island, NY) at an MOI of 1. The virus inoculum was removed after 1.5 h, and fresh medium was added after washing the cells twice with PBS. After 7 d, the medium containing the virus particles was harvested, cleared of cellular debris by low-speed centrifugation, aliquotted, and stored at −80°C.
Transformed B-cell cultures were also screened for antibody production using a modified ELISA (25,29). For the modified assay, 96-well plates were coated with concanavalin A (ConA; Vector Laboratories, Burlingame, CA) at 25 μg/mL in 0.01 M HEPES (Gibco) in a total volume of 100 μL/well for 1 h. The wells were washed, and 50 μL serum-free DENV preparations were added for 18 h. Undiluted supernatant containing murine MAb 3H5 (from ATCC HB-46), which binds to DENV-2 E, was used as a positive control during the screening process. Negative controls consisted of culture fluid grown in parallel with no virus. The plates were blocked with 1% bovine serum albumin (BSA) for 90 min, and dilutions of antibodies #27 and #41 were added to the wells for 1 h. The plates were washed with PBS containing 0.1% Tween-20. Goat anti-human IgG coupled to HRP (A80-104P; Bethyl Laboratories Inc., Montgomery, TX) was added as the secondary antibody at a 1:20,000 dilution. Then, 100 μL TMB (3,3′,5,5′-tetramethylbenzidine) peroxidase substrate (50-76-00; KPL, Gaithersburg, MD) was added as the substrate. After approximately 20 min, the enzyme reaction was stopped by the addition of 1 M hydrochloric acid, and the plates were read at 450 nm.
hMAb recognition of DENV-infected cells by flow cytometry
K562-DC-SIGN cells (a gift from Dr. Vineet Kewal Ramini, National Cancer Institute, Frederick, MD) were infected with DENV-1 or DENV-3 (MOI 0.2), and incubated at 37°C for 1.5 h. The cells were washed and incubated at 37°C in 5% CO2 for 24 h in RPMI/2% FBS. Uninfected and infected K562-DC-SIGN cells were either permeabilized or stained directly with hMAbs. Approximately 2×105 cells were permeabilized in 200 μL Cytofix/Cytoperm (BD Biosciences, San Jose, CA) and stained with antibodies #27 and #41 for 30 min. After washing with FACS buffer (PBS/2% FBS/0.1% sodium azide), the cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-human IgG (F-9006; Sigma-Aldrich). Approximately 2×104 cells were analyzed for each sample. Data were collected on a BD FACSAria (BD Biosciences), and analyzed using FlowJo version 7.5.5 software (Tree Star, Inc., Ashland, OR).
Western blot
The DENV-infected Vero cell lysates were prepared by lysing DENV-1- and DENV-3-infected Vero cells with RIPA buffer (150 mM NaCl, 50 mM Tris, 0.1% sodium dodecyl sulfate [SDS], and 1% Igepal CA-C30) on ice for 1 h. The supernatants were then analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using a 12.5% denaturing gel and pre-stained protein markers on adjacent lanes. Separated proteins were electro-transferred using a Mini Trans-blot Electrophoretic Cell (Bio-Rad Laboratories, Hercules, CA) onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). Electro-blotting was performed in the presence of 200 mM glycine, 24 mM Tris base, and 20% methanol, at a constant current (100 mA) overnight at room temperature. Transfer of proteins onto the membrane was visually verified with pre-stained Precision Plus Kaleidoscope Standards (310010352; Bio-Rad). The membrane was blocked overnight with 5% nonfat milk in 1×PBS with gentle shaking at 4°C. The blocking solution was replaced with the primary antibody solution (1:250 dilution in blocking solution), and incubated overnight at 4°C with gentle shaking. Thereafter, the blot was washed three times with 1×PBST (10 mM phosphate buffer [pH 7.2], 150 mM NaCl, and 0.1% Tween-20) for 10 min, each followed by incubation in anti-human IgG-HRP-conjugated antibody solution (1:5000 dilution in blocking solution, A80-104P; Bethyl Laboratories) for a further 45 min. The blot was washed again as described above. The protein bands were visualized with a chemiluminescent HRP-conjugated antibody detection reagent (Denville Scientific Inc., Metuchen, NJ).
Avidity assays
Two sets of 96-well microplates were coated overnight with a 1:40 dilution of DENV-1 or DENV-3 inactivated lysate, or 50 μL of Con A-immobilized DENV-1 or DENV-3. The plates were blocked with 1% BSA for 90 min, and incubated with 100 μL serially-diluted supernatants from hMAbs #27 or #41 in duplicate for 1 h at 37°C. The plates were washed with PBS/0.1% Tween-20. One set of plates was incubated with 8 M urea for 10 min at 37°C. Both sets of plates were washed and incubated with anti-human IgG-HRP (A80-104P; Bethyl Laboratories) for 1 h at 37°C. TMB solution was then added, and the enzyme reaction was stopped by the addition of 1 M hydrochloric acid. Absorbance values greater than twofold above background were considered positive. Avidity indices were calculated as the ratio of the optical density (OD) with urea to the OD without urea.
