Abstract
Foods that are rich in fat and sugar significantly contribute to over-eating and escalating rates of obesity. The consumption of palatable foods can produce a rewarding effect that strengthens action-outcome associations and reinforces future behavior directed at obtaining these foods. Increasing evidence that the rewarding effects of energy-dense foods play a profound role in overeating and the development of obesity has heightened interest in studying the genes, molecules and neural circuitry that modulate food reward1,2. The rewarding impact of different stimuli can be studied by measuring the willingness to work to obtain them, such as in operant conditioning tasks3. Operant models of food reward measure acquired and voluntary behavioral responses that are directed at obtaining food. A commonly used measure of reward strength is an operant procedure known as the progressive ratio (PR) schedule of reinforcement.4,5 In the PR task, the subject is required to make an increasing number of operant responses for each successive reward. The pioneering study of Hodos (1961) demonstrated that the number of responses made to obtain the last reward, termed the breakpoint, serves as an index of reward strength4. While operant procedures that measure changes in response rate alone cannot separate changes in reward strength from alterations in performance capacity, the breakpoint derived from the PR schedule is a well-validated measure of the rewarding effects of food. The PR task has been used extensively to assess the rewarding impact of drugs of abuse and food in rats (e.g.,6-8), but to a lesser extent in mice9. The increased use of genetically engineered mice and diet-induced obese mouse models has heightened demands for behavioral measures of food reward in mice. In the present article we detail the materials and procedures used to train mice to respond (lever-press) for a high-fat and high-sugar food pellets on a PR schedule of reinforcement. We show that breakpoint response thresholds increase following acute food deprivation and decrease with peripheral administration of the anorectic hormone leptin and thereby validate the use of this food-operant paradigm in mice.
Keywords: Neuroscience, Issue 63, behavioral neuroscience, operant conditioning, food, reward, obesity, leptin, mouse
Protocol
*Most important steps.
1. Equipment and Software
Training and testing is carried out in mouse operant chambers equipped with two ultra-light, retractable levers1 positioned on either side of a food receptacle (see Table 1). A stimulus light is located above each lever and there is a house light at the top of the cage. A pellet dispenser is located outside of the chamber. Each chamber is housed inside a sound-attenuating cubicle with ventilating fan. Chambers are connected to a PC computer with MedPC IV software via a "Smart Control" interface cabinet.
Note: When ultra-light levers are not available it is recommended that nose-poke be used as the operant response for mouse studies. However, with both options available we suggest selection of ultra-light levers as a more reliable measure of motivation given that nose-poke responses can be triggered by exploratory sniffing.
The MedPC programs used to run operant tasks are written in Medstate notation code which a user can learn with the help of the programming manual provided. Programming provides flexibility for the user to modify existing codes or generate unique experimental parameters. Certain codes can be obtained for free from Med Associates "Medstate Notation Repository" http://www.mednr.com. Additional codes may be purchased from Med Associates.
2. Mouse Acclimation and Food Restriction
Upon arrival mice are group-housed for at least 10 days to acclimatize to a reverse 12 h light/dark cycle and provided with ad libitum access to standard chow and water. In our case we use adult C57BL/6 mice purchased from Charles River (St. Constant, QC). All mouse manipulations and behavioral experiments are performed during the dark cycle. All procedures involving the use of animals were approved by the CRCHUM Animal Care Committee.
Mice are subsequently singly-housed and food restricted (70% of their daily food intake) until they reach 90-95% free-feeding body weights in order to facilitate acquisition of lever-press responding. Mice were provided their daily quota of food in the home cage after the termination of session. Upon reaching 5-10% weight loss, the daily food allotted is adjusted to stabilize the lower body weights for the remainder of the training period. Once mice have attained acquisition criteria (see below) they are put back on an ad libitum feeding schedule. Mice regain lost body weight in about 3-4 days.
Mice are handled in the test room for 3 consecutive days prior to the first training session. The day prior to the start of training 10-15 high-fat and high-sugar (HFHS) pellets are placed into the home cage to prevent the potential influence of food neophobia on operant performance. We use 20 mg HFHS, dust-free, precision food pellets (14 mg also available) containing 48.9% Kcal as fat (Bio Serv, Frenchtown, NJ).
3. Operant Training
Mice are initially trained to press the lever on a fixed ratio (FR)-1 reinforcement schedule whereby a single lever press elicits the delivery of a food pellet to the receptacle. Only one lever is designated as "active" (triggering delivery of food reward) and the allocation of right and left levers is counterbalanced between mice. It is important to add at least a 5 second timeout (TO) to the FR1 schedule (FR1/TO-5), during which additional lever-pressing does not result in the delivery of a food pellet. This TO period allows time for mice to consume the food pellet, however longer time outs may be used if the experimenter feels it is necessary for their mouse model. Each FR training session lasts 1 hour or when 50 pellets have been delivered.
