Abstract
In this article, we demonstrate assays to study thermal nociception in Drosophila larvae. One assay involves spatially-restricted (local) stimulation of thermal nociceptors1,2 while the second involves a wholesale (global) activation of most or all such neurons3. Together, these techniques allow visualization and quantification of the behavioral functions of Drosophila nociceptive sensory neurons.
The Drosophila larva is an established model system to study thermal nociception, a sensory response to potentially harmful temperatures that is evolutionarily conserved across species1,2. The advantages of Drosophila for such studies are the relative simplicity of its nervous system and the sophistication of the genetic techniques that can be used to dissect the molecular basis of the underlying biology4-6 In Drosophila, as in all metazoans, the response to noxious thermal stimuli generally involves a "nocifensive" aversive withdrawal to the presented stimulus7. Such stimuli are detected through free nerve endings or nociceptors and the amplitude of the organismal response depends on the number of nociceptors receiving the noxious stimulus8. In Drosophila, it is the class IV dendritic arborization sensory neurons that detect noxious thermal and mechanical stimuli9 in addition to their recently discovered role as photoreceptors10. These neurons, which have been very well studied at the developmental level, arborize over the barrier epidermal sheet and make contacts with nearly all epidermal cells11,12. The single axon of each class IV neuron projects into the ventral nerve cord of the central nervous system11 where they may connect to second-order neurons that project to the brain.
Under baseline conditions, nociceptive sensory neurons will not fire until a relatively high threshold is reached. The assays described here allow the investigator to quantify baseline behavioral responses or, presumably, the sensitization that ensues following tissue damage. Each assay provokes distinct but related locomotory behavioral responses to noxious thermal stimuli and permits the researcher to visualize and quantify various aspects of thermal nociception in Drosophila larvae. The assays can be applied to larvae of desired genotypes or to larvae raised under different environmental conditions that might impact nociception. Since thermal nociception is conserved across species, the findings gleaned from genetic dissection in Drosophila will likely inform our understanding of thermal nociception in other species, including vertebrates.
Keywords: Neuroscience, Issue 63, Drosophila sensory neurons, thermal nociception, nociceptive sensitization, tissue damage, fly behavioral response, dendritic arborization neurons, allodynia, hyperalgesia, behavioral assay
Protocol
A typical outline of the experimental steps for preparing normal or UV-sensitized larvae for the two thermal nociception assays is shown in Figure 1.
1. Preparation of Larvae
Male and female progeny larvae of a desired genotype can be tested directly. Alternatively, a genetic cross can be set up to obtain progeny larvae of a defined genotype of interest. Set up crosses in bottles containing fly food using 20-30 virgin females and 15-20 males.
5 days after egg lay, harvest and clean early third instar larvae by scooping out the mushy fly food and gently straining with running water through a 630 μm (pore size) mesh. Transfer the early third instar larvae (~3-4 mm in length along the anteroposterior axis) to a small moist pad of food to prevent starvation and dessication. If nociceptive sensitization is to be tested, follow the subsequent steps 2.1- 2.11. If larvae are to be tested in the absence of tissue damage, proceed directly to step 3.1.
2. Etherization and UV Irradiation
- Construct an etherization chamber.
- Cut the bottom off a 1.5 ml microcentrifuge tube.
- With a hot metal spatula melt the cut edge of the microcentrifuge tube.
- Affix a fine mesh to the melted tube surface and allow to cool. The etherization chamber will allow entry of ether fumes but prevent larval escape.
Use forceps or a paint brush to gently place 10 early third instar larvae inside a home-made etherization chamber.
Place larvae along the inside of the lid and close.
Note: The next two steps should be performed in a fume hood as ether is a potentially explosive chemical and its fumes can anesthetize a human as well as a larva.
Place the etherization chamber containing larvae inside a Coplin jar containing a 10 ml beaker carrying a cotton ball soaked with ~1.5 ml of diethyl ether.
Screw on the cap of the Coplin jar to expose the larvae to ether fumes for 2 to 2.5 minutes. Note that longer etherization times may adversely affect larval behavior or survival.
