Abstract
Ouabain is a cardiotonic steroid and specific inhibitor of the Na+/K+-ATPase. The relationship between ouabain treatment and the unfolded protein response (UPR) in cells is not precisely understood. Therefore, we studied the possible effects of ouabain on proliferation, apoptosis, and the UPR. HepG2 cells were cultured overnight and then treated with various concentrations of ouabain (0.75 to 750 nM) in the absence or presence of 10 mM 2-deoxyglucose (2-DG) for 48 hours. We also used real-time polymerase chain reaction to obtain quantitative measurements of expression levels of Grp78, Grp94, CHOP, MTJ-1, HKII, MDR-1, MRP-1, HO-1, and Par-4. Cell number, viability, and proliferation of HepG2 cells were monitored with a real-time cell analyzer system (xCELLigence). We show that ouabain modulates the UPR transcription program and induces cell death in glucose-deprived tumor cells. Ouabain at all concentrations showed no cytotoxicity whereas all concentrations were very effective under 2-DG stress conditions. Our findings show that disruption of the UPR during glucose deprivation could be an attractive approach for selective cancer cell killing and could provide a chemical basis for developing UPR-targeting drugs against solid tumors. Ouabain use as an adjunct to conventional cancer therapy also warrants vigorous investigation.
Key words: 2-deoxyglucose, CHOP, Grp78, HepG2, ouabain, unfolded protein response
Introduction
Ouabain is a cardiotonic steroid with positive inotropic effects that is used as a congestive heart failure treatment.1 Ouabain exerts its effects by inhibiting the cell membrane Na+/K+-ATPase pump.2 In addition, because the expression of some subunits of the Na+/K+-ATPase pump increase in cancers, ouabain has a particularly intense effect on cancer cells.3,4 Intracellular ion changes are important in the realization of cell death, and cardiotonic steroids like ouabain, which cause both Ca2+ increase and K+ decrease, promote cell death.4 Ouabain at low doses that do not cause pump inhibition is also claimed to have a proliferative or apoptotic effect, exerted by activating different signal pathways.5–10
The signal pathways of the unfolded protein response (UPR) are activated by the cell under various physiologic and pathologic conditions to overcome endoplasmic reticulum (ER) stress due to corrupted protein folding in the ER and thereby to ensure continuity of its life.11,12 Under ER stress, the proapoptotic BH3 proteins BAX and BAK undergo conformational changes within the ER membrane, which allow the exit of m-calpain, a Ca2+-activator, into the cytosol.13 The Ca2+ released from the ER is taken up by the mitochondria and the mitochondrial internal membrane potential is disrupted. This then initiates apoptosis.8
The glucose analog 2-deoxyglucose (2-DG) inhibits glycolysis and induces ER stress by preventing N-glycosylation of proteins in the cell.14–16 Cancer cells show a greater sensitivity to 2-DG when compared with normal cells, due to the increased glycolytic and GLUT17 activity in cancer cells.
In this study, we examined the effects of ouabain on cell proliferation, apoptosis, and the UPR in a HepG2 cell model where glucose deprivation was created with 2-DG treatment. For this purpose, we studied expressions of glucose-regulated protein 78 (Grp78), glucose-regulated protein 94 (Grp94), murine tumor cell DnaJ-like protein1 (MTJ-1), ccaat/enhancer protein (c/ebp) homolog protein (CHOP), and heme oxygenase1 genes (HO-1) as UPR genes; the prostate apoptosis response4 (Par-4) and hexokinase II (HKII) as genes related to apoptosis; and multidrug resistant gene1 (MDR-1) and multidrug resistance-associated protein1 (MRP-1) as genes related to drug resistance.
Materials and Methods
Cell lines
Passage 17 human hepatic cancer cells (HepG2) were used in this study. HepG2 cells were cultured in a drying oven in a 5% CO2 95% humidity atmosphere at 37°C in DMEM medium (Biological Industries) containing 10% fetal bovine serum (Sigma Aldrich), 4 mM L-Glutamine (Biological Industries), 100 U penicillin/0.1 mg streptomycin (Sigma Aldrich), and 1 g/L D-glucose. When the cells were 70%–80% confluent in the flask, the cells were first washed with phosphate buffered saline (PBS) and then removed from the flask with trypsin-ethylenediaminetetraacetic acid (EDTA; 0.25% trypsin, 0.02% EDTA, Biological Industries). The cells were plated on the cell culture plates at 5×103 cells/per well.
