Abstract
Most known parasitoid wasp species attack the larval or pupal stages of Drosophila. While Trichopria drosophilae infect the pupal stages of the host (Fig. 1A-C), females of the genus Leptopilina (Fig. 1D, 1F, 1G) and Ganaspis (Fig. 1E) attack the larval stages. We use these parasites to study the molecular basis of a biological arms race. Parasitic wasps have tremendous value as biocontrol agents. Most of them carry virulence and other factors that modify host physiology and immunity. Analysis of Drosophila wasps is providing insights into how species-specific interactions shape the genetic structures of natural communities. These studies also serve as a model for understanding the hosts' immune physiology and how coordinated immune reactions are thwarted by this class of parasites.
The larval/pupal cuticle serves as the first line of defense. The wasp ovipositor is a sharp needle-like structure that efficiently delivers eggs into the host hemocoel. Oviposition is followed by a wound healing reaction at the cuticle (Fig. 1C, arrowheads). Some wasps can insert two or more eggs into the same host, although the development of only one egg succeeds. Supernumerary eggs or developing larvae are eliminated by a process that is not yet understood. These wasps are therefore referred to as solitary parasitoids.
Depending on the fly strain and the wasp species, the wasp egg has one of two fates. It is either encapsulated, so that its development is blocked (host emerges; Fig. 2 left); or the wasp egg hatches, develops, molts, and grows into an adult (wasp emerges; Fig. 2 right). L. heterotoma is one of the best-studied species of Drosophila parasitic wasps. It is a "generalist," which means that it can utilize most Drosophila species as hosts1. L. heterotoma and L. victoriae are sister species and they produce virus-like particles that actively interfere with the encapsulation response2. Unlike L. heterotoma, L. boulardi is a specialist parasite and the range of Drosophila species it utilizes is relatively limited1. Strains of L. boulardi also produce virus-like particles3 although they differ significantly in their ability to succeed on D. melanogaster1. Some of these L. boulardi strains are difficult to grow on D. melanogaster1 as the fly host frequently succeeds in encapsulating their eggs. Thus, it is important to have the knowledge of both partners in specific experimental protocols.
In addition to barrier tissues (cuticle, gut and trachea), Drosophila larvae have systemic cellular and humoral immune responses that arise from functions of blood cells and the fat body, respectively. Oviposition by L. boulardi activates both immune arms1,4. Blood cells are found in circulation, in sessile populations under the segmented cuticle, and in the lymph gland. The lymph gland is a small hematopoietic organ on the dorsal side of the larva. Clusters of hematopoietic cells, called lobes, are arranged segmentally in pairs along the dorsal vessel that runs along the anterior-posterior axis of the animal (Fig. 3A). The fat body is a large multifunctional organ (Fig. 3B). It secretes antimicrobial peptides in response to microbial and metazoan infections.
Wasp infection activates immune signaling (Fig. 4)4. At the cellular level, it triggers division and differentiation of blood cells. In self defense, aggregates and capsules develop in the hemocoel of infected animals (Fig. 5)5,6. Activated blood cells migrate toward the wasp egg (or wasp larva) and begin to form a capsule around it (Fig. 5A-F). Some blood cells aggregate to form nodules (Fig. 5G-H). Careful analysis reveals that wasp infection induces the anterior-most lymph gland lobes to disperse at their peripheries (Fig. 6C, D).
We present representative data with Toll signal transduction pathway components Dorsal and Spätzle (Figs. 4,5,7), and its target Drosomycin (Fig. 6), to illustrate how specific changes in the lymph gland and hemocoel can be studied after wasp infection. The dissection protocols described here also yield the wasp eggs (or developing stages of wasps) from the host hemolymph (Fig. 8).
Keywords: Immunology, Issue 63, Parasitoid wasps, innate immunity, encapsulation, hematopoiesis, insect, fat body, Toll-NF-kappaB, molecular biology
Protocol
The entire protocol for the experiment is divided into four steps (Fig. 9). (1) Culturing wasps on fly larvae; (2) Setting up infections and preparing animals for dissection; (3) Isolating and fixing host/parasite structures; (4) Analyzing immune tissues.
1. Culturing Wasps on Drosophila Larvae
Maintenance of wasp cultures requires careful planning. Relative to growing flies, it is fairly labor intensive. We maintain our wasp colonies in the laboratory on larvae or pupae of the y w strain of Drosophila at 24 °C. Culturing wasps involves keeping a continuous source of hosts at the right stage and clearing the "infected" vials of flies that emerge before the wasps do. Wasps follow the haplodiploid method of sex determination and it is therefore important that the females are mated before they infect the hosts.