Following etherization, remove the etherization chamber from the Coplin jar/beaker, giving a few seconds in the hood for ether fumes to dissipate. You may now move from the hood back to the lab.
Use a water squirt bottle to gently rinse larvae from the etherization chamber into a small Petri dish.
Use forceps and a Kimwipe to blot anesthetized larvae dry and gently affix them dorsal side up on a strip of double-sided scotch tape fastened to a 3" x 1" glass microscope slide.
Place the slide on the bottom surface of a UV irradiation chamber and expose the larvae to UV light for 6 seconds at 20 mJ/cm2 intensity.
Immerse the slide in water to gently float the larvae off of the double-sided scotch tape.
Use forceps or a paintbrush to gently place the irradiated larvae in a 15 x 45 mm one dram glass culture vial containing ~ 1.0 ml of fly food where they can recover for a variable period of time (8 -24 hours) at 25 °C or other desired culture temperature before re-harvesting for thermal nociception assays.
3. Local Heat Probe Assay
We deliver a noxious thermal stimulus to an individual larval body segment using a custom-built thermal probe manufactured by Pro-Dev Engineering (see Table of materials). Although this probe has optimal design features (a small metal tip of ~ .07 mm2 area and the ability to precisely maintain a set point temperature from 23 °C to 65 °C) in principle any tool with a small tip that can be heated to a defined temperature for a period of up to 20 seconds should suffice. The probe tip is used to stimulate early 3rd instar larvae precisely on the dorsal midline at abdominal segment A4 (see Figure 2). In response to this thermal stimulus, larvae will generally exhibit an aversive withdrawal behavior of rolling laterally by 360 degrees or more. This behavior is distinct from their light touch response to a non-noxious room temperature metal probe which generally involves a brief pause in their locomotory activity13.
Protocol for the heat probe assay:
Pre-set temperature of the thermal probe to desired set point.
Use a paintbrush or forceps to gently transfer an individual larva onto a flat platform (we usually use a small piece of vinyl cut from a binder) on which the larvae will be subsequently stimulated. The larva should be covered by a thin film of water before contact with the thermal probe. The film of water covering the larva should be as little as possible and completely cover the larva, while ensuring that the larva is not dry when touching the vinyl.
Gently press the probe tip against the larva at segment A4 applying light pressure with the tip at about a 45° angle between the probe and the surface of the larva (see Figure 2). The pressure should cause a slight indentation on the surface of the larva and will usually be sufficient to prevent locomotion. If the larva continues to move, apply slightly more pressure and it will usually stop. Do not record data from larvae that move beyond the field of view or for which constant probe contact cannot be achieved until response or the 20 s cutoff.
Continue stimulating the larva until a withdrawal response is exhibited or until the 20 second cutoff is reached, whichever occurs first. Responding larvae typically first show a preliminary behavior of lifting the head and tail. This is usually followed by the withdrawal behavior of rolling at least 360 degrees. Only a complete roll of 360 degrees is scored as nocifensive behavior (the preliminary head or tail raise is not).
Once the withdrawal behavior is initiated release contact with the probe and record the latency or time to withdrawal. If no withdrawal behavior is observed within 20 seconds, then the larva is a non-responder. The responders can be further divided into 2 categories. If the withdrawal behavior is shown within 5 seconds, then the larva is a fast-responder. If the withdrawal behavior is shown between 5-20 seconds, then the larva is a slow-responder (see Figure 1 in Babcock, et al. 2009)1.
4. Global Heat Plate Assay
The heat plate assay was designed to measure thermal nociception in Drosophila larvae when the whole animal is confronted with a noxious thermal stimulus. Individual mid 3rd instar larvae are placed in an 80 μl drop of water on a 60 x 15 mm Petri dish - see Figure 3 for schematic and photos of the assay setup. The Petri dish is then placed onto a solid heating block (referred to as the "heat plate"). As the temperature of the water drop rises, the larva exhibits a series of five stereotyped behaviors we have termed head thrash, roll, whip, seizure, and paralysis. As these behaviors are to some extent a function of the setpoint temperature of the heat plate and the volume of water in which the larva is immersed we present here what we have found to be the optimum conditions (95 °C heat plate, 80 μl drop of water) for observing all 5 of the behaviors with minimal overlap between them.