Study groups
A total of 10 groups was generated in the study: (1) Control group, (2) 0.75 nM ouabain group, (3) 7.5 nM ouabain group, (4) 75 nM ouabain group, (5) 750 nM ouabain group, (6) 10 mM 2-DG group, (7) 10 mM 2-DG and 0.75 nM ouabain group, (8) 10 mM 2-DG and 7.5 nM ouabain group, (9) 10 mM 2-DG and 75 nM ouabain group, and (10) 10 mM 2-DG and 750 nM ouabain group.
After the cells were plated, they were cultured for an additional 24 hours and then the drugs were applied. Cell proliferation was then monitored for 54 hours by a real-time cell analysis (RTCA) system (xCELLigence). The mean inter-assay coefficient of variation (CV)% and intra-assay CV% for RTCA were 1.2% and 1.8% respectively. The samples were studied in triplicate.
Quantitative real-time polymerase chain reaction and the ΔΔCT (comparative CT) method
After a 48-hour exposure to the drugs, the cell media were removed and the cells were washed with PBS at 37°C and then removed by trypsin-EDTA and transferred to fresh DMEM medium. After centrifuging for 5 minutes at 1000 g, the cells were removed, lysed, and total RNA was extracted using a High Pure PCR RNA Isolation Kit. Isolated RNA was stored at −80°C until use. Gene expression levels were determined by first converting mRNA into cDNA using the Transcriptor First Strand cDNA Synthesis Kit. The obtained cDNAs were used to measure the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), Grp78, Grp94, CHOP, MTJ-1, HKII, MDR-1, MRP-1, HO-1, and Par-4 genes by Quantitative RT-PCR (LightCycler 480 II). The housekeeping gene GAPDH was used as a reference gene. The mean inter-assay CV% and intra-assay CV% for real-time polymerase chain reaction (RT-PCR) analyses were 1.7% and 2% respectively. The efficiency of the RT-PCR was 1. The samples were studied in triplicate.
Gene expression levels were stated as 2–ΔΔCT by using the comparative CT method.
ΔΔCT=ΔCT(target) - ΔCT(calibrator)
ΔCT(target)=CT(target) - CT(reference)
ΔCT(calibrator)=CT(calibrator) - CT(reference)
The control group was used as the calibrator and GAPDH was used as the reference gene.
The forward, reverse primers and probes of the genes used in the study were designed by the Universal Probe Library (UPL) program. (Table 1)
Table 1.
Sequences of Polymerase Chain Reaction Primers
| Genes | Forward primer | Reverse primer | Probes |
|---|---|---|---|
| Grp78 | AGCCTGGCGACAAGAGTG | TCCTTGGGCAGTATTGGATT | ctccacct (probe 39, Roche) |
| Grp94 | CTGGAAATGAGGAACTAACAGTCA | TCTTCTCTGGTCATTCCTACACC | acctgctg (probe 62, Roche) |
| MTJ-1 | GCCAAGCAACTGAAGGATTC | TGTCGATTTGAGTTCGGAGA | ctgctccc (probe 32, Roche) |
| CHOP | CAGAGCTGGAACCTGAGGAG | TGGATCAGTCTGGAAAAGCA | catcacca (probe 9, Roche) |
| HKII | TCCCCTGCCACCAGACTA | TGGACTTGAATCCCTTGGTC | ctggtctc (probe 54, Roche) |
| MDR-1 | ACAGAAAGCGAAGCAGTGGT | ATGGTGGTCCGACCTTTTC | tggctctg (probe 21, Roche) |
| MRP-1 | CTTCGTGTCTTTGGCCTTGT | AGGCGTTTGAGGGAGACAC | catcctcc (probe 88, Roche) |
| HO-1 | GGGTGATAGAAGAGGCCAAGA | AGCTCCTGCAACTCCTCAAA | catccagc (probe 42, Roche) |
| Par-4 | GCAGATCGAGAAGAGGAAGC | CATCATCTTCGTACTCATCTAAGCA | gggagaag (probe 7, Roche) |
| GAPDH | AGCCACATCGCTCAGACAC | GCCCAATACGACCAAATCC | tggggaag (probe 60, Roche) |
Statistical analysis
The gene expression levels were expressed as n times the control by using the GENEX software (GenEx, http://genex.gene-quantification.info/) and the comparative CT method. When compared with the control group, a ≥1.2-fold change was accepted as significant.18
Results
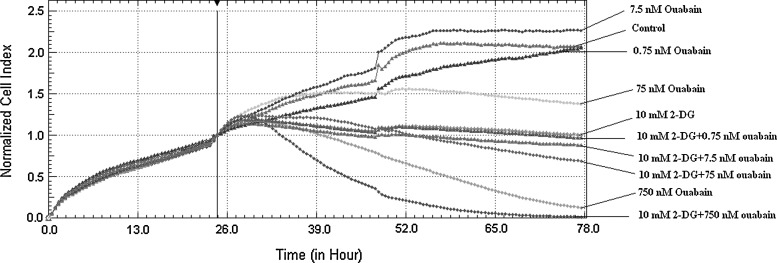
Ouabain groups, alone and combined with 2-DG, presented different growth curves when monitored by RTCA. Ouabain at concentrations of 75 nM and 750 nM showed cytotoxic effects, which started within the first 24 hours and increased by 48 hours. The cytotoxic effect was more apparent when ouabain was combined with 2-DG. No anti-proliferative was seen with 0.75 and 7.5 nM ouabain alone or combined with 2-DG (Fig. 1).
