Abstract
The root of Angelica gigas (Korean angelica) is traditionally used to treat women's ailments that are caused by an impairment of menstrual blood flow and cycle irregularities. This study evaluated the effect particle size of Korean angelica powder on its efficacy for treating estrogen-related symptoms of menopause. Initially, Korean angelica roots were pulverized into ultrafine powder, and orally administered to the rats at a concentration of 500 mg/kg body weight for 8 weeks. The effects of Korean angelica powder particle size on extraction yield, contents of bioactive compounds (decursin and decursinol angelate), levels of serum ovarian hormones (estradiol and progesterone), reproductive hormones (luteinizing hormone and follicle-stimulating hormone), and experimental osteoporosis parameters (mineral density, strength, and histological features) were determined. A significant increase (fivefold) in the contents of decursin and decursinol angelate in the extract of the ultrafine Korean angelica powder was observed compared to coarse Korean angelica powder. Rats were divided into sham-operated or ovariectomized (OVX) groups that were fed coarse (CRS) or ultrafine (UF) ground Korean angelica root. The serum levels of estradiol in the OVX_UF group were 19.2% and 54.1% higher than that of OVX_CRS group. Serum bone-alkaline phosphatase/total-alkaline phosphatase index in the OVX_UF group was half that of the OVX_CRS group. In addition, less trabecular bone loss and thick cortical areas were observed in rats administered ultrafine powder. Therefore, ultrafine grinding may enhance the bioactivity of herbal medicines and be especially useful when their extracted forms lose bioactivity during processing, storage, and oral intake.
Key Words: menopausal symptoms, osteoporosis, ovarian hormone, particle size effect, ultrafine Korean angelica powder
Introduction
During menopause, the loss of natural ovarian function often leads to the development of endocrine disorders, which ultimately lead to serious health consequences in women. During the menopausal transition, the synthesis of ovarian hormones, such as estrogen and progesterone progressively decrease, while the secretion of gonadotropic hormones, including follicle-stimulating hormone (FSH) and luteinizing hormone (LH) increase; consequently, menopausal symptoms prevail.1 The primary symptoms of menopause are hot flashes, sleeping disorder, joint pain, and fatigue.2,3 In addition, disorders, such as osteoporosis, cardiovascular disease, and emotional anxiety are commonly observed.4,5 Osteoporosis, is of particular concern in women due to decreased estrogen levels, which leads to an imbalance between bone resorption and bone synthesis, followed by bone loss and fractures.6 To reduce menopausal symptoms, hormone replacement therapy is generally prescribed with estrogen alone or in combination with progesterone. However, these hormones are often accompanied by side effects, such as endometrial hyperplasia, uterine bleeding, and breast cancer.7,8 Therefore, herbal medicines are becoming increasingly popular as they tend to mitigate the symptoms with fewer side effects.
Angelica gigas (Umbelliferae) root has been traditionally used in Korean folk medicine as a tonic and for treatment of anemia and other common diseases.9 There are also reports of antibacterial and antiamnestic effects, acetylcholinesterase inhibition, depression of cardiac contraction, activation of protein kinase C, and antitumor activity against sarcoma cancer cells.10 Based on its therapeutic potential, efforts have been made to isolate the active compounds from this plant, leading to the isolation of several coumarin compounds.11 It has been reported that coumarins, such as decursin and decursinol angelate are the major active compounds of the A. gigas (Korean angelica) root.12 Recently, it has also been reported that decursinol and decursin exhibit significant neuroprotecitve activity against glutamate-induced neurotoxicity in primary cultures of rat cortical cells and exhibit potent novel antiandrogen receptor signaling activities in vitro.13 In addition, several studies have addressed the effects of decursin and its derivatives on the prevention and mitigation of chronic health disorders.14 It is well known that coumarin derivatives have estrogenic activity, since they bind to intracellular receptors for estradiol and progesterone.15,16
Traditionally, hot water extraction is used to isolate bioactive components from dried Korean angelica roots. However, the method is not considered effective, since decursin and its derivatives are not readily soluble in an aqueous environment. In addition, the bioactivity of decursin and its derivatives can be lost upon heating. Grinding different parts of medicinal plants is a considerable challenge, since the process should both preserve and increase the bioavailability of aqueous soluble and insoluble bioactives of the medicinal plants, but it also retains subsidiary nutrients. Thus, the powdered Korean angelica root is considered to possess innate decursin and its derivatives without any severe activity loss. Recently, various ultrafine grinding processes, such as cryogenic grinding and air classifying grinding, which can reduce the particle size below a few micrometers, have been developed.17,18 It has been reported that ultrafine particles exhibit higher intracellular uptake compared to their macrosized counterparts.10,19 The increased total surface area provides more chance to release bioactives in the gastrointestinal tract and increases absorption through the intestinal wall. However, comparative studies to investigate the effect of particle size on the in vivo bioactivity of medicinal plant powders have rarely been carried out; especially, no study has reported the effect of the particle size of Korean angelica root powders on the mitigation of chronic disorders.
Therefore, the effect of particle size of Korean angelica powder needs to be evaluated. Especially, changes in ovarian hormone levels and osteoporosis risk in vivo are of interest when different sized Korean angelica powders are orally administrated to animals. An ovariectomized experimental menopause-induced rat is a good animal model to investigate the size effect of Korean angelica powder on the changes in ovarian hormones and bone characteristics. In the present study, estradiol, progesterone, LH, and FSH in blood serum and the bone characteristics, such as serum total-alkaline phosphatase (TALP), serum bone-alkaline phosphatase (BALP), bone mineral density (BMD), bone stiffness, and bone histology are evaluated in the ovariectomized rats fed with different-sized Korean angelica powders.
The goals of this study were to prepare ultrafine Korean angelica powder, and to determine the effect of different-sized Korean angelica powders on the change in ovarian hormones, such as estrogen, progesterone, LH, and FSH and experimental osteoporosis parameters, such as serum TALP, serum BALP, BMD, bone stiffness, and bone histology in the ovariectomized rats.
Materials and Methods
Preparation of different-sized A. gigas root powder
Coarse milling
A pin crusher (JIC-P10-2; Myungsung Machine, Seoul, Korea) equipped with a 30-mesh sieve was used for coarse milling. Dried A. gigas Nakai roots harvested from the Jin-bu area (Korea) were milled at 112 g to achieve the required particle size. The milled powder was fractionated using a sieve shaker (CG-213, Ro-Top, Chunggye Industrial Mfg. Co., Seoul, Korea) equipped with a series of sieve standard sieves (Φ 20 cm). The powders were passed through the sieves with 300-μm mesh size, and rejected particles were ground again with the pin crusher. This powder fraction process using the sieve shaker allows the preparation of reproducible samples with similar particle size distribution. The fractionated powders were stored in a desiccator at 25°C before further ultrafine milling.