Flow cytometry-based neutralization assay
Vero cells were seeded in 48-well plates at a density of 0.5×105 cells per well and incubated overnight. The culture supernatants were serially diluted in MEM containing 1% BSA supplemented with penicillin and streptomycin. DENV-1 or DENV-3 was added to antibodies #27 and #41 and incubated at 4°C for 1 h. The virus and MAb mixture was added to the Vero cells to achieve an MOI of 0.2. The plates were incubated at 37°C in 5% CO2 for 1 h. Approximately 1 mL of MEM containing 5% FBS was added to each well, and the plates were incubated at 37°C in 5% CO2 for 24 h. Each well was washed with 1 mL of PBS. The plates were incubated with 0.2 mL of trypsin/well at 37°C for 5 min and washed with 1 mL of PBS containing 10% FBS. The cells were pipetted to break up any clumps and centrifuged at 1000 g for 5 min. The cells were permeabilized using Cytofix/Cytoperm and stained with a 1:100 dilution of the DENV-specific antibody 2H2 (Millipore), followed by a 1:200 dilution of FITC-conjugated anti-mouse IgG as a secondary antibody (Sigma). Approximately 2×104 cells were analyzed for each sample. The percent neutralization was calculated at each dilution using the formula 100 – ([frequency of infected cells in the presence of antibody×100]/frequency of infected cells in the absence of antibody).
Phenotypic staining and flow cytometry
B-cell lines were washed in FACS buffer and stained with LIVE/DEAD Aqua (Molecular Probes, Life Technologies, Grand Island, NY) according to the manufacturer's instructions. The cells were then washed and incubated with the following MAbs: PE-Cy7-αCD19, PerCP-Cy5.5-αCD20, APC-H7-αCD27, APC-αCD38 (BD Biosciences), and PC5-αCD138 (Beckman Coulter, Miami, FL) at 4°C for 30 min. An aliquot of cells was either permeabilized with Cytofix/CytoPerm for 20 min or left unpermeabilized. The cells were stained with MAbs against human IgM and IgG (Dako Systems, Carpenteria, CA). Data were collected on a BD FACSAria and analyzed using FlowJo version 7.5.5.
Results
DENV E- and NS1-specific antibody-secreting cells in PBMCs from DENV-immune donors
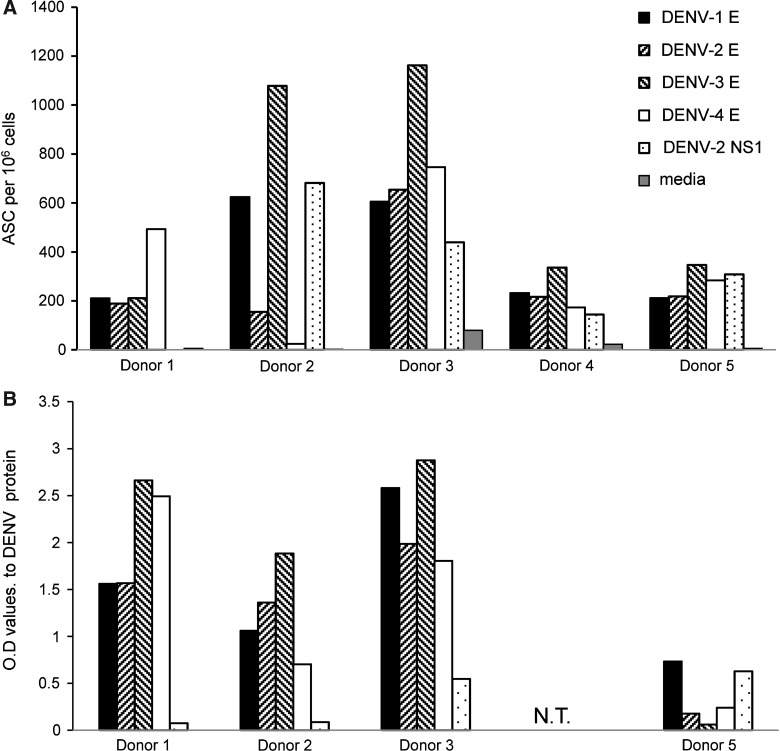
We utilized a B-cell ELISpot assay to enumerate antibody-secreting cells (ASCs) in PBMCs obtained from five adults who either received a live attenuated dengue vaccine or who were naturally exposed to DENV infection (Table 1). The serotype of DENV was identified by RT-PCR for one subject (donor 1) (13); the remaining four subjects were enrolled after acute DENV infection and did not have virus detected. PBMCs were cultured in the presence of the Toll-like receptor (TLR) 7/8 agonist R848 for 7 d to activate memory B cells to secrete antibody. We used recombinant (r) E and NS1 proteins to identify DENV-specific ASCs. We detected DENV E-specific ASCs in PBMCs from 5/5 donors, and NS1-specific ASCs in 4/5 donors (Fig. 1A). We next used supernatants from R848-stimulated PBMCs to assess whether the breadth and serotype cross-reactivity of antibodies to DENV proteins detected by ELISpot were also reflected in the antibodies secreted by memory B cells. We detected secretion of E-specific antibodies by 4/4 donors (one donor was not tested), and NS1-specific antibodies by 2/4 donors (Fig. 1B). These data demonstrate the presence of serotype-cross-reactive antibodies in subjects with varying histories of DENV infection, including those with a single exposure to the virus.
FIG. 1.