Protocol for heat plate assay:
Position the fiber optic light guides to ensure that there is adequate high contrast lighting for viewing the larva. Use a test larva if necessary. The light power should be on low to avoid ambient heat on the larva to be tested.
Place the heating block into the heat plate with flat surface up and place the heat plate onto the microscope base. Turn on the heat plate to the "High" position, allow approximately 15 minutes for the temperature to stabilize, and adjust accordingly to achieve a surface temperature of 95 °C.
Measure 80 μl of distilled water and place the water drop in the middle of the 60 x 15 mm polystyrene Petri dish with a micropipettor.
Use forceps to gently place a clean mid 3rd instar larva into the middle of the water drop. Try to transfer the larva with the minimal possible volume of water so that the drop to be heated stays as close as possible to the original 80 μl.
Gently place the Petri dish containing the water drop and the larva onto the solid heating block on the heat plate. Quickly adjust the location of the dish so the entire larva and drop of water are in view. See Figure 3 and videos 1-6.
Start the timer at the moment the Petri dish is placed onto the solid heating block of the heat plate and record the time of onset of each behavior. If desired, the behaviors can also be video recorded - See Videos 1-5 for representative examples of each behavior. Leica software version 3.7 was used for recording the movies, recording mode was "Continuous", camera resolution was 3 Megapixels (we used 0.63x zoom with which the calculated image resolution is 10 μm/pixel) and frames/ second were 44.5.
5. Representative Results
Local Heat Probe Assay:
Upon contact with the thermal probe, a larva typically shows a preliminary behavior of lifting its head and tail. Typically the lifting of the head is seen first followed by lifting of the tail. A few seconds after this preliminary behavior the larva usually starts rolling laterally which we refer to as the "aversive withdrawal behavior." The time after which the preliminary behavior or the withdrawal behavior is shown may vary by temperature or genetic background. In our initial study we measured the percentage of larvae exhibiting aversive withdrawal at different probe set point temperatures and found that 48 °C was the lowest temperature at which all larvae responded fast (< 5 s). Here, we report that there is a ceiling to the larval thermal nociception response (Figure 4A). 100% fast responders are observed up to a probe temperature of 52 °C. However, at 54 °C and higher, 90 % or more of the larvae fail to respond even after 20 s of contact. These larvae do continue moving following the 20 s cutoff.
As noted previously1, the categories of withdrawal behavior at each temperature can be plotted and compared statistically. Given that this is a behavioral assay there is some variability from larva to larva and between individual users. To account for this we usually measure 3 sets of 30 larvae per test condition. In addition to the simple categorization of withdrawal latency that we have previously reported, we report here that the amplitude (number of rolls or time spent in aversive withdrawal behavior) can also be measured (See Figure 4). Surprisingly, there appears to be an inverse relationship between the input temperature and the robustness of the response because lower temperatures appear to provoke a higher number of rolls (Figure 4B) and more time spent in aversive withdrawal (data not shown). This may indicate that the duration of exposure to a noxious temperature may be the main determinant of robustness.
Heat Plate Assay:
Five stereotyped locomotory behaviors are observed upon transfer of the larva immersed in water to the heat plate. These are described below and shown in the video along with the typical locomotory behavior of an unheated larva in water. The average latencies at which these behaviors are observed are shown in Figure 5A, as are the average water drop temperatures measured for each latency. Under the optimal assay conditions presented here the percentage of larvae showing each distinct behavior ranged from 77 to 100 % (Figure 5B) although occasionally the rolling and whipping behaviors are omitted, overlap, or occur in reverse order. The observed behaviors, in chronological order, are described as follows and can be watched in the video at the times indicated below:
Normal movement in absence of heat: Peristaltic locomotion accompanied by searching head movements. See Video from 6:27.
Head Thrash: Larva moves head quickly in a forward or lateral motion. This motion is similar to their normal locomotion in water (see Video 1) but occurs more jerkily and persistently. See Video from 6:40.