FIG. 1.
Monitoring growth curves of the cells with real-time cell analysis. The first 24 hours is a plating phase for the cells. The drugs were administered at the end of hour 24 and the cells were monitored for 54 hours. The medium was changed once in every 24 hours during the study. The samples were studied in triplicate.
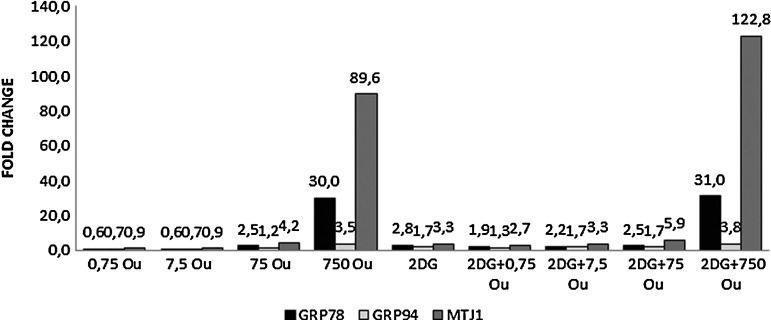
At the end of 48 hours, Grp78 gene expression in groups given the combined treatment was more pronounced than in the groups given ouabain alone, at all ouabain doses.
High ouabain doses (75 and 750 nM) induced a greater expression of the Grp78 gene, a component of the UPR. This effect was more apparent when 750 nM ouabain was combined with 2-DGand indicated a controlled aggravation of ER stress that was compatible with growth curve analysis (Fig. 2).
FIG. 2.
Expression levels of Grp78, Grp94, and MTJ1 genes after 48 hours. RT-PCR was used. GAPDH was used as the reference gene. The samples were studied in triplicate. The efficiency of the RT-PCR was 1.When compared with the control group, a ≥1.2-fold change was accepted as significant. 2-DG, 2- Deoxyglucose; Ou, Ouabain; Grp78, Glucose-regulated protein78; Grp94, Glucose-regulated protein94; MTJ1, Murine transmembrane protein1; RT-PCR, real-time polymerase chain reaction.
When ouabain was applied alone or in combination of 2-DG, Grp94 gene expression was increased, especially at high doses (750 nM; Fig. 2).
MTJ-1 gene expression was upregulated in a dose-dependent manner when ouabain was applied alone and its effect was more pronounced, particularly at the lower doses, when it was applied in combination with 2-DG. The MTJ-1 gene codes for a co-chaperone, which indicated a parallelism with Grp78 expression (Fig. 2).