High frequencies of DENV E-specific memory B cells in immune donors. (A) Frequencies of antigen-specific B cells were measured by ELISpot assay in triplicate in the PBMCs of DENV-immune donors after in vitro stimulation for 7 d with R848 and IL-2. The plates were coated with the indicated DENV rE or rNS1 protein. (B) The undiluted supernatants from the stimulated PBMC cultures were tested by ELISA in triplicate for recognition of recombinant DENV 1–4 E and DENV-2 NS1 proteins.
Isolation of MAbs from DENV-immune PBMCs
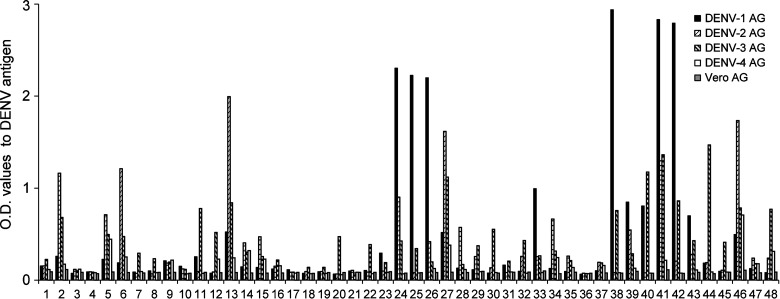
We next wanted to isolate memory B-cell-derived hMAbs from these donors for more in-depth characterization of their serotype-specificity and neutralization potency. CD22+ memory B cells were immortalized with EBV and CpG, as previously described (36), and maintained at 100 cells/well in 96-well plates. In our hands, B-cell lines from donor 2 were maintained in culture and continued to secrete antibodies for a long period; these cells retained CD19 and CD20 expression, consistent with previous reports of EBV-mediated B-cell transformation (22). The immortalized B-cell culture supernatants were screened for IgG antibodies that bound an inactivated lysate of DENV-3-infected cells, since ELISpot assays indicated that responses to DENV-3 were strongest in this subject (Fig. 1). DENV-3 lysate-specific IgG antibodies were detected in 90/1728 (5%) wells (data not shown). We selected wells with OD values≥5 times above background for further analysis. Of the 48 wells that were initially selected, the cells in 40 wells continued to secrete antibodies and underwent more extensive characterization. Supernatants were collected from these wells every 2 wk and tested by ELISA to define the major antigens they recognized.
Serotype specificity of MAbs generated from memory B cells
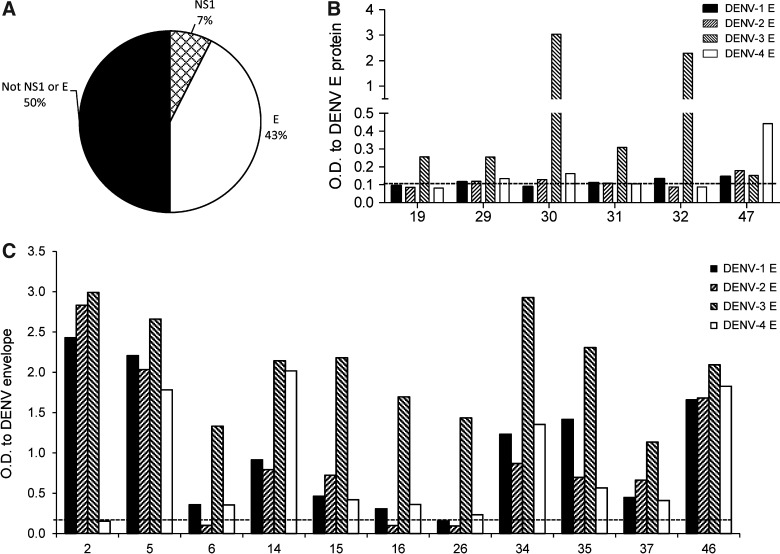
We assessed the serotype-specificity of antibodies in supernatants of DENV-3 lysate-positive wells by using inactivated cell lysates for all four serotypes of DENV. We found that 31/40 hMAbs were able to bind more than one dengue antigen, while 9/40 were serotype-specific and bound DENV-3 antigen only (Fig. 2). Since the inactivated cell lysates contain multiple structural and nonstructural proteins of DENV, we next tested the supernatants for recognition of DENV rE and rNS1 proteins, the major targets of anti-DENV antibodies. We found that 17/40 wells recognized rE protein, and 3/40 supernatants recognized DENV-2 rNS1 protein; 20/40 supernatants did not recognize either rE or rNS1 (Fig. 3A). Of the 17 antibodies that recognized the rE protein, 6 (36%) were serotype-specific with responses predominantly to DENV-3 E, and 11 (64%) were cross-reactive and recognized more than one serotype of E (Fig. 3B and C). The relative concentrations of specific viral components in the cell lysates are unknown, which could explain the qualitative differences in antibody recognition of DENV-inactivated lysates and recombinant proteins. Since B cells were seeded at 100 cells/well, we also cannot exclude the possibility that a change in relative concentration of polyclonal Abs present within the same well over time may have impacted these results. However, given the low frequency of memory B cells in PBMCs, we believe this was unlikely. The data indicated that over 40% of MAbs generated from memory B cells from donor 2 recognized E produced as a soluble recombinant antigen, with the majority of antibodies being serotype-cross-reactive.
FIG. 2.