Roll: Larva rolls laterally at least a full 360°. See Video from 6:50. This can occur a variable number of times and sometimes involves incomplete rolls. For the purposes of scoring the behavior we only count full 360° rolls. This behavior is the most similar to that seen with local application of the heat probe.
Whip: Larva exhibits rapid contraction-release movements along the anteroposterior axis that bring the head very briefly close to the tail. See Video from 7:11. Whipping is often observed in quick succession to or at the same time as rolling.
Seizure: Larva stretches out along the anteroposterior axis and exhibits a high frequency whole-body shaking behavior without bending. See Video from 7:25.
Paralysis: Larva ceases movement. In some larvae this is permanent whereas others then exhibit a low frequency pulsation movement. See Video from 7:36.
These data suggest that the temperature of the water drop and the latency to onset of the five characteristic behaviors observed upon global heating of the larva/water drop are highly correlated.
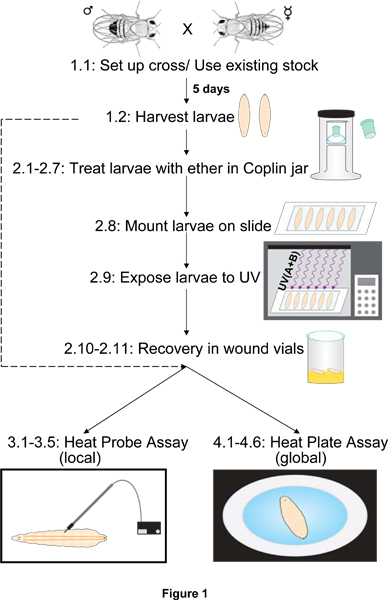 Figure 1. Outline of experimental steps for preparation and assay of larvae. Prior to assaying nociception with the heat probe or the heat plate, larvae derived from a particular stock or genetic cross are harvested and cleaned of food. If nociceptive sensitization (as opposed to baseline nociception) is to be assessed, harvesting is followed by etherization (exposure to ether), mounting, UV irradiation, and a period of recovery on fly food. Harvested or recovered larvae are then subjected to noxious test temperatures using either the heat probe (local) or the heat plate (global) assay. Numbers refer to sections of the methods text that describe the step(s) shown.
Figure 1. Outline of experimental steps for preparation and assay of larvae. Prior to assaying nociception with the heat probe or the heat plate, larvae derived from a particular stock or genetic cross are harvested and cleaned of food. If nociceptive sensitization (as opposed to baseline nociception) is to be assessed, harvesting is followed by etherization (exposure to ether), mounting, UV irradiation, and a period of recovery on fly food. Harvested or recovered larvae are then subjected to noxious test temperatures using either the heat probe (local) or the heat plate (global) assay. Numbers refer to sections of the methods text that describe the step(s) shown.
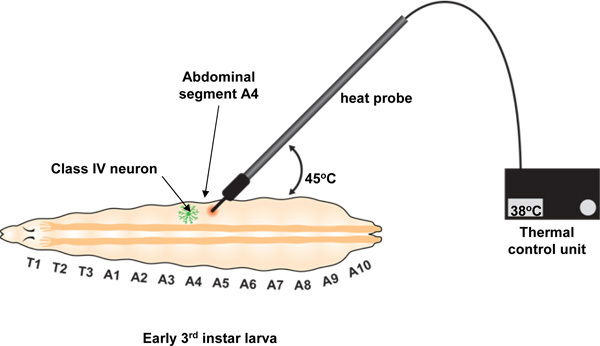 Figure 2. Experimental set up for local heat probe assay. The heat probe is controlled by a thermal control unit which is used to set and maintain the temperature of the probe. The probe is held perpendicular to the anteroposterior axis and used to stimulate the larva at an angle of 45° to the horizontal. Probe contact is made specifically at abdominal segment A4 as shown. The user must maintain this contact with gentle pressure up until the 20 second cutoff or until the rolling behavior commences. If the temperature is perceived as noxious, the larva will show an aversive withdrawal behavior characterized by at least one 360° roll. The number of rolls can be single or multiple (See Figure 4B).