Ultrafine milling
An ultrafine air mill (Turbo Mill, HKP-05; Korea Energy Technology, Seoul, Korea) was used to obtain ultrafine powder. The coarse powders prepared previously were pulverized into ultrafine powder particles with sizes in the range of 0.1–50 μm in the milling chamber with a cooling jacket (−10°C to 25°C). Feeding rate and circumferential velocity of the impeller in the grinding zone were fixed at 3 kg/h and 100 m/s, respectively. Simultaneously, a centrifugal air-classification system classified the powders depending on the particle size. In this system, when the particle size of powders is reduced enough to pass through a classification hole, the particles are transported to an outlet; otherwise the particles remain in the grinding zone. The ultrafine Korean angelica powder obtained was stored in a desiccator until use.
High-performance liquid chromatography analysis of extracts in different-sized A. gigas root powder
One gram of the different-sized Korean angelica root powders was mixed with 20 mL of distilled water. Subsequently, the mixtures were placed in a water bath at 95°C for 3 h under stirring to obtain hot water extracts of the Korean angelica powders. The mixtures were filtered to separate the supernatants and the sediments. The supernatants were dried in a vacuum-evaporator (Korean angelica extracts).
High-performance liquid chromatography (HPLC; LC-20AT, Shimadzu Scientific Instruments, Tokyo, Japan) was used for quantitative analysis of the Korean angelica extracts; the operating conditions are shown in Table 1. The solutions at 5, 10, 20, and 40 μg/mL of decursin and decursinol angelate were prepared to plot their calibration curves. Each solution was injected and the subsequent chromatogram was recorded; the peak areas of decursin and decursinol angelate were converted into corresponding concentrations.
Table 1.
Instrumental and Operating Conditions for Decursin and Decursinol Angelate Analysis by High-Performance Liquid Chromatography
| Instrument | Shimadzu LC-20AT HPLC system |
| Column | Waters Atlantis dC18 (250 mm×4.6 mm, 5 μm) |
| Detector | UV-VIS spectroscope (329 nm) |
| Solvent A | 0.1% acetic acid+H2O |
| Solvent B | 0.1% acetic acid+acetonitrile |
| Solvent C | Absolute ethanol |
| Flow rate | 1 mL/min |
| Injection volume | 10 μL |
| Gradient elution system | % A | % B | % C |
|---|---|---|---|
| Initial | 53 | 30 | 17 |
| 40 m | 60 | 20 | 20 |
| 50 m | 53 | 30 | 17 |
| 60 m | 53 | 30 | 17 |
HPLC, high-performance liquid chromatography; UV-VIS, ultraviolet–visible.
Particle size measurement
Particle sizes of both coarse and ultrafine Korean angelica powders were determined by a particle size analyzer (Mastersizer 2000; Malvern Instruments Ltd., Worcestershire, United Kingdom), which adopted the laser diffraction technique for the size measurement.
For the size measurement, each sample suspension was prepared by mixing the powder with 10 mL of deionized water at a ratio of 1:400 (w/v) and stirring on a magnetic stirrer at 25°C for 30 min. A sample of suspension was placed in a size measurement cell and its particles size was measured at 25°C with the fixed scattering angle at 165°. All samples were measured in triplicate and the mean particle size was calculated on the basis of a volume median diameter.
The polydispersibility representing the range of the particle size distribution of two different-sized Korean angelica powders can be indicated by the span value.20 The span provides information on the degree of homogeneity and reliability of particle distribution. For the calculation of the span, Equation (1) was used:
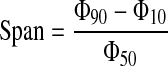 |
(1) |
where Φ represents particle diameter, and subscripts represent percent of cumulative particle size.
Scanning electron microscopy
Micrographic determination of particle size and morphological features of both coarse and ultrafine Korean angelica powders was performed using a field-emission scanning electron microscope (SEM; S-4300; Hitachi, Tokyo, Japan). For the SEM analysis, the equipment was operated at an accelerating voltage of 15.0 kV for the different-sized Korean angelica powders.
In vivo study
Animals and diets
Twenty female Sprague-Dawley rats aged 5 weeks were purchased from DooYeol Biotech (Seoul, Korea). The animals were provided chow diet ad libitum for a week. Each animal was placed into a separate stainless steel cage, and the temperature and humidity were controlled at 20°C–25°C and 30–35%, respectively, with a 12-h light–12-h dark cycle. The animals were fed with 20 g of AIN-93G diet as listed in Table 2 and allowed free access to water. After acclimatization, 15 rats were ovariectomized to induce menopause and 5 rats were sham-operated. The animals were randomly divided into four groups as follows: (1) Sham-operated and non–Korean angelica powder–administered group (SHAM), (2) OVX and non–Korean angelica powder–administered group (OVX), (3) OVX and coarse Korean angelica powder–administered group (OVX_CRS), and (4) OVX and ultrafine Korean angelica powder–administered group (OVX_UF). After 5 weeks, 2 mL of Korean angelica suspensions containing 0.34 g Korean angelica powder in 0.5% carboxyl methyl cellulose solution (Happy Call Co. Ltd., Gyeonggi-do, Korea) were administered orally every day for 8 weeks.
Table 2.
Formulation of AIN-93G Purified Diet for Growing Rodents
| Ingredient | Proportion (g/kg) | Food Energy (kcal/kg) |
|---|---|---|
| Corn starch | 397 | 1590 |
| Casein, 30 mesh | 200 | 800 |
| Maltodextrin 10 | 132 | 528 |
| Sucrose | 100 | 400 |
| Soybean oil | 70 | 630 |
| Cellulose | 50 | 0 |
| Mineral mix S100022Ga | 35 | 0 |
| Vitamin mix V10037b | 10 | 40 |
| L-Cystine | 3 | 12 |
| Choline bitartrate | 2.5 | 0 |
| t-Butylhydroquinone | 0.014 | 0 |
Mineral mix S100022G:
Vitamin mix V10037:
| Ingredient | Proportion (g/kg) | Ingredient | Proportion (g/kg) |
|---|---|---|---|
| Calcium carbonate | 357 | Sucrose | 967 |
| Sucrose (finely powdered) | 221 | Vitamin E acetate (500 IU/g) | 15 |
| Potassium phosphate | 196 | Vitamin K1/dextrose mix (10 mg/g) | 7.5 |
| Sodium chloride | 74 | Niacin | 3 |
| Potassium citrate | 70 | Vitamin B12 (0.1%) | 2.5 |
| Potassium sulfate | 46 | Calcium pantothenate | 1.6 |
| Magnesium oxide | 24 | Vitamin A palmitate (500,000 IU/g) | 0.8 |
| Ferric citrate | 6 | Pyridoxine hydrochloride | 0.7 |
| Smithsonite | 1.7 | Thiamine hydrochloride | 0.6 |
| Sodium metasilicate | 1.45 | Riboflavin | 0.6 |
| Manganous carbonate | 0.63 | Vitamin D3 (400,000 IU/g) | 0.25 |
| Copper carbonate | 0.3 | Folic acid | 0.2 |
| Chromium potassium sulfate | 0.28 | Biotin | 0.02 |
| Boric acid | 0.08 | ||
| Sodium flouride | 0.06 | ||
| Nickel carbonate | 0.03 | ||
| Lithium chloride | 0.02 | ||
| Potassium iodate | 0.01 | ||
| Sodium selenate | 0.01 | ||
| Ammonium paramolybdate | 0.008 | ||
| Ammonium vanadate | 0.007 |
The animal study procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Sejong University (permit# SJ-20100601001).