On day 1, add a small dab of freshly-prepared yeast paste (made by mixing 1 ml of water in roughly 1 g of active dry yeast into a fresh fly food vial. The recipe of the fly food is not critical; we grow flies on the corn-meal/sucrose/agar medium. Place 50-60 young (2-4 day-old) adult y w (or other essentially wild type strain) flies to lay eggs for 48 hours at 24 °C.
On day 3, remove the flies from the vials. Place a buzz plug on the vial with a drop of honey on the inner side of the buzz plug. Honey is diluted 2:1 in distilled water.
Using CO2, anesthetize the wasps and sort 6-8 females and 6-8 males that are roughly 2-3 days old. Add them to the vial containing 0-48 hour old hosts. Note that wasps are more sensitive to CO2 than flies, and you may have to calibrate their exposure to the gas. Drosophila researchers do not use a standard Drosophila anesthesia station design. A good starting point to anesthetize wasps is to set the CO2 pressure to roughly half of what is needed for anesthetizing flies.
Replace the buzz plug (with honey) on the vial. Gently place the vial on its side until the wasps wake up.
Place these vials with fly larvae and wasps in a 24 °C incubator.
Successful infections will delay pupariation slightly. Hosts that have suffered wasp attack but are able to defend themselves (or if they are not infected), continue development following the normal schedule and adult flies emerge from these puparia well ahead of the wasps.
Depending on the wasp species and temperature, wasps eclose in 25-30 days.
Remove the adult flies from the vials in which wasps are developing.
2. Setting up Infections and Preparing Infected Animals for Dissection
Before you start, have a couple of clean glass or plastic Petri dishes, a Pyrex dissecting dish with 9 depressions, Kimwipes, distilled water (in a squirt bottle), 70% ethanol (in a squirt bottle), 1X PBS (in a squirt bottle), microscope slides, and a small, clean spatula handy.
To set up infections, follow steps 1-5 above. Depending on the experiment, it may be necessary to use hosts that are at roughly the same stage of development. For this, egglays of 2-6 hours can be used for infection.
To harvest infected larvae (for dissections of immune tissues or parasites), remove the contents of the fly vials into a small glass or plastic Petri dish.
Using a stereomicroscope and fine forceps (tweezers), carefully pick 6-10 larvae, one-by-one and place them into a depression well with 1X PBS first, then transfer them into water, 70% ethanol, water, and 1X PBS, successively. The purpose of these steps is to free the animals of the fly food and thoroughly clean and sterilize their surface. The rinse in water dilutes the ethanol and the final rinse in PBS removes the ethanol. For Step 3 below, it is best not to leave the animals in PBS for longer than 10 minutes.
3. Isolating and Fixing Host/parasite Structures
Background
The larval lymph gland is a small hematopoietic organ7. At the third larval instar, the lymph gland contains a large pair of anterior lobes that flank the dorsal vessel (Fig. 3A, 6B-C, 7A-D). The anterior lobes are further divided into specialized regions with unique cell properties7. Progenitors of three cell types, plasmatocytes, lamellocytes, and crystal cells reside in the anterior lobes. Pericardial cells separate the anterior lobes from the smaller posterior lobes. The Drosophila lymph gland is a model for insect and mammalian hematopoiesis8,9.
Fat body is functionally similar to the mammalian liver. The humoral response is triggered in the fat body following microbial or wasp infection1,4,10. As a result, a unique combination of antimicrobial peptide genes are activated and the peptides are secreted into the hemolymph1. The fat body is also the primary tissue for glycogen and triglyceride production and storage11. The larval fat body occupies substantial volume of the hemocoel and, so unlike the lymph gland, it is easy to locate. We will now demonstrate how to dissect the lymph gland and the fat body from third instar larvae.
Before you start
Need fine forceps (tweezers) and ethanol-cleaned microscope slides.
Larval lymph gland dissection
Using fine forceps, select a wandering third instar larva kept in 1X PBS.
Place the larva onto a microscope slide.
Using forceps, position the animal with the ventral side up.
Using two pairs of forceps, hold the animal at either side of the posterior end (arrowheads 1, Fig. 3A) and gently pull the cuticle to introduce a small, superficial tear in the cuticle.
The hemolymph containing hemocytes, gut, and fat body will begin to escape from the body cavity. The hemolymph can be taken up with a 10 μl "pipetteman" to make smears, if needed.