Figure 2. Experimental set up for local heat probe assay. The heat probe is controlled by a thermal control unit which is used to set and maintain the temperature of the probe. The probe is held perpendicular to the anteroposterior axis and used to stimulate the larva at an angle of 45° to the horizontal. Probe contact is made specifically at abdominal segment A4 as shown. The user must maintain this contact with gentle pressure up until the 20 second cutoff or until the rolling behavior commences. If the temperature is perceived as noxious, the larva will show an aversive withdrawal behavior characterized by at least one 360° roll. The number of rolls can be single or multiple (See Figure 4B).
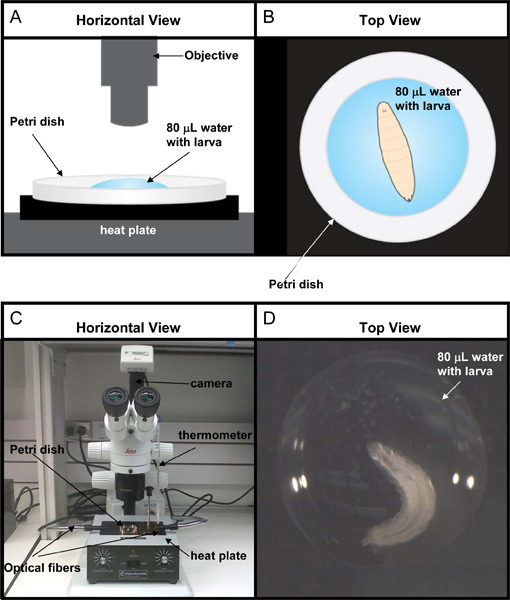 Figure 3. Experimental set-up for heat plate assay. (A) Cartoon of a horizontal view of the 60 x 15 mm Petri dish containing one mid 3rd instar larva in a 80 μl water drop. (B) Cartoon of a top view of the larva placed in the middle of the water drop as viewed through the microscope. (C) Photograph of a horizontal view of the workstation for this assay. (D) Photograph of the larva as viewed through the microscope.
Figure 3. Experimental set-up for heat plate assay. (A) Cartoon of a horizontal view of the 60 x 15 mm Petri dish containing one mid 3rd instar larva in a 80 μl water drop. (B) Cartoon of a top view of the larva placed in the middle of the water drop as viewed through the microscope. (C) Photograph of a horizontal view of the workstation for this assay. (D) Photograph of the larva as viewed through the microscope.
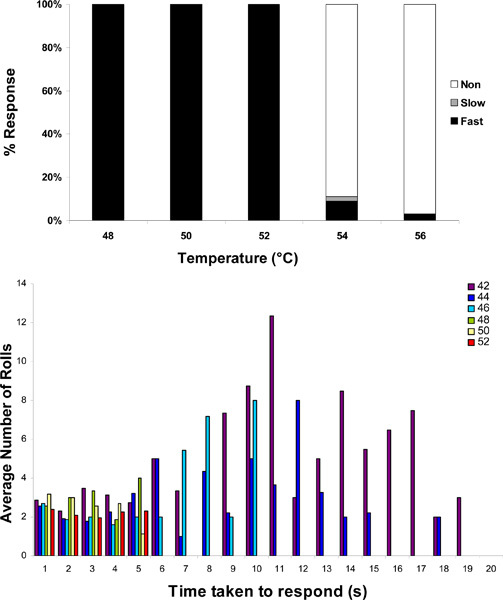 Figure 4. Quantification of behavioral response using the heat probe assay. (A) Plot of the percent of responders belonging to each category (fast-, slow-, and non-responders) versus temperature. (B) Plot of the latency to aversive withdrawal behavior versus the number of rolls each larva exhibited at four different test temperatures of increasing noxiousness (42 °C, 44 °C, 46 °C, 48 °C, 50 °C, and 52 °C).
Figure 4. Quantification of behavioral response using the heat probe assay. (A) Plot of the percent of responders belonging to each category (fast-, slow-, and non-responders) versus temperature. (B) Plot of the latency to aversive withdrawal behavior versus the number of rolls each larva exhibited at four different test temperatures of increasing noxiousness (42 °C, 44 °C, 46 °C, 48 °C, 50 °C, and 52 °C).