Sampling
At the end of the experimental period, all animals were fasted for 12 h and 1 mL of blood from each animal was collected via orbital sinus from the eye. The collected blood samples were centrifuged at 1000 g (High-Performance Personal Table Top Centrifuges CF-10; Daihan Scientific, Seoul, Korea) for 15 min to separate serum from the blood, and all of them were preserved in a deep freezer (−40°C) until analysis. All animals were sacrificed by ether asphyxiation. Left tibias and femurs were dissected cautiously for bone analysis. The amputated tibias were fixed in 10% buffered formalin (Sigma-Aldrich, Steinheim, Germany) and the femurs were frozen until analysis.
Serum hormones
Ovarian hormones, such as estradiol, progesterone, LH, FSH in serum were determined by ELISA kits (Endocrine Technologies, Inc., Newark, CA, USA) according to the manufacturer's protocols. In brief, each serum sample was diluted using a solution provided in the kit. The diluted sample was dispensed in each well and incubated at an appropriate temperature and for a specified time, followed by washing five times with a washing buffer. The diluted sample was mixed with 3,3′,5,5′-tetramethylbenzidine color reagent in each well, and the mixture was incubated. Finally, HCl was added to stop the reaction. The optical density was measured by using a microwell reader (ELx800, Bio-Tek Instrument, Inc., Winooski, VT, USA) at 450 nm. Hormones levels were calculated using the standard curves.
Serum TALP and BALP levels
Serum TALP was determined by the colorimetric method with p-nitrophenyl phosphate (PNPP; Sigma-Aldrich, St. Louis, MO, USA). To determine the serum TALP activity, 50 μL of serum was mixed with 1 mL of 3 mM magnesium acetate solution (Sigma-Aldrich) and incubated in a water bath at 30°C for 5 min, followed by adding 2 mL of 0.5 M PNPP solution. The absorbance of solutions was measured using a spectrophotometer (DU 730, Beckman Coulter, Inc., Fullerton, CA, USA) at 403 nm wavelength for 2 min. Serum TALP activity was calculated by using Equation (2):21
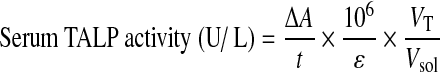 |
(2) |
where ΔA is absorbance change for t min, ɛ is p-nitrophenoxide molar absorptivity (18.8×103 L/mol·cm), 106 is the unit conversion of mol to μmol (106 μmol=1 mol), and VT and Vsol are the total volume of the mixture and the solution volume, respectively.
Serum BALP was also measured by the precipitation method22 through wheat germ agglutinin binding to N-acetylglucosamine (Sigma-Aldrich) in serum. Each serum sample was mixed with the diluted Triton X-100 (Sigma-Aldrich) solution in a ratio of 10:1 and incubated at 37°C for 30 min. The diluted sample was blended with 50 μL of 5 g/L wheat germ agglutinin solution (Lectin from Triticum vulgaris, Sigma-Aldrich) and incubated at 37°C for 30 min. The supernatant of each sample was obtained after centrifugation (5810R, Eppendorf North America, Inc., Westbury, NY, USA) at 500 g for 10 min, and subsequently the absorbance of the supernatant was measured (ASN). Serum BALP activity was calculated by using Equation (3):
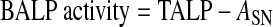 |
(3) |
BMD and stiffness
Piximus mouse densitometer (Lunar Piximus 2, GE Lunar Corporation, Madison, WI, USA) was used to determine BMD and bone mineral content (BMC) of the femur. To determine BMD and BMC, the apparatus was first calibrated according to the manufacturer's instruction. Each femur was placed in the chamber under the X-ray beam path, and the whole region of it was scanned for ∼5 min and its head region was then subsequently measured. The density of each femur was calculated by Lunar Piximus software.
Bone stiffness was measured by using a bone strength meter (EJ-02R, Iwoo Scientific Corporation, Seoul, Korea) and the measurement was performed by a three-point bending machine. Femurs were placed on the 2-point sample holder and the measuring point was located over the center of the sample. The maximum load and stiffness of bone fractions were measured at 10 mm/min velocity. The analysis was performed at the Korean Institute of Toxicology (Daejeon, Korea).
Bone histology
For microscopic histological observation, sectioned trabecular bone and cortical bone of the embedded left tibia were collected and stained with hematoxylin & eosin dye. Images were captured by a photomicroscope (Axiophot Olympus IX70, Zeiss Corporation, Jena, Germany) at 40× magnification.
Statistical analysis
ANOVA and Duncan's multiple tests were performed to analyze the differences between experimental results (SAS Institute, Inc., Cary, NC, USA). All data are expressed as mean±standard deviation and the level of significance is P<.05.
Results and Discussion
Particle size distribution and morphology
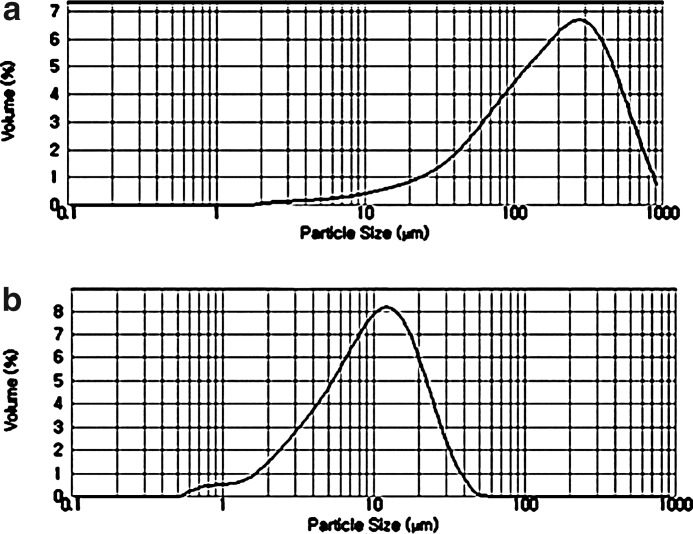
The particle size distribution of different-sized Korean angelica powders is shown in Figure 1. The diagrams show that the coarse Korean angelica powder has a relatively broader size distribution compared to ultrafine powder. The cumulative size curve of coarse powder was under a bias toward large particles, while that of ultrafine powder showed relatively bilateral symmetry. The cumulative particle size distributions D(0.1), D(0.5), and D(0.9) of the Korean angelica powders are listed in Table 3. The mean particle sizes of coarse and ultrafine Korean angelica powders based on D(0.5) were 159.4 and 12.6 μm, respectively. The span values of coarse and ultrafine Korean angelica powders were 2.51 and 2.03, respectively. Ultrafine powder possessed a lower span value, indicating that the sample has a narrow particle size distribution than coarse powder.