*Acquisition Criteria: The criteria used to determine acquisition of food-maintained operant responding includes: (1) a minimum number of active responses and rewards earned; (2) a measure of discrimination between active and inactive levers, and (3) between-session performance stability. Mice exhibiting discrimination of ≥3:1 for the active versus inactive lever and obtaining ≥ 20 rewards per session over three consecutive sessions are considered to achieve acquisition criteria10. We have observed that the majority (~75%) of C57BL6 mice require about 7-10 days of training to achieve acquisition criteria. Mice that do not reach acquisition criteria by this time undergo further training for an additional 5-7 days. We exclude mice from testing (~5% of cases) when there is no progress by the extra 5th training day. It is important to keep in mind that impaired operant acquisition may be an outcome of certain pharmacological and genetic interventions and should therefore be documented.
Following three successive sessions of obtaining ≥ 20 pellets, the schedule is increased to FR5/TO-5 seconds in which 5 active lever presses trigger the delivery of the food pellet. Training on the FR5 schedule lasts three days.
*The mice are then trained in the progressive ratio (PR) schedule of reinforcement. The response ratio schedule during PR testing can be calculated as per Richardson and Roberts (1996)11 using the following formula (rounded to the nearest integer): = [5e (R*0.2)] - 5 where R is equal to the number of food rewards already earned plus 1 (i.e., next reinforcer). Thus, the number of responses required to earn a food reward follow the order: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95 and so on. The final ratio completed is the breakpoint.
A PR session lasts a maximum of 1 hour. Failure to press the lever in any 10 min period results in termination of the session. Performance on the PR schedule of reinforcement is considered stable when the number of rewards earned in a 1 hour session deviates by ≤10% for at least 3 consecutive days.
4. Progressive Ratio Testing and Validation
To validate food-maintained operant responding in the PR task, one can assess breakpoint responding following manipulations known to modulate food reward. A relatively easy test to carry out is food deprivation which will increase the motivational state of the mouse for food and thereby increase breakpoints. Mice must first be tested in the PR task during a period of ad libitum feeding until stable, baseline performance is achieved. The day after the last "baseline" day food is removed from the home cage and mice are then tested in the PR task 24 hours after the start of the fast.
We also investigated whether peripheral administration of the anorectic hormone leptin would have the opposite effect on breakpoint thresholds. Leptin (A.F. Parlow, National Hormone and Peptide Program, NIDDK) was dissolved in sterile PBS. PR testing was carried out following an IP injection of PBS on one day and then following injection of leptin (5 mg/kg) the following day. Leptin was injected one hour prior to PR testing. Breakpoints were compared using a paired t-test (GraphPad Prism).
5. Representative Results
When testing the ability of food deprivation to modulate breakpoint responding on a PR schedule of reinforcement, we expect to significantly increase breakpoints as shown in Figure 1. In this experiment a 24 h period of food deprivation produced a ~3.6-fold increase in breakpoints as compared to those obtained in the free-feeding baseline state thereby suggesting that food deprivation increases the rewarding effects of food. In contrast, peripheral administration of the anorectic hormone leptin (5 mg/kg, IP) one hour prior to testing should decrease breakpoint responding as shown in Figure 2.
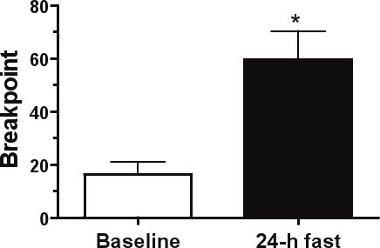 Figure 1. Fasting increases the rewarding effects of food. A 24 h fast significantly increased breakpoints on a progressive ratio (PR) schedule of reinforcement in mice (n=4). Mean±SEM; *p<.05.
Figure 1. Fasting increases the rewarding effects of food. A 24 h fast significantly increased breakpoints on a progressive ratio (PR) schedule of reinforcement in mice (n=4). Mean±SEM; *p<.05.
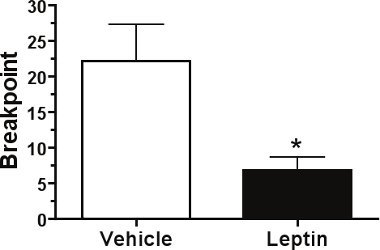 Figure 2. Leptin decreases food reward in mice. Peripheral leptin administration (5 mg/kg) decreased PR breakpoint response thresholds in mice (n=4). Mean±SEM; *p<0.05.