Antibody serotype cross-reactivity to inactivated lysates of DENV. Undiluted supernatants from individual wells of B-cell lines from donor 2 were tested by ELISA for reactivity to inactivated antigen lysate preparations of DENV-1– through DENV-4–infected or uninfected Vero cells.
FIG. 3.
Antibody serotype cross-reactivity to recombinant DENV E and NS1 proteins. (A) Distribution of antibodies from donor 2 that bound to the NS1 and/or E proteins of DENV, as determined by ELISA. Antibodies from donor 2 were determined by ELISA (n=3) to be (B) serotype-specific, or (C) serotype-cross-reactive to DENV E protein.
MAbs recognize conformation-dependent, reduction-sensitive epitopes on E
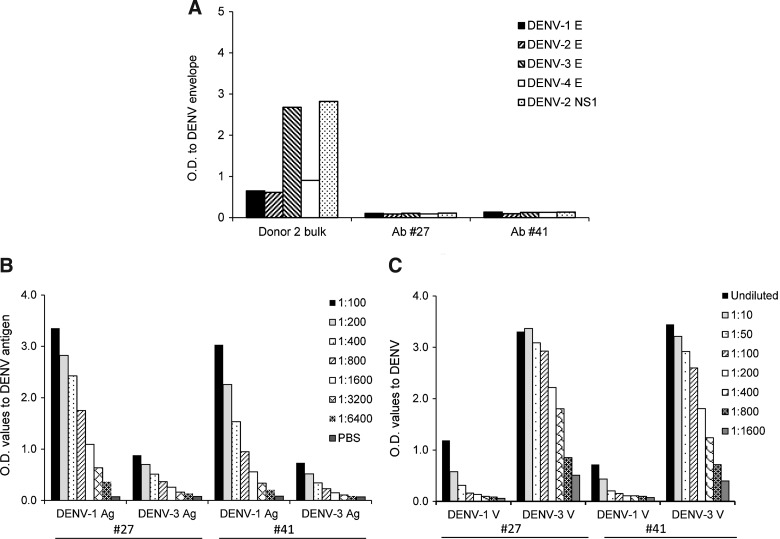
Recently, several groups have identified the prM protein as a dominant target of anti-DENV human antibodies (1,8). We further characterized two MAbs (#27 and #41) as potentially binding to the prM protein, since they did not recognize rE or rNS1 by ELISA (Fig. 4A). Both hMAbs #27 and #41 were able to recognize the inactivated lysates of DENV-1 and −3, but not DENV-2 or −4 antigens (Figs. 2 and 4B and data not shown). MAbs #27 and #41 were able to recognize DENV-1–inactivated lysates better than DENV-3–inactivated lysates consistently. We next tested these antibodies in our standard ELISA for their ability to bind to infectious DENV virus or DENV virus produced in LoVo cells, which is known to be enriched for immature virus particles expressing prM (26); neither #27 nor #41 bound infectious virus or immature virus produced in LoVo cells (data not shown). We then modified the ELISA to affinity-immobilize viral glycoproteins using ConA (25,29), and we detected substantial binding of MAbs #27 and #41 to DENV-3, with less binding to DENV-1 (Fig. 4C). These data suggest that MAb #27 and #41 bound to a conformation-sensitive epitope on the E protein that is not presented by the rE protein, which was produced in Drosophila cells.
FIG. 4.
Monoclonal antibodies from donor 2 recognize DENV. (A) Lack of recognition of rE and rNS1 proteins of DENV by MAbs #27 and #41 (n=3). (B) Dose-dependent recognition of DENV-1– and DENV-3–inactivated lysates by MAbs #27 and #41 (n=3). (C) Dose-dependent recognition of DENV-1 and DENV-3 whole virus by MAbs #27 and #41 using a modified ELISA (n=2).
Supernatants from wells #27 and #41 were next tested for reactivity to DENV in a non-reducing Western blot. Dengue virions were solubilized and subjected to Western blot analysis to identify the viral structural proteins recognized by MAbs. An inactivated lysate of dengue virus was also used to identify both structural and nonstructural proteins. MAbs #27 and #41 both recognized a band of ∼55–60 kDa, the expected molecular weight of the E protein under non-reducing conditions (8,10). Previous studies of mouse anti-E and anti-NS1 MAbs have shown that most MAbs lost reactivity upon treatment with β-mercaptoethanol (under reducing conditions), and therefore were sensitive to the protein conformation supported by disulfide bridges (11,18,27). Binding of MAbs #27 and #41 to E was abolished when virus and lysates were subject to reducing conditions (Supplementary Figure S1; see online supplementary material at http://www.liebertonline.com).