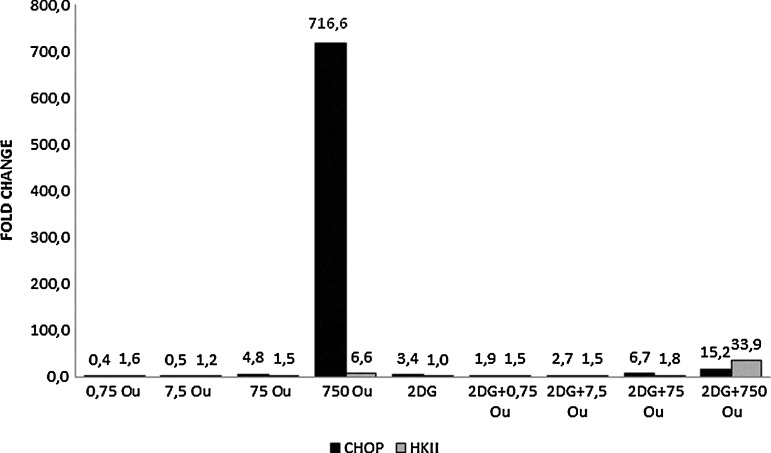
Ouabain alone at the lower doses (0.75 and 7.5 nM) resulted in downregulation of CHOP gene expression. In contrast, the higher doses (75 and 750 nM) caused a dose-dependent upregulation of CHOP. Ouabain supplied alone at the higher doses (750 nM in particular) showed cytotoxic effects, in agreement with the data obtained from the growth curve analysis. When ouabain was combined with 2-DG, it showed a dose-dependent upregulation of CHOP gene expression at all doses and a parallel increase in the cytotoxic effect (Fig. 3).
FIG. 3.
Expression levels of CHOP and HKII genes after 48 hours. RT-PCR was used. GAPDH was used as the reference gene.The samples were studied in triplicate. The efficiency of the RT-PCR was 1.When compared with the control group, a ≥1.2-fold change was accepted as significant. CHOP, C/EBP homologous protein; HKII, Hexokinase II.
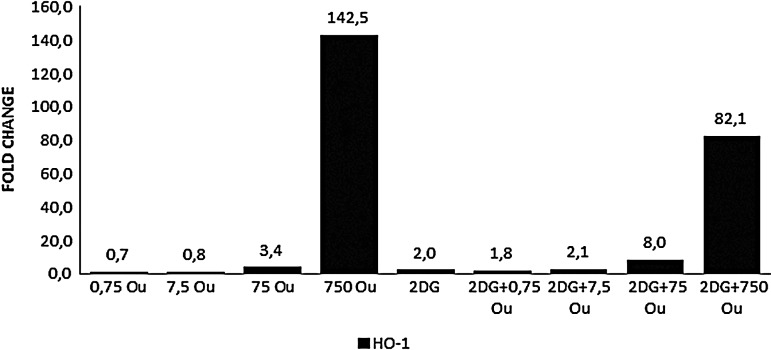
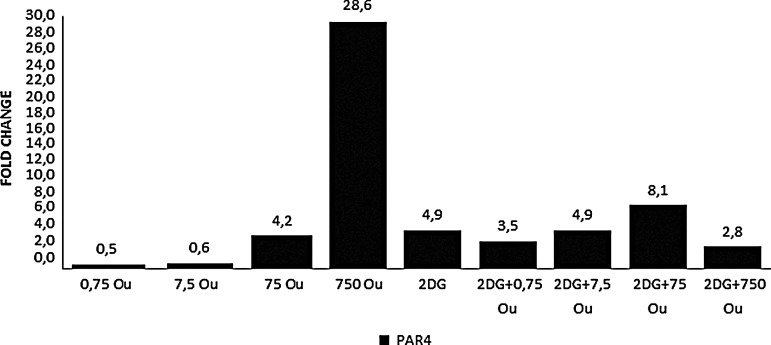
Ouabain alone and ouabain+2-DG combinations showed dose-dependent upregulation of HKII gene expression in HepG2 cells cultured for 48 hours, with the 750 nM treatments being much more effective (Fig. 3). Ouabain treatment at the higher doses (75 and 750 nM), alone and combined with 2-DG, increased HO-1 gene expression (Fig. 4). When ouabain was applied alone, a dose-dependent upregulation of Par-4 gene expression was also observed and was further promoted by the combined treatment with 2-DG. Growth-curve analysis correlated with the Par-4 gene expression analysis (Fig. 5).
FIG. 4.
Expression levels of HO-1 gene after 48 hours. RT-PCR was used. GAPDH was used as the reference gene. The samples were studied in triplicate. The efficiency of the RT-PCR was 1.When compared with the control group, a ≥1.2-fold change was accepted as significant. HO-1, Heme oxygenase-I.
FIG. 5.
Expression levels of Par-4 gene after 48 hours. RT-PCR was used. GAPDH was used as the reference gene. The samples were studied in triplicate. The efficiency of the RT-PCR was 1.When compared with the control group, a ≥1.2-fold change was accepted as significant. PAR4, Prostate apoptosis response protein-4.