Figure 2. Leptin decreases food reward in mice. Peripheral leptin administration (5 mg/kg) decreased PR breakpoint response thresholds in mice (n=4). Mean±SEM; *p<0.05.
Discussion
Operant conditioning tasks provide an effective means to evaluate changes in the motivational properties of food. The experimental procedures detailed in the present article permit training mice to lever press for food rewards and stably respond on a PR schedule of reinforcement.
Measuring the amount of food consumed does not provide the same readout as effort-based response measures since not all factors that affect food intake modulate the rewarding effects of food. For example, depleting utilizable glucose by peripheral 2-deoxyglucose administration in rats increases the amount of freely available food consumed but fails to alter break points in a PR task for food12. PR breakpoint responding can also reflect changes in the quality of food rewards. For example, break points are positively correlated with sucrose concentration demonstrating that motivation for sucrose increases in a manner that follows sweet taste8. Here we demonstrate that 24h food deprivation substantially increases PR breakpoints for HFHS food in mice. In contrast, we found that peripheral administration of the anorectic hormone leptin significantly decreases breakpoints for HFHS food in mice. These findings are consistent with previously reported actions of food deprivation and leptin on reward thresholds in rats12-14.
When using this protocol it is important to keep in mind that acquisition of food-maintained operant responding is significantly enhanced by regular training (every 1-2 days) and by increasing the motivational state of the mouse by implementing a food restriction regimen. In addition, ensuring that mice exhibit relatively stable responding on one reinforcement schedule before moving on to more complex schedules (e.g, FR1 to FR5) is key to minimizing variable performance during testing. Despite the application of these steps, it is not unusual to obtain some variability especially between different mice. For this reason, it is highly recommended to use a within-subjects (repeated measures) design when possible to remove across subject variability. Although within-subject testing is not possible for investigating stable genetic modifications (i.e., knockout versus control mice) it can be used to examine the influence of drugs, lesions or acute genetic (e.g., optogenetic) manipulations.
Obesity and obesity-related illnesses are a growing problem that has increased the urgency to identify the molecules and neural pathways involved. Accumulating evidence highlights the important role of brain reward circuitry in food craving, increased caloric intake and weight gain. The PR task is a valuable tool for measuring the impact of neurobiological, genetic or pharmacological manipulations on the rewarding effects of food.
Disclosures
Production and Free Access of this video-article is sponsored by Med Associates, Inc.
Acknowledgments
This project was supported by grants from the Natural Sciences and Engineering Research Council of Canada (355881) and the Canadian Foundation for Innovation.
References
- Fulton S. Appetite and reward. Front. Neuroendocrinol. 2010;31:85–103. doi: 10.1016/j.yfrne.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. International journal of Obesity. 2009;33(Suppl 2):S8–S13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF. The Behavior of Organisms: An Experimental Analysis. Appelton-Century; 1938. [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. J. Exp. Anal. Behav. 1963;6:387–392. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998. pp. 139–169. [DOI] [PubMed]
- Glass MJ, O'Hare E, Cleary JP, Billington CJ, Levine AS. The effect of naloxone on food-motivated behavior in the obese Zucker rat. Psychopharmacology (Berl) 1999;141:378–384. doi: 10.1007/s002130050847. [DOI] [PubMed] [Google Scholar]
- Brennan K, Roberts DC, Anisman H, Merali Z. Individual differences in sucrose consumption in the rat: motivational and neurochemical correlates of hedonia. Psychopharmacology (Berl) 2001;157:269–276. doi: 10.1007/s002130100805. [DOI] [PubMed] [Google Scholar]
- Vaughan C, Moore M, Haskell-Luevano C, Rowland NE. Food motivated behavior of melanocortin-4 receptor knockout mice under a progressive ratio schedule. Peptides. 2006;27:2829–2835. doi: 10.1016/j.peptides.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Haluk DM, Wickman K. Evaluation of study design variables and their impact on food-maintained operant responding in mice. Behav. Brain Res. 2010;207:394–401. doi: 10.1016/j.bbr.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Jewett DC, Cleary J, Levine AS, Schaal DW, Thompson T. Effects of neuropeptide Y, insulin, 2-deoxyglucose, and food deprivation on food-motivated behavior. Psychopharmacology (Berl) 1995;120:267–271. doi: 10.1007/BF02311173. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol. Behav. 2006;89:611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]