Placing both forceps on the right side of the animal beneath the mouth hooks (arrowheads 2, Fig. 3A), gently tear the cuticle.
Move both forceps to the left side of the animal beneath the mouth hooks (arrowheads 2, Fig. 3A) and carefully make another small tear.
While holding the mouth hooks of the animal, gently pull the cuticle down towards the posterior end of the animal (as though peeling it back).
As the cuticle is pulled back, the brain, fat body, imaginal discs, salivary glands, and proventriculus will become exposed. The detached cuticle is now at the posterior end but will still have the dorsal vessel with the lymph gland attached to it.
Cut the proventriculus and move it away from the lymph gland area. The lymph gland will be underneath the brain even though you may not be able to see it.
Carefully tease away the fat body, gut, salivary glands, and other structures that may be sitting on the gland. Be careful not to detach the lymph gland from the ring gland and the dorsal vessel located posterior to the brain. The lymph gland will be positioned flat against the microscope slide.
Carefully remove all additional tissues away from the lymph gland until the entire gland unfolds from the anterior lobes to the final set of pericardial cells.
Note: A properly dissected lymph gland will have one pair of anterior lobes and two sets of posterior lobes along the dorsal vessel (Fig. 6B). The lobes can be damaged easily or can fall off from the dorsal vessel. The samples should therefore be handled with great care. The gland also has a tendency to shrivel or contract. Gently straightening out the organ at its posterior end allows for all parts and cells to be presented well. This is especially necessary for the immunostaining protocols.
Fay body dissection
On the dissecting stage of the Zeiss 1000 dissecting microscope (any stereo dissecting microscope can be used), place a light box that allows incident light to be transmitted through the sample. Position a third instar larva from Step 2 on a microscope slide in 200 μl of 1X PBS. Tilt the light source away from the sample so that the organism appears transparent and therefore it is easy to visualize its internal organs.
Using forceps, position the animal so that the anterior end is away from you.
Using forceps, hold the cuticle of the larva on the left side of its mouth hooks (arrowhead 1, Fig. 3B). Using the other forceps, gently tear the cuticle all the way to the posterior end (arrowhead 2, Fig 3B). Do not tear the cuticle away from the body at the posterior end.
Very gently start removing the gut to one side.
Gently remove the brain, imaginal discs, and the ring gland with the lymph gland running through the dorsal vessel.
Do not remove the salivary glands since there is fat body adhering to these glands.
Gently remove the cuticle and leave the fat body on the slide in 1X PBS.
Note 1: The fat body has only one cell layer and it is therefore important to have all cells flattened in the same plane on the glass slide. Cells are endopolyploid and easy to visualize. A well-dissected fat body sample should maintain normal cell contacts, and should have minimal fat globules around the dissected sample (Fig. 6I, J).
Note 2: If wasp eggs remain unaffected by the host immune system, they will initiate development almost immediately. Early developmental stages of the parasite (from the host larva) are easily accessible from the dissected hemocoel (Fig. 8). Parasite eggs or larvae either adhere to the fat body or other organs, or simply slip onto the glass slide during the dissection.
4. Analyzing Immune Tissues
Air dry lymph glands for 5-10 min and remove 1X PBS from the fat body slide before fixing for 5-10 minutes with 4% paraformaldehyde prepared in PBS. Each slide can have several dissected lymph glands for staining. Move slides with the fixed samples into a homemade humidified chamber for subsequent steps. This chamber is prepared by placing two pieces of folded, damp paper towels at the bottom of an empty plastic micropipette tip box. One or more slides with samples can be placed on top of the divider used for holding the tips.
As such, the analysis of the immune tissues is done following standard methods that combine in vivo cell labeling and indirect immunohistochemistry4.
5. Representative Results
Wasp infection activates expression of the Toll pathway reporter Drosomycin-GFP (Fig. 6G, J). In this experiment, the effect of wasp-infestation on gene expression is visualized in vivo using a GFP-reporter linked to the Drs promoter12. In uninfected control animals, the GFP expression is not detected. The GFP signal is clearly detected in the cytoplasm and nucleus of the cells of the fat body, dissected from animals infected with L. victoriae. To obtain the best results, the ratio of wasps : hosts should be 1:10. The infection should last for at least 2 hours. Post infestation, the Drs-GFP signal intensifies and many fat body cells are GFP-positive even up to 72 hours 1,4.