FIG. 1.
Cumulative particle size distribution of different-sized Korean angelica powders: (a) coarse Korean angelica powder; (b) ultrafine Korean angelica powder.
Table 3.
Particle Size Distribution of Different-Sized Korean Angelica Powders
| |
Particle size (μm) |
|
||
|---|---|---|---|---|
| Korean angelica powder | D(0.1) | D(0.5) | D(0.9) | Span value |
| Coarse | 29.3±0.24a | 159.4±6.05a | 429.5±9.31a | 2.51±0.15a |
| Ultrafine | 3.5±0.04b | 12.6±0.25b | 29.0±0.35b | 2.03±0.02b |
Means with different superscript letters in the same column are significantly different (P<.05).
The micrographs of all Korean angelica powders obtained from field-emission SEM are shown in Figure 2. Both coarse and ultrafine powder particles exhibited irregular shapes with a rugged surface.
FIG. 2.
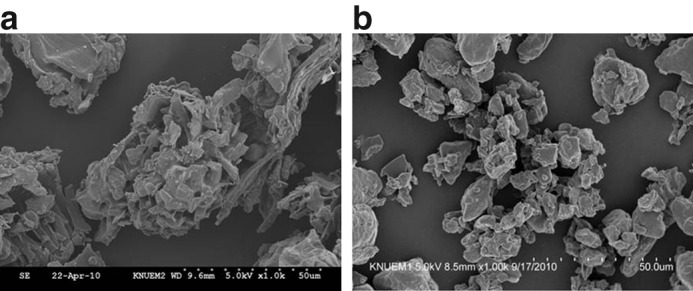
Scanning electron micrographs of different-sized Korean angelica powders: (a) coarse Korean angelica powder; (b) ultrafine Korean angelica powder.
Contents of decursin and decursinol angelate
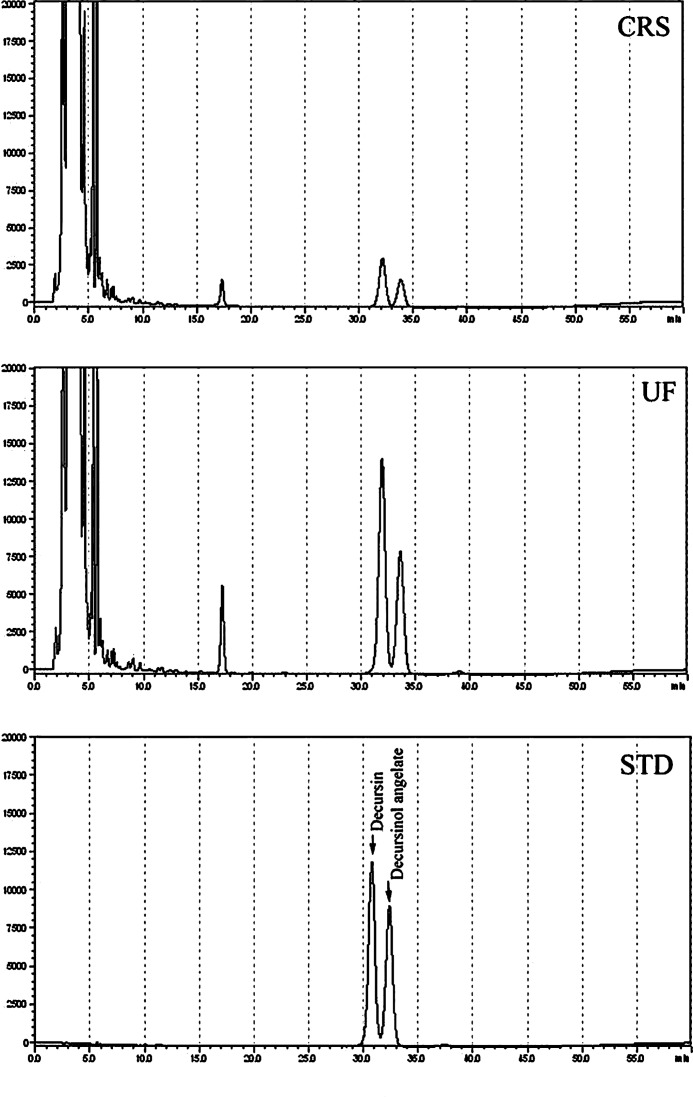
Contents of decursin and decursinol angelate were determined by HPLC (Fig. 3). The contents of decursin and decursinol angelate were not uniform in different-sized Korean angelica powders. Analytical determinations show that the extract from the ultrafine Korean angelica powder is higher in yield and contains higher concentrations of decursin and decursinol angelate than those from the coarse powder. The contents of decursin and decursinol angelate in the extract of the ultrafine Korean angelica powder were 14.70 and 9.17 μg/g of dry weight of Korean angelica powder, respectively, whereas those in the coarse Korean angelica powder were 2.84 and 1.81 μg/g of dry weight of Korean angelica powder, respectively (Table 4). The contents of decursin and decursinol angelate were significantly higher in ultrafine Korean angelica powder than in coarse powder, since in the coarse powder they remained bound within the intact cell wall structure. The relatively high shear rate during ultrafine milling increased the breakdown of cell wall structure, and subsequently, the decursin and decursinol angelate eluted easily from the Korean angelica powders. These results demonstrate that the milling process affects the physicochemical properties and texture of medicinal plants and grain of cereal plants.23
FIG. 3.
High-performance liquid chromatography chromatogram of decursin and decursinol angelate in water extract of different-sized Korean angelica powders.
Table 4.
Contents of Decursin and Decursinol Angelate in Water Extracts of Different-Sized Korean Angelica Powders
| |
|
Contents (μg/g EX) |
Contents (μg/g DW) |
||
|---|---|---|---|---|---|
| Korean angelica powder | Extraction yield (%) | Decursin | Decursinol angelate | Decursin | Decursinol angelate |
| Coarse | 40.67±1.87 | 6.99±0.12 | 4.44±0.24 | 2.84±0.07 | 1.81±0.10 |
| Ultrafine | 46.56±0.74 | 31.57±0.18 | 19.70±0.31 | 14.70±0.08 | 9.17±0.14 |
EX, weight of Korean angelica extract; DW, dry weight of Korean angelica powder.