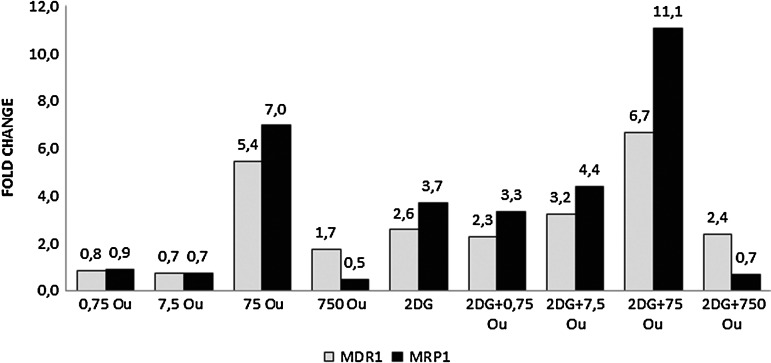
The only downregulated genes were MDR-1 and MRP-1, which showed decreased expressions in response to 750 nM ouabain, either with or without 2-DG. This effect was consistent with the marked cytotoxicity at that dose seen in the RTCA study (Fig. 6).
FIG. 6.
Expression levels of MDR1 and MRP1 genes after 48 hours. A dose-dependent increase was observed for all ouabain doses combined with 2-DG. RT-PCR was used. GAPDH was used as the reference gene. The samples were studied in triplicate. The efficiency of the RT-PCR was 1. When compared with the control group, a ≥1.2-fold change was accepted as significant. MDR1, Multidrug resistant gene1; MRP-1, Multidrug resistance-associated protein1.
Discussion
Specific UPR signal pathways, including adaptive routes, are activated in cells in response to ER stress.19 The CHOP, Grp78, Grp94, XBP-1, MTJ-1, and HO-1 genes all show the activation level of UPR and the cellular response can be specified by the determination of expression of these genes.
In poorly vascularized solid tumor cells, glucose insufficiency occurs in addition to the continuous or intermittent hypoxia.20 This glucose deficiency can be mimicked under experimental conditions by treatment of cells with 2-DG. Studies performed with different cell models have shown that 2-DG also activates the UPR signal pathway. Breast cancer (SKBR3) and pancreas tumor (1420) cells have been shown to respond to treatment with 4 mmol/L 2-DG by upregulation of the UPR genes, Grp78, Grp94, and CHOP, under normoxic conditions.21 In our study, 2-DG was used primarily to provide hypoglycemic conditions similar to those found in the center of solid tumors and secondarily as a UPR stimulating agent; its dose was specified based on existing literature.22 When supplied at 10 mM, 2-DG induced a 2.8-fold increase in Grp78 expression when compared with the control group and this increase was taken as evidence of UPR pathway induction (Fig. 2).
Hypoxia and hypoglycemia are more commonly observed in cells in the center of tumors when compared with cells at the periphery, and the cells in the center reproduce more slowly (i.e., they have longer doubling times). We used 2-DG here to mimic hypoxia and hypoglycemia and we found evidence to support previous literature findings, as a decrease in cell proliferation from the control group rate occurred only in the group administered 2-DG (Fig. 1).
Administration of ouabain, either alone or combined with 2-DG, resulted in a decrease in cell viability and this effect was exacerbated by combination with 2-DG. The decrease in cell viability was most significant at 75 nm and 750 nm ouabain, both alone and combined with 2-DG. These results indicate that ouabain kills HepG2 cells dose dependently and that its impact is more significant when combined with the effects of 2-DG (Fig. 1).
Resistance to drugs is a major difficulty that limits the success of chemotherapy in treating neoplastic diseases.23 The MRP-1 and MDR-1 genes are excessively expressed in hepatic cell carcinoma (HCC)24 and in human HCC, and MDR-1 gene expression develops resistance against doxorubicin.25 In our study, similar effects were seen for the lower concentrations of ouabain (0.75 and 7.5 nM), where parallel increases in expression of MDR-1 and MRP-1 were seen. This also shows that resistance is developed against both ouabain and 2-DG with time. (Figs. 1 and 6).