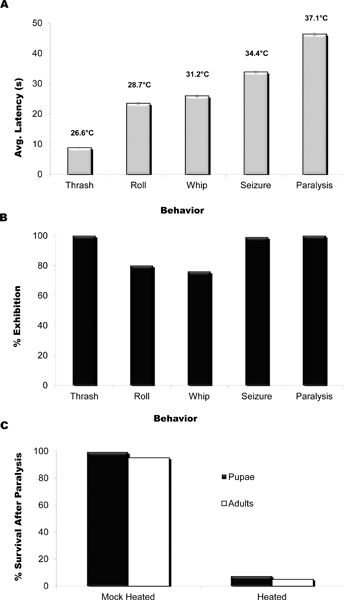 Figure 5. Heat plate assay: Latency and Temperature vs. Behavior. (A) Latency and average water drop temperature to each behavioral response under the optimal conditions of 95 °C (hot plate surface temperature) and 80 μl drop of water (n = 150). Note that each behavior is observed within a particular time interval. Water drop temperatures were measured using a thermocouple inserted into either the top or bottom of the drop (n = 10 drops for each location) and these measurements were averaged. (B) Percent of larvae exhibiting each behavioral response under optimal assay conditions. Thrashing, seizing, and paralysis are observed almost 100 % of the time while rolling and whipping are observed 77 and 80 % of the time, respectively. At lower surface temperature set points seizure and paralysis are not observed within 200 s and at higher set points rolling and whipping can be skipped or may occur simultaneously. n = 150. (C) Percentage of larvae that survive following initiation of the paralysis behavior. Larvae were heated until beginning paralysis and then removed to standard culture conditions to recover. Mock-treated larvae received equivalent treatment except for heat exposure. Formation of pupae and viable adults were quantified on days 7-13. n = 120.
Figure 5. Heat plate assay: Latency and Temperature vs. Behavior. (A) Latency and average water drop temperature to each behavioral response under the optimal conditions of 95 °C (hot plate surface temperature) and 80 μl drop of water (n = 150). Note that each behavior is observed within a particular time interval. Water drop temperatures were measured using a thermocouple inserted into either the top or bottom of the drop (n = 10 drops for each location) and these measurements were averaged. (B) Percent of larvae exhibiting each behavioral response under optimal assay conditions. Thrashing, seizing, and paralysis are observed almost 100 % of the time while rolling and whipping are observed 77 and 80 % of the time, respectively. At lower surface temperature set points seizure and paralysis are not observed within 200 s and at higher set points rolling and whipping can be skipped or may occur simultaneously. n = 150. (C) Percentage of larvae that survive following initiation of the paralysis behavior. Larvae were heated until beginning paralysis and then removed to standard culture conditions to recover. Mock-treated larvae received equivalent treatment except for heat exposure. Formation of pupae and viable adults were quantified on days 7-13. n = 120.
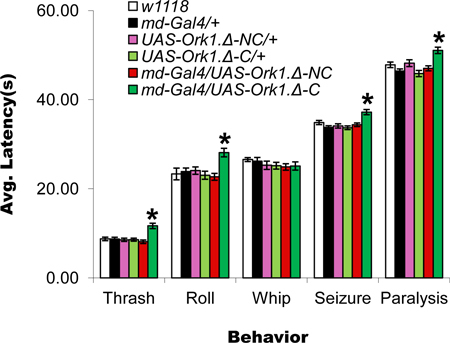 Figure 6.Inactivation of larval nociceptive sensory neurons increases behavioral response latencies. Plot of the latency to each behavior for the indicated genotypes: w1118, Gal4109(2)80 = md-Gal4/+, UAS-Ork1.Δ-C/+, UAS-Ork1.Δ-NC /+, md-Gal4/UAS-Ork1.Δ-C, and md-Gal4/UAS-Ork1.Δ-NC. md-Gal4 drives expression of UAS-regulated transgenes in all four classes of multidendritic peripheral sensory neurons; UAS-Ork1.Δ-C14 expresses a modified version of the Drosophila open rectifier K+ channel required for synaptic transmission, and UAS-Ork1.Δ-NC14 expresses a further modified version of this same channel that does not interfere with synaptic transmission. Note that only larvae bearing both the md-Gal4 driver6 and the UAS-Ork1.Δ-C transgenes show increased latencies for four of the five observed behaviors (thrash, roll, seizure, and paralysis). Note also that because of the increased latency to rolling these animals whip before they roll. Asterisk denotes p < 0.05 by Student's t-test. n = 30 larvae per genotype.