Body weight and food intake efficiency
The effects of the different-sized Korean angelica powders on the transition of the body weight and total weight gain are shown in Table 5. Several studies have reported that an ovariectomy leads to increased body weight and food intake by decreasing ovarian hormones levels.24,25 Initial body weights were not significantly different between the four groups, but total body weights of ovariectomized rats were significantly higher compared with sham-operated rats at the end of experiment, probably due to insufficient ovarian hormone secretion. There were no significant differences in the total body weight gain among ovariectomized rats. From these results, it can be concluded that Korean angelica powder has no effect on ovariectomy-induced weight gain, which is consistent with observations that isoflavones have no effect on the body weight gain of ovariectomized rats.26,27
Table 5.
Effect of the Different-Sized Korean Angelica Powders on the Body Weight in Experimental Rat Groups
| Group | Initial body weight (g) | Final body weight (g) | Total weight gain (g) |
|---|---|---|---|
| SHAM | 125.6±1.6a | 262.8±6.9b | 137.2±7.9b |
| OVX | 122.0±3.9a | 303.0±16.8a | 180.9±13.1a |
| OVX_CRS | 123.1±4.2a | 308.4±12.6a | 185.3±8.6a |
| OVX_UF | 124.3±0.1a | 309.5±6.4a | 185.2±6.3a |
Values are mean±SD of five rats per group.
Means with different superscript letters in the same column are significantly different (P<.05).
SHAM, nonovariectomized+no Korean angelica supplement; OVX, ovariectomized+no Korean angelica supplement; OVX_CRS, ovariectomized+coarse Korean angelica powder supplement; OVX_UF, ovariectomized rat+ultrafine Korean angelica powder supplement; SD, standard deviation.
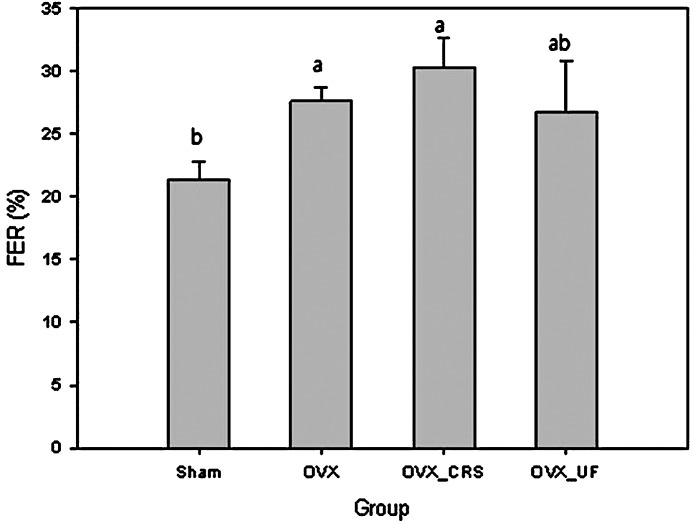
The food efficiency ratio (FER) defined as a body weight gain against total diet intake could give more reliable data on the body weight transition (Fig. 4). FERs of ovariectomized groups were relatively high compared to the sham-operated group because ovariectomy leads to more body weight gain. Among ovariectomized groups, rats fed with ultrafine powder had significantly lower FERs compared even though body weight gains were not significantly different.
FIG. 4.
FER in ovariectomized rats. abThe different superscript letters represent significant difference (P<.05). FER, food efficiency ratio; SHAM, sham-operated rats; OVX, ovariectomized rats; OVX_CRS, ovariectomized and coarse Korean angelica powder–supplemented rats; OVX_UF, ovariectomized and ultrafine Korean angelica powder–supplemented.
Serum hormones
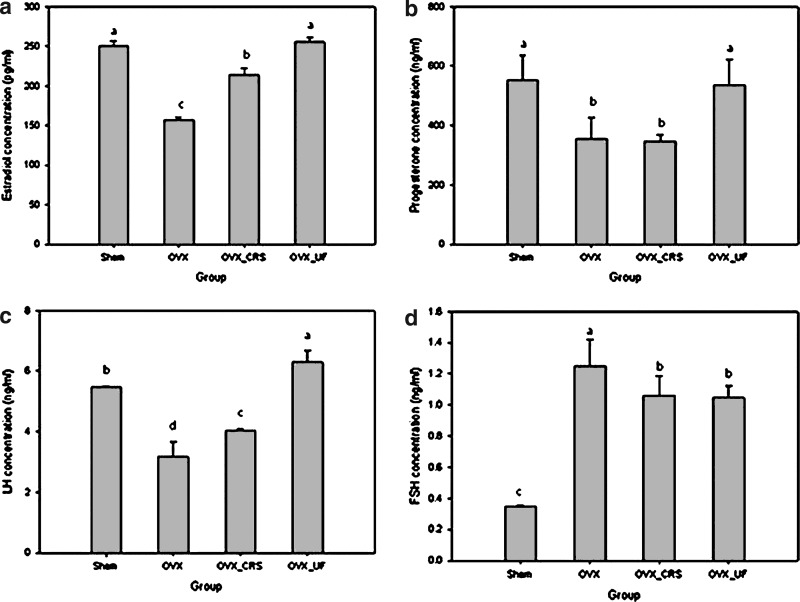
The effects of the different-sized Korean angelica powders on serum hormones levels in ovariectomized rats are shown in Figure 5. During menopausal transition, the serum levels of ovarian hormones, such as estradiol and progesterone, decrease rapidly by the decline in ovarian function.28 The serum levels of the estradiol among SHAM, OVX, OVX_CRS, and OVX_UF were 250, 156.6, 214.2, and 255.3 pg/mL, respectively. The serum levels of estradiol in the OVX_UF and OVX_CRS groups were 63% and 41% higher, respectively, compared with the OVX group (P<.05). The serum level of the estradiol in the OVX_UF group was the highest among all the groups. The OVX_CRS group represented the higher estradiol level than the OVX group despite the lower level than OVX_UF. The serum levels of the progesterone among SHAM, OVX, OVX_CRS, and OVX_UF were 552.4, 353.6, 347.1, and 534.9 ng/mL, respectively. The serum level of progesterone in the OVX_UF was 51% higher compared with the OVX group, but there was no significant difference between OVX_CRS and OVX. In general, insoluble food materials have low bioavailability due to their poor solubility. Particle size reduction can lead to the improved bioavailability of food materials by increasing the solubility and providing greater surface areas for biochemical reactions.29 In addition, the relatively high shear rate during ultrafine milling increased the breakdown of cell wall structure, and subsequently the useful bioactive components, such as decursin and decursinol angelate, eluted easily from the Korean angelica powders, which may be responsible for the increased effectiveness of the ultrafine Korean angelica powder on the serum levels of estradiol and progesterone than coarse Korean angelica powders. Decursin and decursinol angelate stimulate the estrogen and progesterone receptors by acting as natural estrogen and progesterone. In this study, decursin and its derivatives might act as estradiol and progesterone analogues resulting in the stimulation of those receptors.15,16
FIG. 5.