The Na+/K+-ATPase pump acts as a signal transmitting receptor for ouabain.26,27 Ouabain can show both proliferative and apoptotic effect depending on the dosage28 and these effects are related to other factors such as the cell type, dosage, exposure time of the cell, and the pump isoforms.29 Ouabain suppresses NF-kB activation, induces apoptosis, and increases the proapoptotic effects of cytotoxic drugs in glioma cells.30 In our study, ouabain also showed an apoptotic effect in HepG2 cells. Further studies are required to uncover the mechanism underlying this effect. (Fig. 1).
The Grp78 gene increases tumor proliferation, promotes tumor metastasis, and is important in the development of drug resistance.31 Increased Grp78 expression is shown in various cancer cell lines including the lung, breast, melanoma, prostate, hepatocellular, and gastric cancers.12,32 Similarly, Grp94 overexpression leads to cancer progression and drug resistance.33 In our study, in all the groups except the 0.75 and 7.5 nm ouabain groups, a significant increase was seen in Grp78, Grp94, and MTJ1 expression levels when compared with the control group. The groups treated with 750 nm ouabain, alone or in combination with 2-DG, showed especially high Grp78 and MTJ1 expression when compared with the other groups. The Grp78 gene and its co-chaperone MTJ1 gene typically are studied in parallel with each other. These results show that Grp78 plays a role in the cell apoptosis (Fig. 2).
In a study performed by Huang et al.6 on independent androgen PC-3 cells, a low dose of ouabain (<10 nM) was found to decrease mitochondrial activity, increase Par-4 expression, and increase the sensitivity of the cells to cytotoxicity. High doses of ouabain (>300 nM) caused loss of the mitochondrial membrane potential and decreased mitochondrial function, increased the generation of reactive oxygen species (ROS), and stimulated the Par-4 gene expression and apoptosis. We have seen that ouabain, alone or combined with 2-DG, caused a dose-dependent increase in Par-4 expression and cell death (Fig. 5).
Hepatoma cells transfected into the rats (AS-30D) show increased HKII expression and, in hypoxic situations, HKII expression is stimulated by HIF-1.34 In transformed cells, the glycolytic path is very active because of the increased HKII expression and more than 70% of HKII in these cells is related to mitochondria.16 In our study, in all the groups except the 0.75 and 7.5 nM ouabain groups, a significant dose-dependent increase was seen in the HK-II expression (Fig. 3). The upregulation of hexokinase II in the HepG2 cell increases its binding frequency to voltage-dependent anion channels (VDACs) and detrimentally affects the permeability of the mitochondrial outer membrane. Blocking the VDAC-HKII interaction can increase OXPHOS function.35 VDAC function is further debilitated by strong binding to HKII. Energy production is decreased and this is exacerbated by the preferential access of ATP by mitochondrially bound HKII. Therefore, the dependence on glycolysis is perpetuated.36
In the present study, after 12 hours of incubation in the medium without glucose, the expression of HO-1 mRNA in HepG2 cells increased 25 times when compared with the control cells. Glucose starvation therefore stimulated the HO-1 gene expression through a different path than the UPR pathway. When glutamine is removed from the medium, the HO-1 increase promoted by glucose starvation disappears, indicating that ROS formation is important for the HO-1 increase. The increase in HO-1 mRNA protects the cells from glucose starvation and it is important in the formation of adaptive cell responses.37 In all of the groups except the 0.75 and 7.5 nM ouabain groups, and especially in the 750 nM ouabain and 750 nM ouabain combined with 2-DG groups, HO-1 expression was significantly increased (Fig. 4). This indicates a substantial stress level in the cell, as this is a survival reaction and a sign of UPR induction.
The role of CHOP changes from cell to cell. Whereas CHOP expression promotes demyelinization in Schwann cells, it is antiapoptotic in oligodendrocytes.38 In all of the groups except the 0.75 and 7.5 nM ouabain groups, CHOP expression showed a dose-dependent increase (Fig. 3). An analogy is seen between the cell death viewed by RTCA and CHOP expression (Figs. 1 and 3). These results show that cell death increases in parallel with the CHOP expression increases in HepG2 cells.
Studies have shown that digitalis drugs can be useful as an anticancer drug. Stenkvist et al.39 followed up 175 breast cancer patients, 32 who were receiving digitalis therapy, for 22 years, and reported a death ratio of 6% in the digitalis group and 34% in the nondigitalis group. When compared with the general population, a decreased risk was determined in the development of leukemia/lymphoma and renal-urinary system cancers due to digitoxin because of its use in cardiac diseases.39,40
In our study, we showed that UPR gene expressions in HepG2 cell culture changed depending on the ouabain dose. Changes were seen in the gene expressions that are the indicators of UPR. When the central role of UPR is considered in the adjustment of cell life and death, further research is required to understand the underlying mechanisms better. This can also be useful for the development of UPR-targeted drugs in solid tumors.
