Abstract
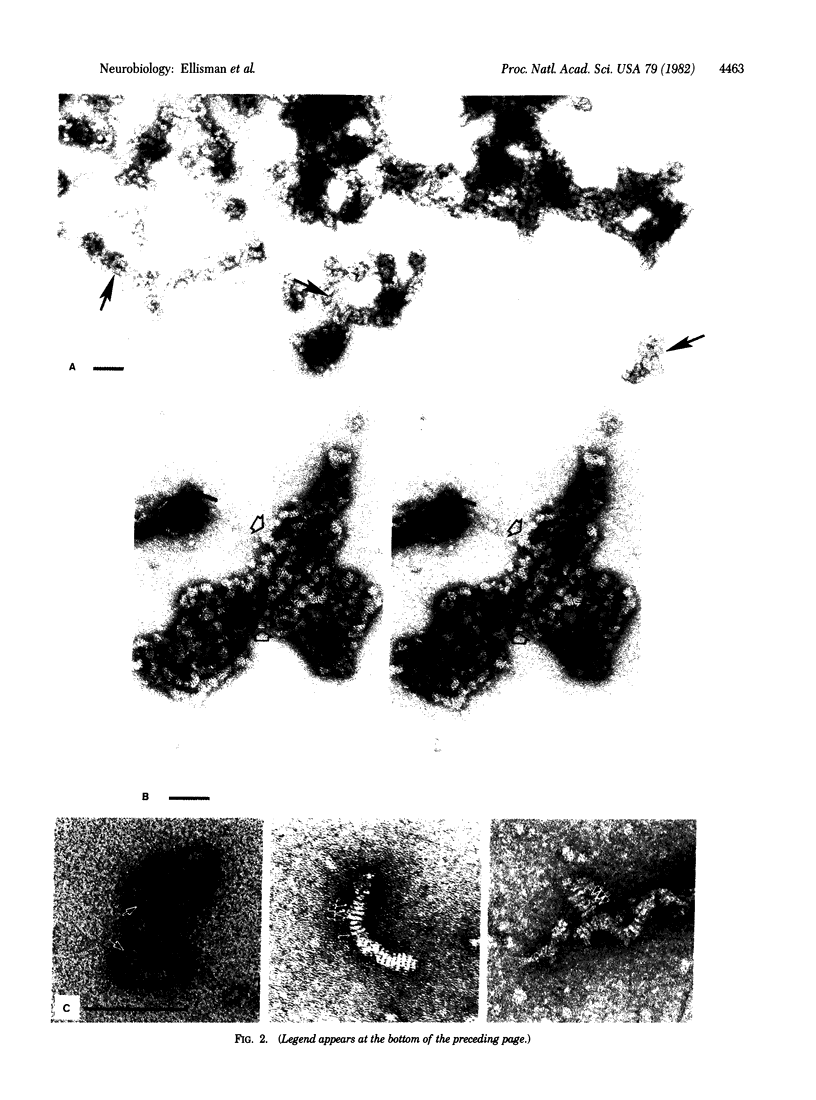
Preparations of highly purified tetrodotoxin-binding protein (sodium channel) from the electric organ of the eel Electrophorus electricus were examined in negatively stained preparations. Structures observed in preparations exhibiting the highest tetrodotoxin binding tended to aggregate into ordered clusters with a unique ribbon-like conformation. The individual particles of these aggregates are elongated or rod-shaped, approximately 40 A wide and 170 A long. Stereoscopic imaging of the three-dimensional aspects of the structures revealed that the rod-like image is not an edge view of a flattened disc but represents a cylindrical structure. Individual rods in nonclustered forms were also observed but with greater frequency in preparations with lower specific activity. The dimensions of the particles suggest that they represent a protein core formed by perhaps one copy of the large glycopeptide previously identified as being part of the sodium channel. The structure of the sodium channel component visualized by negative staining is discussed in the context of the excitable properties it contributes to biological membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Levinson S. R., Brabson J. S., Raftery M. A. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2606–2610. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Ionic pores, gates, and gating currents. Q Rev Biophys. 1974 May;7(2):179–210. doi: 10.1017/s0033583500001402. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Murphy L. E. Estimate of the molecular weight of the sarcolemmal sodium channel using H2O-D2O centrifugation. J Neurochem. 1981 Jun;36(6):2097–2100. doi: 10.1111/j.1471-4159.1981.tb10842.x. [DOI] [PubMed] [Google Scholar]
- Beneski D. A., Catterall W. A. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc Natl Acad Sci U S A. 1980 Jan;77(1):639–643. doi: 10.1073/pnas.77.1.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer T. I., Raftery M. A. Partial characterization of a tetrodotoxin-binding component from nerve membrane. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3634–3637. doi: 10.1073/pnas.69.12.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Darszon A., Vandenberg C. A., Schönfeld M., Ellisman M. H., Spitzer N. C., Montal M. Reassembly of protein-lipid complexes into large bilayer vesicles: perspectives for membrane reconstitution. Proc Natl Acad Sci U S A. 1980 Jan;77(1):239–243. doi: 10.1073/pnas.77.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M. H. Molecular specializations of the axon membrane at nodes of Ranvier are not dependent upon myelination. J Neurocytol. 1979 Dec;8(6):719–735. doi: 10.1007/BF01206672. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. Purification of the saxitoxin receptor of the sodium channel from rat brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4620–4624. doi: 10.1073/pnas.78.7.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. An essential ionized acid group in sodium channels. Fed Proc. 1975 Apr;34(5):1318–1321. [PubMed] [Google Scholar]
- Kao C. Y., Nishiyama A. Actions of saxitoxin on peripheral neuromuscular systems. J Physiol. 1965 Sep;180(1):50–66. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson S. R., Curatalo C. J., Reed J., Raftery M. A. A rapid and precise assay for tetrodotoxin binding to detergent extracts of excitable tissues. Anal Biochem. 1979 Oct 15;99(1):72–84. doi: 10.1016/0003-2697(79)90045-9. [DOI] [PubMed] [Google Scholar]
- Levinson S. R., Ellory J. C. Molecular size of the tetrodotoxin binding site estimated by irradiation inactivation. Nat New Biol. 1973 Sep 26;245(143):122–123. doi: 10.1038/newbio245122a0. [DOI] [PubMed] [Google Scholar]
- Levinson S. R. The purity of tritiated tetrodotoxin as determined by bioassay. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):337–348. doi: 10.1098/rstb.1975.0013. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Gullick W., Conti-Tronconi B., Ellisman M. Proteolytic nicking of the acetylcholine receptor. Biochemistry. 1980 Oct 14;19(21):4791–4795. doi: 10.1021/bi00562a012. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axons of the frog brain. J Neurocytol. 1976 Dec;5(6):731–745. doi: 10.1007/BF01181584. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]