Wasp infection induces differentiation of lamellocytes in the lymph gland (Fig. 6D). Lamellocytes surround and block wasp development (Fig. 6H). Lymph glands are dissected to visualize the cellular changes induced in the anterior lobes after L. victoriae infection. Newly-differentiated lamellocytes are present at the periphery of the dissected lobes; lamellocytes are marked with a GFP transgene that is driven by the misshapen enhancer (MSNF9-GFP). All cells are visualized with filamentous actin using rhodamine-labeled phalloidin. Notice that the integrity of the anterior lobes is disrupted in these glands as the basement membrane that normally surrounds the lobe continuously (Fig. 6C), is now discontinuous (Fig. 6D). From the same dissections, lamellocytes in the hemolymph can also be visualized in smears (Fig. 6G); some of which are associated with the capsule structure formed to block parasite development (Fig. 6H).
Spätzle protein is expressed in most cells of the larval lymph gland (Fig. 7C,D). To detect Spz in the dissected lymph glands, we followed a standard protocol for staining cells of the lymph gland with polyclonal anti-Spz antibodies4. In vivo, Spz levels increase in lymph gland cells after wasp infection (compare panels Fig. 7C, E with 7G, I, and panels 7D, F with 7H, J).
Early and late stages of wasp development from host larvae and pupae (Fig. 8). To examine developmental stages of wasps, infected larvae are dissected at various time points after oviposition. Here we show a sampling of these stages from Leptopilina spp. from infected wild type D. melanogaster hosts.
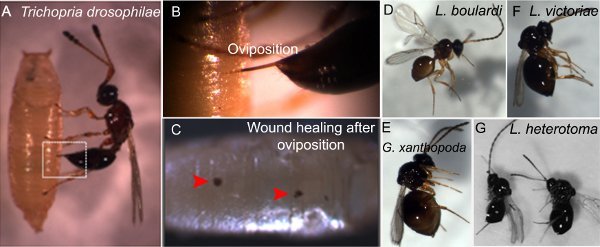 Figure 1. Different wasp species and oviposition of wasp egg. All images were obtained using a Leica stereomicroscope. A
Trichopria drosophilae female wasp oviposits egg into a D. melanogaster pupa. B Magnification of oviposition of Trichopria drosophilae into host pupa. C Melanized spots indicate wound healing at the site of oviposition. D
L. boulardi male. E
G. xanthopoda female. F
L. victoriae female. G
L. heterotoma male (left) and female.
Figure 1. Different wasp species and oviposition of wasp egg. All images were obtained using a Leica stereomicroscope. A
Trichopria drosophilae female wasp oviposits egg into a D. melanogaster pupa. B Magnification of oviposition of Trichopria drosophilae into host pupa. C Melanized spots indicate wound healing at the site of oviposition. D
L. boulardi male. E
G. xanthopoda female. F
L. victoriae female. G
L. heterotoma male (left) and female.
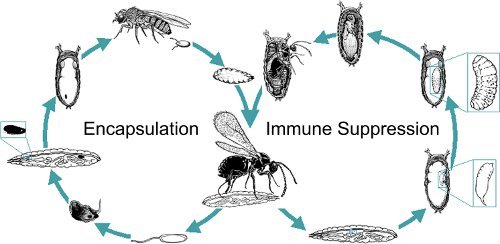 Figure 2. Life cycles showing the fly/wasp arms race. Oviposition results in one of two outcomes. Either host immune reactions succeed to block the development of the wasp (left), or the wasp thwarts the host's immune responses and succeeds (right). Modified from Melk and Govind, 199918.
Figure 2. Life cycles showing the fly/wasp arms race. Oviposition results in one of two outcomes. Either host immune reactions succeed to block the development of the wasp (left), or the wasp thwarts the host's immune responses and succeeds (right). Modified from Melk and Govind, 199918.
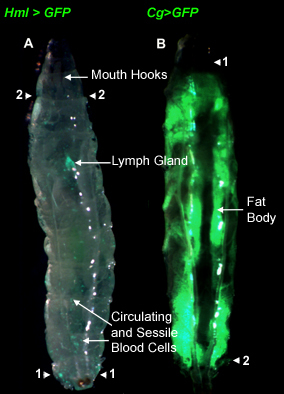 Figure 3. Third instar larvae showing the organs of interest prior to dissection. All images were obtained using a Leica stereomicroscope. A Hml>GFP larva with GFP (green) fluorescence (arrow) in individual cells of the lymph gland indicates the general location of the lymph gland in an intact animal. The larva was imaged with fluorescence and bright field optics. Arrowheads point to locations important for the lymph gland dissection protocol described in the text. B
Cg>GFP larva with GFP expression in the fat body to indicate the location of the organ in an intact animal. Arrowheads point to locations important for the fat body dissection protocol described in the text.