Figure 6.Inactivation of larval nociceptive sensory neurons increases behavioral response latencies. Plot of the latency to each behavior for the indicated genotypes: w1118, Gal4109(2)80 = md-Gal4/+, UAS-Ork1.Δ-C/+, UAS-Ork1.Δ-NC /+, md-Gal4/UAS-Ork1.Δ-C, and md-Gal4/UAS-Ork1.Δ-NC. md-Gal4 drives expression of UAS-regulated transgenes in all four classes of multidendritic peripheral sensory neurons; UAS-Ork1.Δ-C14 expresses a modified version of the Drosophila open rectifier K+ channel required for synaptic transmission, and UAS-Ork1.Δ-NC14 expresses a further modified version of this same channel that does not interfere with synaptic transmission. Note that only larvae bearing both the md-Gal4 driver6 and the UAS-Ork1.Δ-C transgenes show increased latencies for four of the five observed behaviors (thrash, roll, seizure, and paralysis). Note also that because of the increased latency to rolling these animals whip before they roll. Asterisk denotes p < 0.05 by Student's t-test. n = 30 larvae per genotype.
Supplemental Video 1.Click here to watch video.
Supplemental Video 2.Click here to watch video.
Supplemental Video 3.Click here to watch video.
Supplemental Video 4.Click here to watch video.
Supplemental Video 5.Click here to watch video.
Supplemental Video 6.Click here to watch video.
Discussion
The assays described here can be used to qualitatively and quantitatively assess larvae of different genotypes for responsiveness to noxious thermal stimuli. A main feature of the heat probe assay is that the stimulus is given only at a single locus. This presumably leads to firing of only a small subset of class IV neurons- the ones in the segment contacted by the probe and perhaps those in immediately adjacent segments11. Because of the local stimulation, the heat probe assay mimics the common sensory experience of detecting a noxious stimulus that is localized to a particular body region- such as a hand contacting a hot stove. A disadvantage of the heat probe assay is that it has some user-to-user variability that can likely be attributed to three factors: i) the pressure with which the user applies the probe to the larva, ii) the precise location of the probe on the larva relative to the underlying nociceptive neurons, and, iii) the precise angle at which the probe contacts the surface of the larva.
We previously reported a quantitative strategy of categorizing larvae into non-responders, slow responders, and fast responders based on their withdrawal latency to a given temperature1. Here we report on larval responsiveness to even higher temperatures. Interestingly, we find that there is a ceiling to larval thermal nociceptive responses and that this ceiling lies between 52 and 54 °C. This may indicate that larvae do not possess a transient receptor potential (TRP) channel capable of gating at temperatures higher than 52 °C. Alternatively, it could suggest that the neurons or muscles used to initiate or carry out the motor response become damaged before they can even function in aversive withdrawal. We also report a different analysis of the amplitude of the withdrawal response- using either the number of rolls as an indicator of the "robustness" of the response. Naively, one would expect that these parameters would increase with increasing temperature or time of stimulation. Surprisingly, we find that this is not the case. Larvae stimulated for a longer time at a temperature at the low end of the noxious range (42 °C) show more rolls and more time spent rolling than larvae probed at higher temperatures (48-52 °C). This suggests that within the noxious temperature window it is primarily the duration of exposure that determines the amplitude of the response. Since larvae exposed to highly noxious temperatures (48-52 °C) respond on average very quickly, they do not exhibit as many rolls as larvae exposed to a less noxious temperature for a longer length of time. The analysis of response amplitude reported here adds another quantitative dimension along which different genotypes or environmental manipulations can be compared.