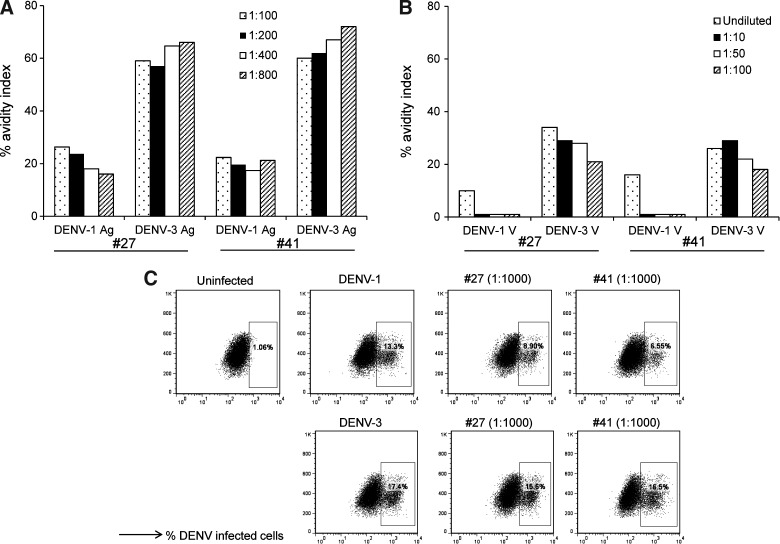
We speculated that MAbs #27 and #41 would have different avidities to DENV-1– and DENV-3–inactivated lysates, which could reflect the serotype of virus the donor was infected with. We calculated the avidity index and present the values obtained at different concentrations of MAbs showing a positive ELISA result. Our data indicated that both MAbs #27 and #41 had a higher avidity index to DENV-3–inactivated lysate (Fig. 5A). While the overall avidity index to ConA-immobilized infectious DENV was lower compared to the inactivated lysates, both MAbs #27 and #41 also had a higher avidity index to DENV-3 virions (Fig. 5B).
FIG. 5.
Antibody avidity and neutralization activity of MAbs #27 and #41. Avidity indices of MAbs #27 and #41 to (A) DENV-1– and DENV-3–inactivated lysates, and (B) ConA-immobilized DENV-1 and DENV-3 virions (n=2). The indicated dilutions of MAbs #27 and #41 (total concentration of undiluted supernatant 2.5 mg/ml) were used. The avidity index was calculated as the ratio of the OD with urea to the OD without urea multiplied by 100. (C) Neutralization activity of MAbs #27 and #41. Vero cells were infected with DENV-1 or DENV-3. Representative dot plots show DENV-1– and DENV-3–infected cells in the absence or presence of the indicated dilutions of MAbs #27 and #41.
We next tested the neutralizing activity of MAbs #27 and #41 using a flow-based neutralization assay with Vero cells. Neutralization did not reach 100%, but plateaued between 40 and 50% for MAb #27, and between 50 and 60% for MAb #41, when incubated with DENV-1 or DENV-3 at various dilutions (1:1000 to 1:160,000). Shown are representative dot plots depicting infection in the presence of 1:1000 dilutions of MAbs #27 and #41 (Fig. 5C). Together, these data indicate that MAbs #27 and #41 both bound reduction-sensitive epitopes on the E protein of DENV. Both MAbs had higher avidity to DENV-3 compared to DENV-1, with poor neutralizing activity in vitro.
MAbs #27 and #41 recognize DENV-infected cells
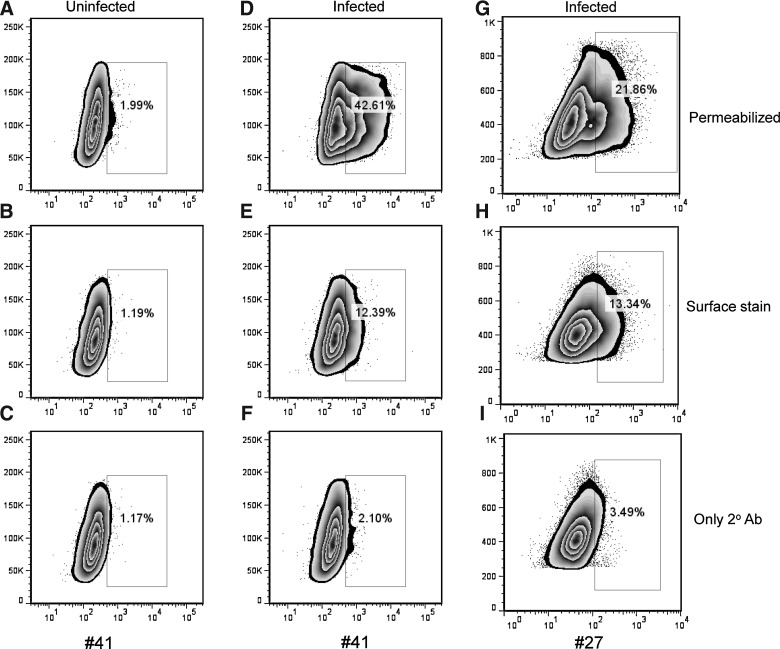
Since the DENV E protein is predominantly expressed in the cytoplasm and then transported to the cell surface of virus-infected cells, we next determined whether MAbs #27 and #41 were able to bind non-permeabilized and/or permeabilized virus-infected cells. K562-DC-SIGN cells were infected with DENV-1 virus at an MOI of 0.2, and 24 h later, we stained permeabilized and non-permeabilized cells with MAbs #27 and #41. Both antibodies bound to permeabilized and non-permeabilized virus-infected cells, although their staining of permeabilized infected cells (Fig. 6D and G) was greater than that of non-permeabilized cells (Fig. 6E and H). Uninfected cells (Fig. 6A–C), and cells stained with only the secondary antibody (Fig. 6C, F, and I), had low-level background staining.
FIG. 6.