Acknowledgments
This study was supported by grants from the Ondokuz Mayis University Research Fund, project number PYO.TIP. 1901 09 001.
Disclosure Statement
There is no conflict of interest between any authors. All authors agree on this topic.
References
- 1.Hauptman PJ. Kelly RA. Digitalis. Circulation. 1999;99:1265. doi: 10.1161/01.cir.99.9.1265. [DOI] [PubMed] [Google Scholar]
- 2.Nesher M. Shpolansky U. Rosen H, et al. The digitalis-like steroid hormones: New mechanisms of action and biological significance. Life Sci. 2007;80:2093. doi: 10.1016/j.lfs.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M. Adams KF., Jr. Colucci WS. Digoxin in the management of cardiovascular disorders. Circulation. 2004;109:2959. doi: 10.1161/01.CIR.0000132482.95686.87. [DOI] [PubMed] [Google Scholar]
- 4.Xu ZW, et al. Targeting the Na(+)/K(+)-ATPase alpha1 subunit of hepatoma HepG2 cell line to induce apoptosis and cell cycle arresting. Biol Pharm Bull. 2010;33:743. doi: 10.1248/bpb.33.743. [DOI] [PubMed] [Google Scholar]
- 5.Harwood S. Yaqoob MM. Ouabain-induced cell signaling. Front Biosci. 2005;10:2011. doi: 10.2741/1676. [DOI] [PubMed] [Google Scholar]
- 6.Huang YT. Chueh SC. Teng CM, et al. Investigation of ouabain-induced anticancer effect in human androgen-independent prostate cancer PC-3 cells. Biochem Pharmacol. 2004;67:727. doi: 10.1016/j.bcp.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Kulikov A. Eva A. Kirch U, et al. Ouabain activates signaling pathways associated with cell death in human neuroblastoma. Biochim Biophys Acta. 2007;1768:1691. doi: 10.1016/j.bbamem.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Larre I, et al. Ouabain modulates epithelial cell tight junction. Proc Natl Acad Sci U S A. 2010;107:11387. doi: 10.1073/pnas.1000500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheiner-Bobis G. Schoner W. A fresh facet for ouabain action. Nat Med. 2001;7:1288. doi: 10.1038/nm1201-1288. [DOI] [PubMed] [Google Scholar]
- 10.Simpson CD, et al. Inhibition of the sodium potassium adenosine triphosphatase pump sensitizes cancer cells to anoikis and prevents distant tumor formation. Cancer Res. 2009;69:2739. doi: 10.1158/0008-5472.CAN-08-2530. [DOI] [PubMed] [Google Scholar]
- 11.Oyadomari S. Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, et al. Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ. 2010;17:488. doi: 10.1038/cdd.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong ML. Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39:75. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- 14.Kang HT. Hwang ES. 2-Deoxyglucose: An anticancer and antiviral therapeutic, but not any more a low glucose mimetic. Life Sci. 2006;78:1392. doi: 10.1016/j.lfs.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Saito S, et al. Chemical genomics identifies the unfolded protein response as a target for selective cancer cell killing during glucose deprivation. Cancer Res. 2009;69:4225. doi: 10.1158/0008-5472.CAN-08-2689. [DOI] [PubMed] [Google Scholar]
- 16.Pelicano H. Martin DS. Xu RH, et al. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 17.Aft RL. Zhang FW. Gius D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: Mechanism of cell death. Brit J Cancer. 2002;87:805. doi: 10.1038/sj.bjc.6600547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasdemir C, et al. Transcriptional profiling of septal wall of the right ventricular outflow tract in patients with idiopathic ventricular arrhythmias. Pacing Clin Electrophysiol. 2010;33:159. doi: 10.1111/j.1540-8159.2009.02606.x. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y. Shimizu Y. Mann MJ, et al. Plasma cell differentiation initiates a limited ER stress response by specifically suppressing the PERK-dependent branch of the unfolded protein response. Cell Stress Chaperones. 2010;15:281. doi: 10.1007/s12192-009-0142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JG, et al. MDR1 gene expression: İts effect on drug resistance to doxorubicin in human hepatocellular carcinoma cell lines. J Natl Cancer Inst. 1994;86:700. doi: 10.1093/jnci/86.9.700. [DOI] [PubMed] [Google Scholar]