Figure 3. Third instar larvae showing the organs of interest prior to dissection. All images were obtained using a Leica stereomicroscope. A Hml>GFP larva with GFP (green) fluorescence (arrow) in individual cells of the lymph gland indicates the general location of the lymph gland in an intact animal. The larva was imaged with fluorescence and bright field optics. Arrowheads point to locations important for the lymph gland dissection protocol described in the text. B
Cg>GFP larva with GFP expression in the fat body to indicate the location of the organ in an intact animal. Arrowheads point to locations important for the fat body dissection protocol described in the text.
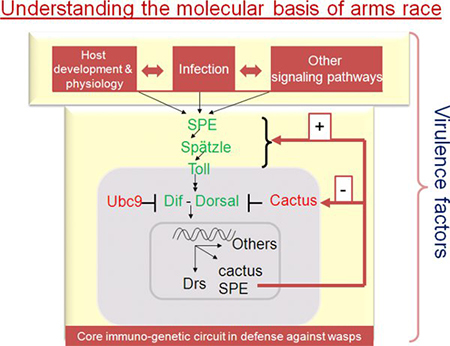 Figure 4. Immuno-genetic circuit of host immune response against parasitoid attack. Core components of the Toll pathway are shown. Spätzle (Spz) is activated by the protease Spätzle processing enzyme, SPE. Activated Spz serves as the ligand for Toll. Intracellular signaling leads to the activation of NF-κB transcription factors, Dorsal and Dif. Cactus serves as the IκB inhibitor of the pathway. Activation of Drosomycin, a canonical Toll pathway target gene, can be monitored using transgenic fly strains with the GFP reporter (see Fig. 6I, J).
Figure 4. Immuno-genetic circuit of host immune response against parasitoid attack. Core components of the Toll pathway are shown. Spätzle (Spz) is activated by the protease Spätzle processing enzyme, SPE. Activated Spz serves as the ligand for Toll. Intracellular signaling leads to the activation of NF-κB transcription factors, Dorsal and Dif. Cactus serves as the IκB inhibitor of the pathway. Activation of Drosomycin, a canonical Toll pathway target gene, can be monitored using transgenic fly strains with the GFP reporter (see Fig. 6I, J).
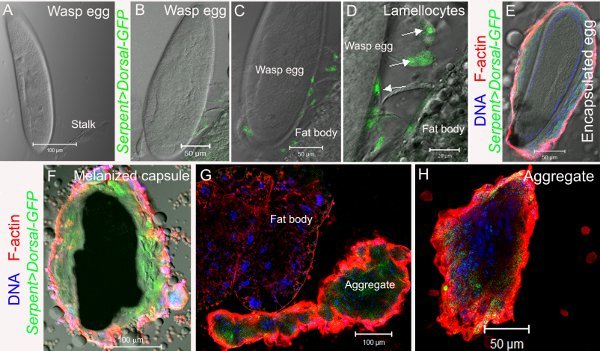 Figure 5. Encapsulation in response to L. victoriae infestation in Serpent>GFP-dl Drosophila hosts. An enhancer in the Serpent gene is expressed in blood cells13. When this enhancer drives the expression of the GFP-Dorsal fusion protein in a transgene, Dorsal is detected via the green fluorescence of GFP in some plasmatocytes and lamellocytes that make up the capsules and aggregates. All images were obtained using a Zeiss LSM confocal microscope. A Egg of L. victoriae. B-D Lamellocytes (white arrows) at the posterior end of the egg express GFP-Dorsal. C-D Higher magnifications of the sample in panel B. E-H Samples are counterstained with rhodamine-labeled phalloidin to visualize filamentous actin (F-actin, red) and with Hoechst 33258 to visualize DNA (blue). E Encapsulated egg of L. victoriae. F Melanized capsule of L. victoriae. G-H