Human MAbs #27 and #41 recognize virus-infected cells. (A–C) Uninfected and (D–I) infected K562-DC-SIGN cells were either permeabilized (A, D, and G), or stained directly (B, E, and H), with hMAbs #41 (A–F) or #27 (G–I). Following staining with the primary MAb, the cells were stained with a secondary FITC-conjugated anti-human IgG antibody. Representative staining of uninfected (C) and infected (F and I) cells with the secondary FITC-conjugated antibody alone is shown.
Phenotype of B cells that secrete DENV-specific antibodies
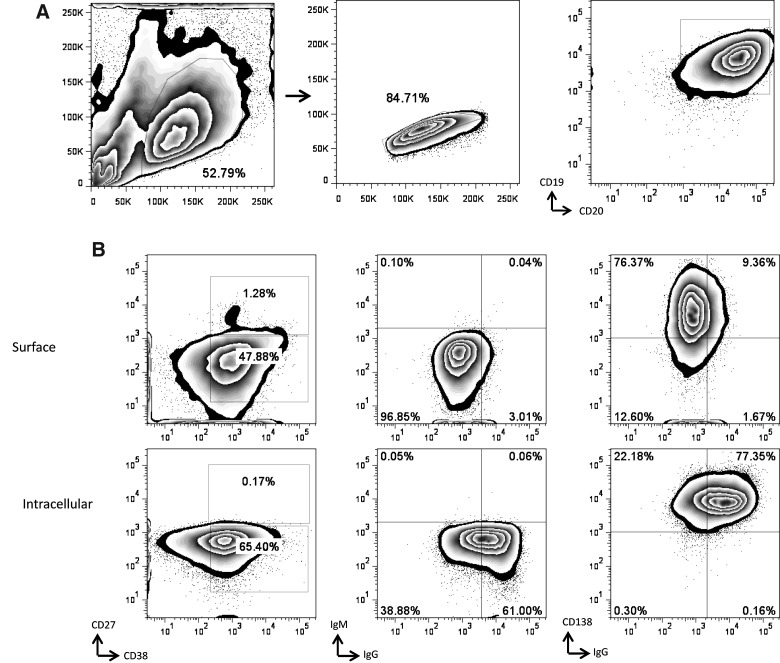
Human B cells express a number of markers that aid in distinguishing naive B cells, memory B cells, antibody-secreting plasmablasts, and plasma cells (17). We examined the phenotype of the EBV-transformed B cells from donor 2 that proliferated and maintained their secretion of DENV-specific antibodies. The B cell lines that secreted MAbs #27 and #41 both expressed CD19 and CD20 (Fig. 7), but did not express CD27, a marker associated with memory B cells. Plasma cells differentiate from activated memory B cells, and this differentiation is linked to proliferation induced either by antigen binding or by triggering of CD40 or TLRs (17,24,35). Our findings indicated that #27 and #41 expressed activation markers (CD38), and markers associated with plasma cells (CD138). In addition, these cell lines expressed high levels of intracellular IgG, but little cell-surface IgG. Together, these data suggest that B cells from donor 2 that maintained their ability to secrete DENV-specific antibodies were CD19+CD20+CD27−CD38+CD138+IgG+ B cells.
FIG. 7.
Phenotype of the B-cell line that produced MAb #41. (A) The gating strategy to identify cells in the lymphocyte gate was based on forward- and side-scatter profiles. Next, singlet cells were selected, and B cells were identified using antibodies directed against CD19 and CD20. (B) CD27, CD38, CD138, IgM, and IgG expression was assessed both on the surface (top panel) and intracellularly (bottom panel).
Discussion
We assessed the breadth and magnitude of antibodies secreted by human memory B-cell lines from a single donor with prior exposure to DENV. While all antibodies bound an inactivated lysate of DENV, only 50% of antibodies bound rE or rNS1 protein. Most DENV-specific hMAbs we identified were serotype cross-reactive, as has been shown by other groups (1,6,8). We characterized in detail two antibodies that did not bind rE protein produced in Drosophila cells, but bound reduction-sensitive epitopes on the E protein derived from whole virus or virus-infected cell lysates. As the rE protein used in these studies contained only ∼80% of the full-length protein, lacking the membrane-proximal region and the transmembrane domains, it is possible that MAbs #27 and #41 target these regions (3). It is also possible that these antibodies bind epitopes that are poorly accessible on the surface of the mature virion, are cryptic, or are formed by multiple E proteins in close proximity. These findings are in accord with several recent reports that have characterized hMAbs from DENV-immune donors (1,6,8,29,32).
Studies by Beltramello et al. and Dejnirattisai et al. indicated that DENV E protein domain III (EDIII)-binding antibodies represent a fraction of antibodies generated in immune subjects (1,8). Work done by Wahala et al. indicated that only a small fraction of the neutralizing activity in human DENV immune sera is EDIII-specific (40). We have not assessed whether the antibodies generated by memory B cells are directed against specific domains of the E protein. MAbs #27 and #41 recognized DENV-1–inactivated lysates better than DENV-3 lysates. However, both Abs recognized ConA-immobilized DENV-3 virions better than DENV-1 virions. Avidity assays indicated stronger binding to DENV-3 lysate and virions compared to DENV-1. These findings are interesting since this donor reported travel to Northern Thailand in 1987 where there was an ongoing outbreak of DENV-3 (38). The MAbs that we characterized only poorly neutralized DENV infection in vitro, and we do not know if they play any role in vivo. De Alwis et al. characterized three MAbs that strongly neutralized DENV infection in vitro, and found that they bound complex epitopes that were expressed only when the E protein was assembled as a virus particle (7). Our data add to the existing literature that a number of neutralizing and non-neutralizing DENV-specific antibodies bind epitopes that may recognize quarternary structures on intact virions of DENV.