- 21.Lai E. Teodoro T. Volchuk A. Endoplasmic reticulum stress: Signaling the unfolded protein response. Physiology (Bethesda) 2007;22:193. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hamamura RS, et al. Induction of heme oxygenase-1 by cobalt protoporphyrin enhances the antitumour effect of bortezomib in adult T-cell leukaemia cells. Brit J Cancer. 2007;97:1099. doi: 10.1038/sj.bjc.6604003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouillard F. Tondelier D. Edelman A, et al. Drug resistance induced by ouabain via the stimulation of MDR1 gene expression in human carcinomatous pulmonary cells. Cancer Res. 2001;61:1693. [PubMed] [Google Scholar]
- 24.Bonin S. Pascolo L. Croce LS, et al. Gene expression of ABC proteins in hepatocellular carcinoma, perineoplastic tissue, and liver diseases. Mol Med. 2002;8:316. [PMC free article] [PubMed] [Google Scholar]
- 25.Pastorino JG. Hoek JB. Hexokinase II: The integration of energy metabolism and control of apoptosis. Curr Med Chem. 2003;10:1535. doi: 10.2174/0929867033457269. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, et al. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem. 2004;279:17250. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, et al. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol. 2003;284:C1550. doi: 10.1152/ajpcell.00555.2002. [DOI] [PubMed] [Google Scholar]
- 28.Chueh SC. Guh JH. Chen J, et al. Dual effects of ouabain on the regulation of proliferation and apoptosis in human prostatic smooth muscle cells. J Urol. 2001;166:347. [PubMed] [Google Scholar]
- 29.Ledoux S. Yang R. Friedlander G, et al. Glucose depletion enhances P-glycoprotein expression in hepatoma cells: Role of endoplasmic reticulum stress response. Cancer Res. 2003;63:7284. [PubMed] [Google Scholar]
- 30.Younes M. Lechago LV. Somoano JR, et al. Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res. 1996;56:1164. [PubMed] [Google Scholar]
- 31.Lee AS. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem Sci. 2001;26:504. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 32.Park HR, et al. Effect on tumor cells of blocking survival response to glucose deprivation. J Natl Cancer Inst. 2004;96:1300. doi: 10.1093/jnci/djh243. [DOI] [PubMed] [Google Scholar]
- 33.Gaddameedhi S. Chatterjee S. Association between the unfolded protein response, induced by 2-deoxyglucose, and hypersensitivity to cisplatin: A mechanistic study employing molecular genomics. J Cancer Res Ther. 2009;5:61. doi: 10.4103/0973-1482.55146. [DOI] [PubMed] [Google Scholar]
- 34.McConkey DJ. Lin Y. Nutt LK, et al. Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer Res. 2000;60:3807. [PubMed] [Google Scholar]
- 35.Campbell AM. Chan SHP. The voltage dependent anion channel affects mitochondrial cholesterol distribution and function. Arch Biochem Biophys. 2007;466:203. doi: 10.1016/j.abb.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Campbell AM. Chan SHP. Mitochondrial membrane cholesterol, the voltage dependent anion channel (VDAC), and the Warburg effect. J Bioenerg Biomembr. 2008;40:193. doi: 10.1007/s10863-008-9138-x. [DOI] [PubMed] [Google Scholar]
- 37.Chang SH. Barbosa-Tessmann I. Chen C, et al. Glucose deprivation induces heme oxygenase-1 gene expression by a pathway independent of the unfolded protein response. J Biol Chem. 2002;277:1933. doi: 10.1074/jbc.M108921200. [DOI] [PubMed] [Google Scholar]
- 38.Gozzelino R. Jeney V. Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 39.Stenkvist B, et al. Analysis of reproducibility of subjective grading systems for breast carcinoma. J Clin Pathol. 1979;32:979. doi: 10.1136/jcp.32.10.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JQ, et al. Sodium/potassium ATPase (Na+, K+-ATPase) and ouabain/related cardiac glycosides: A new paradigm for development of anti-breast cancer drugs? Breast Cancer Res Treat. 2006;96:1. doi: 10.1007/s10549-005-9053-3. [DOI] [PubMed] [Google Scholar]