Effect of the different-sized Korean angelica powders on the serum levels of ovarian hormones in ovariectomized rats. (a) Estradiol, (b) progesterone, (c) LH, (d) FSH. abcdThe different superscript letters represent significant difference (P<.05). FSH, follicle-stimulating hormone; LH, luteinizing hormone.
The serum levels of LH and FSH among groups of SHAM, OVX, OVX_CRS, and OVX_UF were 5.5, 3.2, 4.0, and 6.3 ng/mL (LH) and 0.34, 1.25, 1.06, and 1.05 ng/mL (FSH), respectively. There have been some reports indicating that the serum levels of LH and FSH increase during the postmenopausal period1,30 depending on ethnicity, age, body size, and lifestyle factors. In this study, the serum level of FSH increased in OVX groups compared to the sham group. FSH levels were lower in the Korean angelica administered group, but there was no significant difference (P<.05) between the OVX_CRS and OVX_UF groups. The serum level of LH increased in the Korean angelica powder-administered groups. Moreover, the serum level of LH increased with decreasing particle size.
Serum TALP and BALP
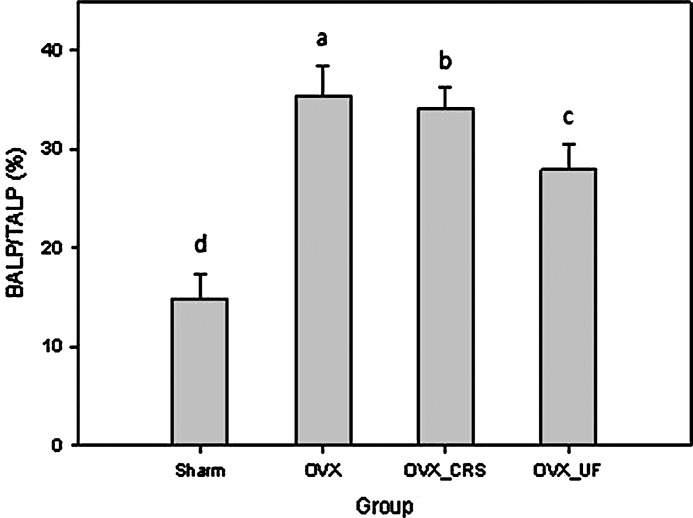
Serum TALP and BALP activities in ovariectomized rats are shown in Figure 6. ALP is made in the liver, bone, kidney, and placenta, and is known to be released during injury, bone growth, and pregnancy. In this study, the serum TALP levels of SHAM, OVX, OVX_CRS, and OVX_UF groups were 103.83, 162.23, 165.48, and 107.07 U/L, respectively. Although serum TALP activity is a good marker of bone formation, its activity can have an inaccurate result in the presence of another disease, such as hepatobiliary. Elevated serum BALP activity is observed in osteoporosis patients, which is due to the imbalance between bone resorption and bone formation. Serum BALP activity has been gradually recognized as a potential bone formation index, which could indicate accurate activity of serum ALP at specific conditions.31 The serum BALP levels of SHAM, OVX, OVX_CRS, and OVX_UF groups were 10.53, 49.25, 56.42, and 24.3 U/L. The serum levels of TALP and BALP were higher in OVX groups than the SHAM group (P<.05), which is due to the ovariectomy in OVX groups resulting in the increase of serum ALP concentration in blood.32 In addition, the ratio of BALP to TALP (BALP/TALP) was suggested that serum BALP/TALP can be a useful index for bone formation.31 The lowest serum BALP/TALP ratio was observed in the SHAM group, whereas the highest one was shown in the OVX group (Fig. 6). The serum BALP/TALP ratio of the ovariectomized rats fed with ultrafine Korean angelica powder was lower compared with the OVX and OVX_CRS groups. The result is in good agreement with the report33 that reduction of estradiol secretion during menopausal period increases serum BALP concentration. It is indicated that severe bone regeneration during the menopausal period caused by ovariectomy increases serum BALP concentration. Therefore, intake of Korean angelica powder, especially ultrafine, improves bone formation of ovariectomized rats by preventing estrogen reduction.
FIG. 6.
Effect of the different-sized Korean angelica powders on the ratio of the BALP/TALP in ovariectomized rats. abcdThe different superscript letters represent significant difference (P<.05). BALP, bone-alkaline phosphatase; TALP, total-alkaline phosphatase.
BMD and mineral content
BMD and BMC values for left femur of ovariectomized rats are listed in Table 6. BMC values were similar between all experimental groups. BMD is considered to be an indicator of osteoporosis in postmenopausal women without undergoing a bone fracture, because BMD is closely associated with bone fractures.34 According to the National Osteoporosis Risk Assessment (NORA) study,35 risk of bone fracture is 2.74-fold higher in women with osteoporosis and 1.73-fold higher in women with osteopenia than normal women. In this study, BMC was the same among all experimental groups at the end of experiment. BMD between groups of SHAM, OVX, OVX_CRS, and OVX_UF were 0.154, 0.141, 0.136, and 0.152 g/cm2, respectively. The group fed with ultrafine Korean angelica powder showed similar BMD levels as the SHAM group, but higher levels than the OVX and OVX_CRS groups. Given the ovarian hormone levels, these results indicate that a high BMD level in the OVX_UF group is strongly related to the amount of ovarian hormone secretion.
Table 6.
Effect of the Different-Sized Korean Angelica Powders on the Bone Mineral Density and Bone Mineral Content in Experimental Rat Groups
| Group | BMC (g) | BMD (g/cm2) |
|---|---|---|
| SHAM | 0.324±0.01a | 0.154±0.003a |
| OVX | 0.318±0.01a | 0.141±0.001b |
| OVX_CRS | 0.320±0.03a | 0.136±0.004b |
| OVX_UF | 0.342±0.01a | 0.152±0.002a |
Values are mean±SD of five rats per group.
Means with different superscript letters in the same column are significantly different (P<.05).
BMC, bone mineral content; BMD, bone mineral density.
Bone strength and stiffness
Bone stiffness, maximum load, and fracture load for left femur are shown in Table 7. In general, evaluation of BMD is effective for indicating the bone loss rate in osteoporosis, but it has limitations for predicting an actual bone fracture, and bone stiffness is also important for predicting the risk of fracture.36,37 In this study, bone stiffness values in the groups fed with coarse and ultrafine Korean angelica powders were 123.94 and 120.87 N/mm, respectively, and were higher than that in the OVX group.
Table 7.