A main feature of the heat plate assay is that it involves a global exposure to the noxious heat. As such, it is more akin to the animal sitting in a heating cauldron than touching a hot stove. Although it is not clear when a larva might experience a globally noxious stimulus in the wild, in the lab the behavioral responses to this global exposure are more complex than those observed upon local stimulation. A strength of the heat plate assay, also noted by others3, is that it has little user-to-user variability since touching the larva is not a component of the protocol. The only substantial variance seems to be in defining when each behavior commences and this can be minimized with repeated viewing/familiarity. An interesting difference between the assays is the temperatures at which the aversive behaviors commence. These are much lower in the heat plate assay than with the heat probe. The preliminary behavior exhibited by larvae contacted with the heat probe (head and tail raise) may be a correlate of the head thrash observed at ~27 °C in the heat plate assay. It is possible that this response reflects "discomfort" more than "pain". We have not observed a correlate of whipping, seizure, and paralysis even at high (up to 48°C) temperatures in the heat probe assay and it may be that a critical mass of sensory neuron firing from more than one region of the body is needed to bring on these behaviors. Interestingly, the seizure and paralysis behaviors are observed at temperatures (~34 – 37 °C) below the bottom end of the nociceptive threshold observed with the heat probe indicating that global stimulation may involve summing of neuronal responses that are insufficient to trigger behavior with local application of the heat probe. That these temperatures are actually perceived as noxious to the larvae is supported by the observation that larvae that begin the paralysis behavior and are subsequently allowed to recover on fly food do not in most cases survive (Figure 5C). Further supporting the argument that the heat plate assay is reading out nociceptive responses is the fact that blocking synaptic transmission in known nociceptive sensory neurons increases the latency of most of the observed behaviors (Figure 6). The observation that there is no increase in latency for the higher-temperature whipping behavior suggests that other sensory neurons that do not express md-Gal4 may be required for this behavior.
In sum, both assays involve exposing an individual larva to a noxious thermal stimulus of defined temperature - the hot tip of a small metal probe in the local assay and immersion in a drop of rapidly heating water in the global assay. Behavioral responses of Drosophila larvae of varying genetic backgrounds and/or exposed to varying environmental conditions (for instance plus or minus tissue damage), can be studied and quantified using these assays. Ultimately, results from these assays will help us better understand genetic networks controlling nociception in Drosophila and other related species.
Disclosures
We have nothing to disclose.
Acknowledgments
We thank Christian Landry for heat probe design, Daniel Babcock for developing the larval heat probe assay, Sean Sweeney for suggesting the heat plate assay, the Bloomington Drosophila Stock Center for fly stocks, and Galko lab members for critical reading of the manuscript. This work was supported by NIH R01 NS069828 to MJG and an NIH MARC U-STAR Training Grant (T34GM079088 to the University of Houston-Downtown Scholars Academy) for minority access to research careers (AVG).
References
- Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr. Biol. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WD, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Oswald M, Rymarczyk B, Chatters A, Sweeney S. A novel thermosensitive escape behavior in Drosophila larvae. Fly (Austin) 2011;5:17810. doi: 10.4161/fly.5.4.17810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Moore AW, Jan LY, Jan YN. hamlet, a binary genetic switch between single- and multiple- dendrite neuron morphology. Science. 2002;297:1355–1358. doi: 10.1126/science.1072387. [DOI] [PubMed] [Google Scholar]
- Gao FB, Brenman JE, Jan LY, Jan YN. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 1999;13:2549–2561. doi: 10.1101/gad.13.19.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Illich PA, Weeks JC, Lewin MR. Defensive responses of larval Manduca sexta and their sensitization by noxious stimuli in the laboratory and field. J. Exp. Biol. 2001;204:457–469. doi: 10.1242/jeb.204.3.457. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Hwang RY. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB. Projections of Drosophila multidendritic neurons in the central nervous system: links with peripheral dendrite morphology. Development. 2007;134:55–64. doi: 10.1242/dev.02666. [DOI] [PubMed] [Google Scholar]
- Sweeney NT, Li W, Gao FB. Genetic manipulation of single neurons in vivo reveals specific roles of flamingo in neuronal morphogenesis. Dev. Biol. 2002;247:76–88. doi: 10.1006/dbio.2002.0702. [DOI] [PubMed] [Google Scholar]
- Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]