L. victoriae infection induces blood cell (plasmatocyte and lamellocyte) aggregation.
Figure 5. Encapsulation in response to L. victoriae infestation in Serpent>GFP-dl Drosophila hosts. An enhancer in the Serpent gene is expressed in blood cells13. When this enhancer drives the expression of the GFP-Dorsal fusion protein in a transgene, Dorsal is detected via the green fluorescence of GFP in some plasmatocytes and lamellocytes that make up the capsules and aggregates. All images were obtained using a Zeiss LSM confocal microscope. A Egg of L. victoriae. B-D Lamellocytes (white arrows) at the posterior end of the egg express GFP-Dorsal. C-D Higher magnifications of the sample in panel B. E-H Samples are counterstained with rhodamine-labeled phalloidin to visualize filamentous actin (F-actin, red) and with Hoechst 33258 to visualize DNA (blue). E Encapsulated egg of L. victoriae. F Melanized capsule of L. victoriae. G-H
L. victoriae infection induces blood cell (plasmatocyte and lamellocyte) aggregation.
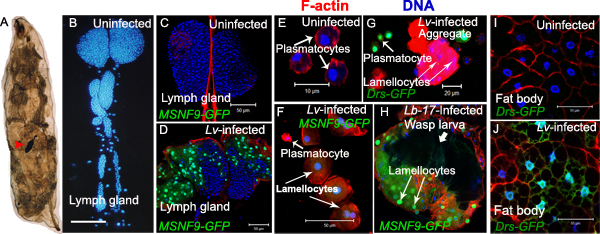 Figure 6. Immune responses against wasp infection. Images in panels A and B were obtained using a Zeiss Axio Scope. Images in panels C-J were obtained using a Zeiss LSM confocal microscope. A Host larva showing melanized encapsulated wasp egg (arrowhead) through the transparent cuticle. B Representative third instar larval lymph gland stained with Hoechst 33258 reveals cells of all lobes and interspersed pericardial cells. Scale bar represents 100 μ. C-J All samples were counterstained with Hoechst 33258 (to label nuclei) and rhodamine phalloidin (to label F-actin). C-D Anterior lymph gland lobes from control (C) and L. victoriae-infected (D) MSNF9-GFP animals. In infected animals, MSNF enhancer expression, specific to lamellocytes14, is detected with a nuclear GFP reporter. E Plasmatocytes isolated from uninfected animals do not express GFP. These animals have very few if any lamellocytes. F Plasmatocytes (GFP-negative, short arrow) and newly-differentiated, larger lamellocytes (long arrows) with weak nuclear GFP expression from L. victoriae-infected MSNF9-GFP larva. G Free and aggregated plasmatocytes and lamellocytes from the hemocoel of a L. victoriae-infected Drs-GFP larva. Expression of the Drs-GFP reporter is activated in some plasmatocytes (short arrow), but not in lamellocytes (long arrows) post-infection. H Encapsulated and melanized wasp larva from L. boulardi-infected MSNF9-GFP host larva. Numerous MSNF9-GFP-positive lamelloctyes surround the wasp larva (dark structure; thick arrow) and exhibit strong nuclear GFP expression (long arrows). This panel previously published in PLoS One15. I-J Fat body cells dissected from uninfected (I) and L. victoriae-infected (J) Drs-GFP larva. GFP is nuclear and cytoplasmic in fat body cells of infected animals. Click here to view larger figure.
Figure 6. Immune responses against wasp infection. Images in panels A and B were obtained using a Zeiss Axio Scope. Images in panels C-J were obtained using a Zeiss LSM confocal microscope. A Host larva showing melanized encapsulated wasp egg (arrowhead) through the transparent cuticle. B Representative third instar larval lymph gland stained with Hoechst 33258 reveals cells of all lobes and interspersed pericardial cells. Scale bar represents 100 μ. C-J All samples were counterstained with Hoechst 33258 (to label nuclei) and rhodamine phalloidin (to label F-actin). C-D Anterior lymph gland lobes from control (C) and L. victoriae-infected (D) MSNF9-GFP animals. In infected animals, MSNF enhancer expression, specific to lamellocytes14, is detected with a nuclear GFP reporter. E Plasmatocytes isolated from uninfected animals do not express GFP. These animals have very few if any lamellocytes. F Plasmatocytes (GFP-negative, short arrow) and newly-differentiated, larger lamellocytes (long arrows) with weak nuclear GFP expression from L. victoriae-infected MSNF9-GFP larva. G Free and aggregated plasmatocytes and lamellocytes from the hemocoel of a L. victoriae-infected Drs-GFP larva. Expression of the Drs-GFP reporter is activated in some plasmatocytes (short arrow), but not in lamellocytes (long arrows) post-infection. H Encapsulated and melanized wasp larva from L. boulardi-infected MSNF9-GFP host larva. Numerous MSNF9-GFP-positive lamelloctyes surround the wasp larva (dark structure; thick arrow) and exhibit strong nuclear GFP expression (long arrows). This panel previously published in PLoS One15. I-J Fat body cells dissected from uninfected (I) and L. victoriae-infected (J) Drs-GFP larva. GFP is nuclear and cytoplasmic in fat body cells of infected animals. Click here to view larger figure.