We recently compared DENV-specific B-cell responses by ELISpot in a cohort of Thai children with primary or secondary DENV infection (19). Our data indicated that there was a weak and inconsistent correlation between DENV-specific antibody titers in the serum and the frequency of DENV-specific memory B cells in the blood. We do not know if our results with hMAbs correlate with the neutralization properties of polyclonal immune serum from this donor. The abundance of non-neutralizing and weakly neutralizing hMAbs generated from DENV-specific memory B cells suggests that only a small fraction of DENV-specific antibodies in immune sera are responsible for the majority of in vitro neutralization (1,6,8). However, individual antibodies that are weakly neutralizing may function differently in a polyclonal milieu (2). On the other hand, these antibodies may actually serve to enhance viral replication of FcR-expressing cells, as has been shown by several groups (1,8). The humoral immune system has many mechanisms that contribute to viral control. Antibodies may redirect virus into noninfective environments or trigger immune complexes or lysis by antibody-dependent cell-mediated cytotoxicity (ADCC), or by local cytokine secretion (9,21). Further studies are needed to understand how the properties of hMAbs from DENV-immune donors relate to the properties of serum antibody.
The B cells that secrete the DENV-specific antibodies characterized in this study have undergone activation and proliferation under in vitro conditions. We used a modified approach to stimulate memory B cells to secrete antibodies, as has been used previously in the literature (36,37). While this method has been very effective in our hands to screen for DENV-specific antibodies in supernatants from stimulated cells, the maintenance of B cells that continue to secrete antibodies long-term has been difficult. Alternate approaches whereby immunoglobulin genes are molecularly cloned result in stable antibody production by promising B-cell lines and should be considered (41).
The phenotype of B cells that secreted anti-DENV antibodies that recognized conformation-sensitive E epitopes was CD19+CD20+CD27−CD38+CD138+IgG+. We have phenotyped B cells from donor 2 prior to and at multiple time points (2 wk, 3 mo, and >5 mo) post-EBV immortalization. CD19 and CD20 expression was maintained on B cells during the process of immortalization (data not shown). We were able to see a distinct but small population of CD27+ cells in PBMCs from donor 2; however, we found low expression on bulk B cells and in lines #27 and #41. The finding that these ASCs do not express CD27, a marker used to phenotype memory B cells, was unexpected. While CD27 expression was initially used as an exclusive marker of memory B cells, recent reports suggest that CD27 expression is more diverse than previously appreciated (17). CD27 expression has been reported to be downregulated following EBV transformation (22); therefore, it is possible that memory B cells secreting MAbs #27 and #41 downregulated CD27. TLR9 stimulation of naive (CD27−) and memory (CD27+) human B cells in vitro converts them to CD138+ cells, a marker used for bone marrow–resident plasma cells (15,16). Whether markers on DENV-specific ASCs are reflective of atypical memory B cells that exist in vivo, or whether the in vitro conditions used to stimulate human B cells altered their phenotype, is unclear.
We only characterized hMAbs from a single donor in this study. The difficulty of obtaining samples from immune donors with a well-characterized natural history of DENV infection is well known. Nevertheless, our data support recent findings in the literature that anti-DENV hMAbs are cross-reactive and directed against multiple proteins (1,6,8,29,32). Further studies in larger cohorts of donors are required to draw more generalizable conclusions about the repertoire of B cells available in the peripheral blood of DENV-immune donors.
Supplementary Material
Acknowledgment
This work was funded by the National Institutes of Health, grant P01 AI34533.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Beltramello M. Williams KL. Simmons CP, et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8:271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavacini L. Posner M. Native HIV type 1 virion surface structures: relationships between antibody binding and neutralization or lessons from the viral capture assay. AIDS Res Hum Retroviruses. 2004;20:435–441. doi: 10.1089/088922204323048186. [DOI] [PubMed] [Google Scholar]
- 3.Clements DE. Coller BA. Lieberman MM, et al. Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine. 2010;28:2705–2715. doi: 10.1016/j.vaccine.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crill WD. Hughes HR. Delorey MJ. Chang GJ. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One. 2009;4:e4991. doi: 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crill WD. Roehrig JT. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol. 2001;75:7769–7773. doi: 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Alwis R. Beltramello M. Messer WB, et al. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis. 2010;5:e1188. doi: 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Alwis R. Smith SA. Olivarez NP, et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dejnirattisai W. Jumnainsong A. Onsirisakul N, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowd KA. Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology. 2011;411:306–315. doi: 10.1016/j.virol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falconar AK. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch Virol. 1999;144:2313–2330. doi: 10.1007/s007050050646. [DOI] [PubMed] [Google Scholar]
- 11.Falconar AK. Young PR. Production of dimer-specific and dengue virus group cross-reactive mouse monoclonal antibodies to the dengue 2 virus non-structural glycoprotein NS1. J Gen Virol. 1991;72(Pt 4):961–965. doi: 10.1099/0022-1317-72-4-961. [DOI] [PubMed] [Google Scholar]