Effect of the Different-Sized Korean Angelica Powders on the Bone Stiffness in Experimental Rat Groups
| Group | Maximum load (N) | Fracture load (N) | Stiffness (N/mm) |
|---|---|---|---|
| SHAM | 96.95±4.56a | 94.49±3.95a | 169.54±5.94a |
| OVX | 86.41±18.83a | 77.97±8.49b | 96.50±4.47c |
| OVX_CRS | 95.64±16.36a | 91.20±14.55ab | 123.94±4.45b |
| OVX_UF | 102.27±13.94a | 102.27±13.94a | 120.87±4.51b |
Values are mean±SD of five rats per group.
Means with different superscript letters in the same column are significantly different (P<.05).
Bone strength can be predicted by measuring the maximum load or the fracture load.38 Bones of the rats fed with ultrafine Korean angelica powder showed the highest maximum load-bearing of 102.27 N, but there was no significant difference among all groups (P<.05). On the contrary, the fracture load showed a significant difference between groups. The fracture load in the rats fed with ultrafine Korean angelica powder was similar to that in the sham-operated rats. However, the OVX group showed the lowest fracture load as expected. The differences in the fracture load among the groups exhibited a similar pattern to BMD, indicating these results are consistent with an earlier report39 of a close relationship between BMD and the fracture load.
Bone histology
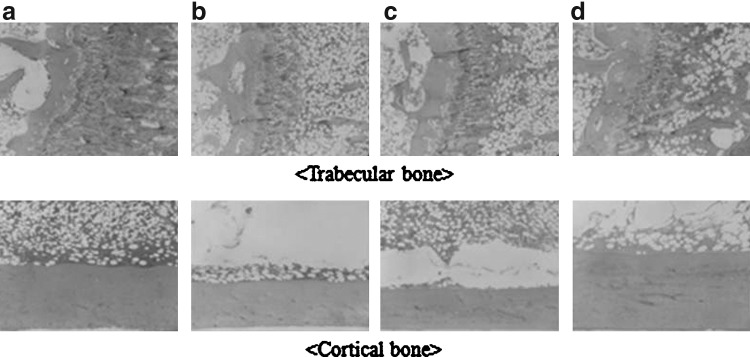
Figure 7 shows the change of distribution of bone matrices observed from a trabecular bone and a cortical bone in the left tibia of ovariectomized rats. Ovariectomy impedes the formation of bone tissues, making a trabecular bone thin and dwindling, and further inducing low BMD.40 Trabecular bones of a sham-operated rat showed a little loss in the right side of the epiphyseal plate compared to the other groups, while ovariectomized groups showed a severe loss of trabecular bone tissue sporadically. Plenty of trabecular loss was observed in ovariectomized rats. Although a similar trabecular bone loss was observed in the rats fed with coarse Korean angelica powder, an enlarged trabecular area was established near the epiphyseal plate compared to the OVX group. Compared to other ovariectomized groups, a more spacious and thick trabecular area was observed in the rats fed with ultrafine Korean angelica powder. This tendency is similar to the BMD result, which indicates that the structural integrity of the trabecular bone is strongly associated with BMD.
FIG. 7.
Histological images of right tibia in ovariectomized rats: (a) sham-operated rats, (b) ovariectomized rats, (c) ovariectomized and coarse Korean angelica powder supplemented rats, (d) ovariectomized and ultrafine Korean angelica powder supplemented rats.
Loss of cortical bone is caused predominantly by decreased ovarian function, rather than aging in the menopausal period.41 Also, it is reported that ovariectomy affects both formation and resorption of bone tissue, resulting in decreased bone strength and cortical thickness.42 Figure 7 indicates that cortical bone thickness and area decreased in both ovariectomized and coarse Korean angelica powder administered rats. On the contrary, the cortical distribution in the rats fed with ultrafine Korean angelica powder was almost the same as in the sham-operated rats. The cortical bone distribution is related to factors, such as the maximum load or the fracture load, reflecting bone strength.43 In this study, the cortical bone thickness was proportional to the increase of bone strength.
Summary
A. gigas has long been used as a treatment of women's ailments. In this study, different-sized Korean angelica powders were administered to ovariectomized rats to demonstrate the effect of particle size on the reversal of the physiological outcomes of decreased estrogen levels during menopause. It is expected that a smaller particle size could improve bioavailability by increasing the surface area. Consequently, the ovarian hormones, including estrogen and progesterone, increased significantly in the ovariectomized rats fed with ultrafine Korean angelica powder, and also increased bone metabolism. In conclusion, ultrafine grinding is a good technology to make herbal powder products for enhancing the bioavailability and bioactivity in various food and nutraceutical applications.
Acknowledgments
This study was carried out with the support of the Cooperative Research Program for Agricultural Science & Technology Development (Project No. 20070401080043), Rural Development Adminstration, Suwon, Republic of Korea, and a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MEST) (grant code: F1706).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Burger HG. Dudley EC. Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84:4025–4030. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg F. Hot flashes: epidemiology and physiology. Ann NY Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu J. Burdette JE. Xu H, et al. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem. 2001;49:2472–2479. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- 4.Brzezinski A. Debi A. Phytoestrogens: the “natural” selective estrogen receptor modulators. Eur J Obstet Gynecol R B. 1999;85:47–51. doi: 10.1016/s0301-2115(98)00281-4. [DOI] [PubMed] [Google Scholar]
- 5.Malacara JM. Canto de Cetina T. Bassol S, et al. Symptoms at pre- and postmenopause in rural and urban women from three states of Mexico. Maturitas. 2002;43:11–19. doi: 10.1016/s0378-5122(02)00077-4. [DOI] [PubMed] [Google Scholar]
- 6.Marcus R. Clinical review 76: The nature of osteoporosis. J Clin Endocrinol Metab. 1996;81:1–5. doi: 10.1210/jcem.81.1.8550734. [DOI] [PubMed] [Google Scholar]
- 7.Recker R. Clinical review 41: Current therapy for osteoporosis. J Clin Endocrinol Metab. 1993;76:14–16. doi: 10.1210/jcem.76.1.8421079. [DOI] [PubMed] [Google Scholar]
- 8.Schairer C. Lubin J. Troisi R. Sturgeon S. Brinton L. Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485–491. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 9.Chi HJ. Kim HS. Studies on the components of Umbelliferae plants in Korea: pharmacological study of decursin, decursinol and nodakenin. Korean J Pharmacol. 1970;1:25–32. [Google Scholar]
- 10.Lee S. Lee Y. Jung S. Shin K. Kim B-K. Kang S. Anti-tumor activities of decursinol angelate and decursin from Angelica gigas. Arch Pharm Res. 2003;26:727–730. doi: 10.1007/BF02976682. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH. Kang SS. Shin KH. Coumarins and a pyrimidine from Angelica gigas roots. Nat Prod Sci. 2002;8:58–61. [Google Scholar]