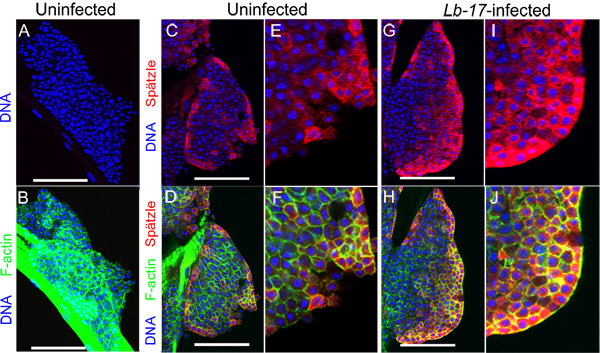 Figure 7. Spätzle expression after parasitoid wasp infection. A-J Samples were counterstained with Hoechst 33258 to visualize DNA. All images were obtained using a Zeiss LSM confocal microscope. A-F Anterior lobes dissected from uninfected y w larvae. Samples were processed for indirect immunostaining without primary antibody (A and B) or with anti-Spz antibody (red; C, D, E, F) and counterstained with Alexa Flour-phalloidin to label F-actin in cells (green; B, D, F). G-J Anterior lobes dissected from infected y w larvae and stained with anti-Spz antibody (red; G, H, I, J) and Alexa Fluor-phalloidin (green; panels H, J). E-F Higher magnifications of samples in C and D, respectively. I-J Higher magnifications of the samples in G and H, respectively. Scale bars in panels A-D, G, H represent 50 μm.
Figure 7. Spätzle expression after parasitoid wasp infection. A-J Samples were counterstained with Hoechst 33258 to visualize DNA. All images were obtained using a Zeiss LSM confocal microscope. A-F Anterior lobes dissected from uninfected y w larvae. Samples were processed for indirect immunostaining without primary antibody (A and B) or with anti-Spz antibody (red; C, D, E, F) and counterstained with Alexa Flour-phalloidin to label F-actin in cells (green; B, D, F). G-J Anterior lobes dissected from infected y w larvae and stained with anti-Spz antibody (red; G, H, I, J) and Alexa Fluor-phalloidin (green; panels H, J). E-F Higher magnifications of samples in C and D, respectively. I-J Higher magnifications of the samples in G and H, respectively. Scale bars in panels A-D, G, H represent 50 μm.
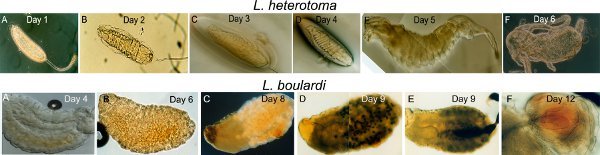 Figure 8. Larval and pupal stages of L. heterotoma and L. boulardi. Individual stages were isolated from larval or pupal fly hosts post infestation, as indicated. All images were obtained using a Zeiss Axio Scope. Top row A-F
L. heterotoma stages, 1-6 days after infection. Bottom row A-F Postembryonic L. boulardi stages, 4 to 12 days after infection.
Figure 8. Larval and pupal stages of L. heterotoma and L. boulardi. Individual stages were isolated from larval or pupal fly hosts post infestation, as indicated. All images were obtained using a Zeiss Axio Scope. Top row A-F
L. heterotoma stages, 1-6 days after infection. Bottom row A-F Postembryonic L. boulardi stages, 4 to 12 days after infection.
 Figure 9. Flow chart of the experimental protocol.
Figure 9. Flow chart of the experimental protocol.
Discussion
Interest in parasitic wasps of Drosophila is surging as molecular techniques to decode whole genomes become efficient and cost-effective. However, relative to their exceptionally well-studied hosts, many fascinating aspects of wasp biology remain obscure. These include issues related to host range, immune suppression, superparasitism, and behavior. The focus of this presentation was to demonstrate the effects of infection on the fly's immune tissues. The dissection techniques demonstrated here can be used for the analysis of gene expression at the RNA level (in situ hybridization), or for extraction of nucleic acid for microarrays or PCR, or for Western analyses of proteins. A vast variety of fly strains are available from the stock centers and individual research labs to manipulate and label immune cells. The choice of fly strains is dictated by the experimental questions. These dissection techniques can also be used for the analysis of immune tissues of other Drosophila species.