- 12.Falconar AK. Young PR. Miles MA. Precise location of sequential dengue virus subcomplex and complex B cell epitopes on the nonstructural-1 glycoprotein. Arch Virol. 1994;137:315–326. doi: 10.1007/BF01309478. [DOI] [PubMed] [Google Scholar]
- 13.Gagnon SJ. Zeng W. Kurane I. Ennis FA. Identification of two epitopes on the dengue 4 virus capsid protein recognized by a serotype-specific and a panel of serotype-cross-reactive human CD4+ cytotoxic T-lymphocyte clones. J Virol. 1996;70:141–147. doi: 10.1128/jvi.70.1.141-147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzman MG. Alvarez M. Rodriguez-Roche R, et al. Neutralizing antibodies after infection with dengue 1 virus. Emerg Infect Dis. 2007;13:282–286. doi: 10.3201/eid1302.060539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henn AD. Rebhahn J. Brown MA, et al. Modulation of single-cell IgG secretion frequency and rates in human memory B cells by CpG DNA, CD40L, IL-21, and cell division. J Immunol. 2009;183:3177–3187. doi: 10.4049/jimmunol.0804233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huggins J. Pellegrin T. Felgar RE, et al. CpG DNA activation and plasma-cell differentiation of CD27-naive human B cells. Blood. 2007;109:1611–1619. doi: 10.1182/blood-2006-03-008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson SM. Wilson PC. James JA. Capra JD. Human B cell subsets. Adv Immunol. 2008;98:151–224. doi: 10.1016/S0065-2776(08)00405-7. [DOI] [PubMed] [Google Scholar]
- 18.Lai CY. Tsai WY. Lin SR, et al. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol. 2008;82:6631–6643. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathew A. West K. Kalayanarooj S, et al. B-cell responses during primary and secondary dengue virus infections in humans. J Infect Dis. 2011;204:1514–1522. doi: 10.1093/infdis/jir607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modis Y. Ogata S. Clements D. Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci USA. 2003;100:6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy BR. Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 22.O'Nions J. Allday MJ. Proliferation and differentiation in isogenic populations of peripheral B cells activated by Epstein-Barr virus or T cell-derived mitogens. J Gen Virol. 2004;85:881–895. doi: 10.1099/vir.0.19704-0. [DOI] [PubMed] [Google Scholar]
- 23.Oliphant T. Nybakken GE. Engle M, et al. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol. 2006;80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Andres M. Paiva B. Nieto WG, et al. Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin Cytom. 2010;78(Suppl 1):S47–S60. doi: 10.1002/cyto.b.20547. [DOI] [PubMed] [Google Scholar]
- 25.Robinson JE. Holton D. Liu J. McMurdo H. Murciano A. Gohd R. A novel enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to HIV-1 envelope glycoproteins based on immobilization of viral glycoproteins in microtiter wells coated with concanavalin A. J Immunol Methods. 1990;132:63–71. doi: 10.1016/0022-1759(90)90399-g. [DOI] [PubMed] [Google Scholar]
- 26.Rodenhuis-Zybert IA. van der Schaar HM. da Silva Voorham JM, et al. Immature dengue virus: a veiled pathogen? PLoS Pathog. 2010;6:e1000718. doi: 10.1371/journal.ppat.1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roehrig JT. Bolin RA. Kelly RG. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology. 1998;246:317–328. doi: 10.1006/viro.1998.9200. [DOI] [PubMed] [Google Scholar]
- 28.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 29.Schieffelin JS. Costin JM. Nicholson CO, et al. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol J. 2010;7:28. doi: 10.1186/1743-422X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrestha B. Brien JD. Sukupolvi-Petty S, et al. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog. 2010;6:e1000823. doi: 10.1371/journal.ppat.1000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons CP. Chau TN. Thuy TT, et al. Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis. 2007;196:416–424. doi: 10.1086/519170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SA. Zhou Y. Olivarez NP. Broadwater AH. de Silva AM. Crowe JE., Jr Persistence of circulating B memory cell clones with potential for dengue virus disease enhancement for decades following infection. J Virol. 2011 doi: 10.1128/JVI.06335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sukupolvi-Petty S. Austin SK. Engle M, et al. Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J Virol. 2010;84:9227–9239. doi: 10.1128/JVI.01087-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sukupolvi-Petty S. Austin SK. Purtha WE, et al. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol. 2007;81:12816–12826. doi: 10.1128/JVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tangye SG. Tarlinton DM. Memory B cells: effectors of long-lived immune responses. Eur J Immunol. 2009;39:2065–2075. doi: 10.1002/eji.200939531. [DOI] [PubMed] [Google Scholar]
- 36.Traggiai E. Becker S. Subbarao K, et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traggiai E. Chicha L. Mazzucchelli L, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 38.Ungchusak K. Kunasol P. Dengue haemorrhagic fever in Thailand, 1987. Southeast Asian J Trop Med Public Health. 1988;19:487–490. [PubMed] [Google Scholar]
- 39.Valdes K. Alvarez M. Pupo M. Vazquez S. Rodriguez R. Guzman MG. Human Dengue antibodies against structural and nonstructural proteins. Clin Diagn Lab Immunol. 2000;7:856–857. doi: 10.1128/cdli.7.5.856-857.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahala WM. Kraus AA. Haymore LB. Accavitti-Loper MA. de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology. 2009;392:103–113. doi: 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wrammert J. Smith K. Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.