- 12.Konoshima M. Chi HJ. Hata K. Coumarins from the root of Angelica gigas Nakai. Chem Pharm Bull. 1968;16:1139–1140. doi: 10.1248/cpb.16.1139. [DOI] [PubMed] [Google Scholar]
- 13.Guo J. Jiang C. Wang Z, et al. A novel class of pyranocoumarin anti-androgen receptor signaling compounds. Mol Cancer Ther. 2007;6:907–917. doi: 10.1158/1535-7163.MCT-06-0231. [DOI] [PubMed] [Google Scholar]
- 14.Qin LP. Zhang QY. Tian YP. Zheng HC. Haung M. Huang BK. Total coumarins from fruits of Cnidium monnieri inhibit formation and differentiation of multinucleated osteoclasts of rats. Acta Pharmacol Sin. 2003;24:181–186. [PubMed] [Google Scholar]
- 15.Branham WS. Dial SL. Moland CL, et al. Phytoestrogens and mycoestrogens bind to the rat uterine estrogen receptor. J Nutr. 2002;132:658–664. doi: 10.1093/jn/132.4.658. [DOI] [PubMed] [Google Scholar]
- 16.Zava DT. Dollbaum CM. Blen M. Estrogen and progestin bioactivity of foods, herbs, and spices. Proc Soc Exp Biol Med. 1998;217:369–378. doi: 10.3181/00379727-217-44247. [DOI] [PubMed] [Google Scholar]
- 17.Lee KY. Jang TG. Kang WS. Air classification of buckwheat using impeller of turbo mill. Korean J Food Eng Prog. 2006;10:14–22. [Google Scholar]
- 18.Li S. Ge S. Huang Z. Wang Q. Zhao H. Pan H. Cryogenic grinding technology for traditional Chinese herbal medicine. Cryogenics. 1991;31:136–137. [Google Scholar]
- 19.Yim TK. Wu WK. Pak WF. Mak DHF. Liang SM. Ko KM. Myocardial protection against ischaemia-reperfusion injury by a Polygonum multiflorum extract supplemented ‘Dang-Gui decoction for enriching blood’, a compound formulation, ex vivo. Phytother Res. 2000;14:195–199. doi: 10.1002/(sici)1099-1573(200005)14:3<195::aid-ptr629>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Chew NYK. Chan HK. Effect of powder poly dispersibility on aerosol generation. J Pharm Pharm Sci. 2002;5:162–168. [PubMed] [Google Scholar]
- 21.Bowers GN., Jr. McComb RB. Measurement of total alkaline phosphatase activity in human serum. Clin Chem. 1975;21:1988–1995. [PubMed] [Google Scholar]
- 22.Behr W. Barnert J. Quantification of bone alkaline phosphatase in serum by precipitation with wheat-germ lectin: a simplified method and its clinical plausibility. Clin Chem. 1986;32:1960–1966. [PubMed] [Google Scholar]
- 23.Cadden AM. Comparative effects of particle-size reduction on physical structure and water binding-properties of several plant fibers. J Food Sci. 1987;52:1595–1599. [Google Scholar]
- 24.Mayes JS. Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5:197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 25.Musatov S. Chen W. Pfaff DW, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deyhim F. Stoecker BJ. Brusewitz GH. Arjmandi BH. The effects of estrogen depletion and isoflavones on bone metabolism in rats. Nutr Res. 2003;23:123–130. [Google Scholar]
- 27.Ishimi Y. Arai N. Wang X, et al. Difference in effective dosage of genistein on bone and uterus in ovariectomized mice. Biochem Biophys Res Commun. 2000;274:697–701. doi: 10.1006/bbrc.2000.3175. [DOI] [PubMed] [Google Scholar]
- 28.Karim R. Hodis HN. Stanczyk FZ. Lobo RA. Mack WJ. Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. J Clin Endocrinol Metab. 2008;93:131–138. doi: 10.1210/jc.2007-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liversidge GG. Cundy KC. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int J Pharm. 1995;125:91–97. [Google Scholar]
- 30.Randolph JF. Sowers M. Bondarenko IV. Harlow SD. Luborsky JL. Little RJ. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89:1555–1561. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 31.Ramaswamy G. Rao V. Krishnamoorthy L. Ramesh G. Gomathy R. Renukadevi D. Serum levels of bone alkaline phosphatase in breast and prostate cancers with bone metastasis. Indian J Clin Biochem. 2000;15:110–113. doi: 10.1007/BF02883737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalu DN. Liu CC. Salerno E. Hollis B. Echon R. Ray M. Skeletal response of ovariectomized rats to low and high doses of 17[beta]-estradiol. Bone Miner. 1991;14:175–187. doi: 10.1016/0169-6009(91)90021-q. [DOI] [PubMed] [Google Scholar]
- 33.Gundberg CM. Looker AC. Nieman SD. Calvo MS. Patterns of osteocalcin and bone specific alkaline phosphatase by age, gender, and race or ethnicity. Bone. 2002;31:703–708. doi: 10.1016/s8756-3282(02)00902-x. [DOI] [PubMed] [Google Scholar]
- 34.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 35.Siris ES. Miller PD. Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women—Results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 36.Delmas PD. Seeman E. Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone. 2004;34:599–604. doi: 10.1016/j.bone.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi S. Inoue S. Hosoi T. Ouchi Y. Shiraki M. Orimo H. Association of bone mineral density with polymorphism of the estrogen receptor gene. J Bone Miner Res. 1996;11:306–311. doi: 10.1002/jbmr.5650110304. [DOI] [PubMed] [Google Scholar]
- 38.Zioupos P. Currey JD. Changes in the stiffness, strength, and toughness of human cortical bone with age. Bone. 1998;22:57–66. doi: 10.1016/s8756-3282(97)00228-7. [DOI] [PubMed] [Google Scholar]
- 39.Beason DP. Dakin GJ. Lopez RR. Alonso JE. Bandak FA. Eberhardt AW. Bone mineral density correlates with fracture load in experimental side impacts of the pelvis. J Biomech. 2003;36:219–227. doi: 10.1016/s0021-9290(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 40.Mori S. Jee WS. Li XJ. Production of new trabecular bone in osteopenic ovariectomized rats by prostaglandin E2. Calcified Tissue Int. 1992;50:80–87. doi: 10.1007/BF00297302. [DOI] [PubMed] [Google Scholar]
- 41.Meema HE. Bunker ML. Meema S. Loss of compact bone due to menopause. Obstet Gynecol. 1965;26:333–343. [PubMed] [Google Scholar]
- 42.Han SM. Szarzanowicz TE. Ziv I. Effect of ovariectomy and calcium deficiency on the ultrasound velocity, mineral density and strength in the rat femur. Clin Biomech. 1998;13:480–484. doi: 10.1016/s0268-0033(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 43.Ammann P. Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14:13–18. doi: 10.1007/s00198-002-1345-4. [DOI] [PubMed] [Google Scholar]