Disclosures
No conflicts of interest declared.
Acknowledgments
We are grateful to Prof. Todd Schlenke for Trichopria drosophilae, Prof. Tony Ip for transgenic fly strains, and Prof. Carl Hashimoto for anti-Spätzle antibodies. We thank present and past members of the lab for their contributions to this presentation. This work was supported by the following grants: from NIH (S06 GM08168, RISE 41399-009, and G12-RR03060), USDA (NRI/USDA CSREES 2006-03817 and 2009-35302-05277) and PSC-CUNY.
References
- Schlenke TA, Morales J, Govind S, Clark AG. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathog. 2007;3:1486–1501. doi: 10.1371/journal.ppat.0030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H, Morales J, Govind S. Identification and immuno-electron microscopy localization of p40, a protein component of immunosuppressive virus-like particles from Leptopilina heterotoma, a virulent parasitoid wasp of Drosophila. J. Gen. Virol. 2006;87:461–470. doi: 10.1099/vir.0.81474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueguen G, Rajwani R, Paddibhatla I, Morales J, Govind S. VLPs of Leptopilina boulardi share biogenesis and overall stellate morphology with VLPs of the heterotoma clade. Virus Res. 2011;160:159–165. doi: 10.1016/j.virusres.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddibhatla I, Lee MJ, Kalamarz ME, Ferrarese R, Govind S. Role for sumoylation in systemic inflammation and immune homeostasis in Drosophila larvae. PLoS Pathog. 2010;6:e1001234. doi: 10.1371/journal.ppat.1001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino RP, Carton Y, Govind S. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev. Biol. 2002. pp. 243–265. [DOI] [PubMed]
- Sorrentino RP, Melk JP, Govind S. Genetic analysis of contributions of dorsal group and JAK-Stat92E pathway genes to larval hemocyte concentration and the egg encapsulation response in Drosophila. Genetics. 2004;166:1343–1356. doi: 10.1534/genetics.166.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Crozatier M, Vincent A. Ontogeny of the Drosophila larval hematopoietic organ, hemocyte homeostasis and the dedicated cellular immune response to parasitism. Int. J. Dev. Biol. 2010;54:1117–1125. doi: 10.1387/ijdb.093053jk. [DOI] [PubMed] [Google Scholar]
- Martinez-Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 2007;21:3044–3060. doi: 10.1101/gad.1602607. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Schlegel A, Stainier DY. Lessons from "lower" organisms: what worms, flies, and zebrafish can teach us about human energy metabolism. PLoS Genet. 2007;3:e199. doi: 10.1371/journal.pgen.0030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Jung AC, Criqui M, Lemaitre B, Uttenweiler-Joseph S, Michaut L, Reichhart J, Hoffmann JA. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 1998;17:1217–1227. doi: 10.1093/emboj/17.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner K, Kockel L, Duchek P, Luque CM, Rorth P, Perrimon N. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev. Cell. 2004;7:73–84. doi: 10.1016/j.devcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Tokusumi T, Shoue DA, Tokusumi Y, Stoller JR, Schulz RA. New hemocyte-specific enhancer-reporter transgenes for the analysis of hematopoiesis in Drosophila. Genesis. 2009;47:771–774. doi: 10.1002/dvg.20561. [DOI] [PubMed] [Google Scholar]
- Tokusumi T, Sorrentino RP, Russell M, Ferrarese R, Govind S, Schulz RA. Characterization of a lamellocyte transcriptional enhancer located within the misshapen gene of Drosophila melanogaster. PLoS One. 2009;4:e6429. doi: 10.1371/journal.pone.0006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Chiu H, Oo T, Plaza R, Hoskins S, Govind S. Biogenesis, structure, and immune-suppressive effects of virus-like particles of a Drosophila parasitoid, Leptopilina victoriae. J. Insect Physiol. 2005;51:181–195. doi: 10.1016/j.jinsphys.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Bettencourt R, Asha H, Dearolf C, Ip YT. Hemolymph-dependent and -independent responses in Drosophila immune tissue. J. Cell Biochem. 2004;92:849–863. doi: 10.1002/jcb.20123. [DOI] [PubMed] [Google Scholar]
- Melk JP, Govind S. Developmental analysis of Ganaspis xanthopoda, a larval parasitoid of Drosophila melanogaster. J. Exp. Biol. 1999;202:1885–1896. doi: 10.1242/jeb.202.14.1885. [DOI] [PubMed] [Google Scholar]


