Abstract
Background: The pathogenic factors that account for the development of diabetes condition in Chinese women with polycystic ovary syndrome (PCOS) remain elusive. Aim: To clarify the pathogenic features by evaluating the levels of insulin sensitivity and β cell function in these women with PCOS, either separately or by using of a disposition indexes (DIs). Methods: Cross-sectional study involving 137 Chinese women with PCOS and 123 normal women were examined by anthropometry, lipid profile, sex hormone, high-sensitivity C reactive protein, oral glucose tolerance tests and insulin tolerance tests. Results: After controlling for BMI status, the Matsuda Index was significantly lower in women with PCOS in comparison to those of normal women (p<0.000). The early phase of insulin secretion (insulinogenic index) remained significantly lower in lean women with PCOS(LP) than those of both lean and obese women of control group (p=0.007, and p = 0.01, respectively). The mean HOMA-F values were significantly lower (p =0.045) in obese women with PCOS (OP) than those of BMI-matched women. Further, all DIs derived from non-fasting state indexes in women with PCOS were significantly lower than those of BMI-matched control women (p<0.001 for all). Lastly, DIs derived from fasting states indexes in OP were significantly lower than those of LP. Conclusion: Early impaired β cell function was detected in both LP and OP. However, more serious primary defect in insulin action was detected in LP compared to OP. These findings imply that early screening and intervention for PCOS would be therapeutic for Chinese women.
Keywords: Polycystic ovary syndrome, insulin resistance, early phase secretion of insulin
Introduction
Polycystic ovary syndrome (PCOS) is known to affect 6-10% of reproductive-aged women. It is also a common cause for menstrual dysfunction, infertility, and hirsutism [1]. About 30% of women with PCOS had impaired glucose tolerance (IGT) in China [2]. Approximately 20% of women with PCOS were observed, by OGTT screening, to have IGT in USA [3]. Taken together these findings suggest that this subgroup of patients is at risk in developing type 2 diabetes.
Insulin resistance (IR) and hyperinsulinemia play a significant role in the predisposition to diabetes in PCOS [4]. A substantial portion of IR women with PCOS develop IGT or diabetes. However, the precise mechanism responsible for the IR intrinsic to the syndrome remains unclear. Some investigators showed not only IR but also a primary defect in beta cell function (βF) in women in the early stage of PCOS [5-7]. Others were concerned about the increased insulin response to compensate a peripheral defect in insulin action [8,9] or inactivated acute insulin secretion [10]. Although the risk factors in type 2 diabetes are similar among ethnically diverse populations, the ethnic differences in insulin sensitivity (IS) and βF do exist among groups at high risk for type 2 diabetes [11]. A recent study showed that diabetes and prediabetes are highly prevalent in the general population among Chinese adults [12]. Evidence also showed that the Chinese obese women with lack of compensatory increase in βF, are at higher risk for type 2 diabetes than the non-Hispanic whites [13]. The findings of Dunaif et al [14] suggested that the impaired insulin activity in women with PCOS might differ from that seen in type 2 diabetes or in obese women without the classical features of PCOS. Therefore, an important question was raised whether other pathogenic factors could account for the development of IGT or diabetes in Chinese women with PCOS. We hypothesized that the lean women with PCOS were affiliated with the more serious primary defect in insulin activity in comparison to the obese women with PCOS. We further speculated that this defect could be detected or prevented early on in ethnic-specific type 2 diabetes in Chinese women with PCOS.
With regard to the methods in determining the IS in humans, the hyperinsulinemic euglycemic “glucose clamp” is the most reliable one which directly measures the whole body glucose disposal. On the other hand, “glucose clamp” is also known to be labor intensive, technically demanding, as well as time consuming, making it less ideal for use in a large epidemiological and clinical studies. Alternatively a number of simple surrogate indices of insulin sensitivity/resistance derived from OGTT or fasting blood insulin and glucose concentration have been developed [15]. In this study we evaluated IS and βF in Chinese women both with and without PCOS by using the surrogate indices derived from OGTT and estimated the women with PCOS in terms of IS and/or βF either separately or with the tool of disposition indexes (DIs).
Materials and methods
A total of 260 patients were included in this study. They were recruited from the Outpatient Department of Endocrinology and Gynecology of Shanghai Renji Hospital between 2007 and 2009. Of these patients, 137 women were with PCOS, 59 lean [lean PCOS (LP)] [body mass index (BMI) <25kg/m2], and 78 obese PCOS (OP) [BMI ≥25kg/m2]. The control group composed of 123 volunteers, between 16 and 35 years of age, who were age- and weightmatched. Of this group 91 were lean (LC) [ BMI <25kg/m2 ], and 32 obese(OC) [ BMI ≥25kg/m2].
The PCOS diagnosis was based on the Rotterdam Criteria (2003) and obesity was defined as those with a BMI of ≥25kg/m2 according to the 2000 WHO-WPR criteria [16]. All PCOS women enrolled in this study were without a family history of type 2 diabetes. IGT was diagnosed in four patients according to the World health organization criteria. All control women were without family history of PCOS and type 2 diabetes and had regular menstrual cycles (<35 days) and normal androgen levels. In addition, women had normal thyroid function and prolactin levels. Any late-onset nonclassic congenital hyperplasia would be excluded if a basal 17-α hydroxyprogesterone less than 300 ng/dl was detected [17]. Women with other known chronic diseases, or on oral contraceptives or other drugs known to alter glucose and insulin metabolism within the last 3 months were also excluded from the study. All women were evaluated by transvaginal ultrasonography to define ovarian morphology [18].Control subjects were screened for medical history, physical examination, laboratory evaluation and transvaginal ultrasound.
All study evaluations and procedures were conducted in accordance with the guidelines of Helsinki Declaration on human experimentation. The study was approved by the ethics committee of Shanghai Renji hospital and all subjects provided written informed consent.
Anthropometric measurements
The height and weight of each subject wearing light clothing were measured to the nearest 0.1cm and 0.1kg, respectively, using a digital scale and stadiometer. BMI was calculated as body weight (kg) divided by height (m) squared. Waist circumference (WC) and hip circumference (HC) were measured by a single individual. WC was determined by measuring the circumference at the narrowest point between the lower border of the rib cage and the iliac crest. HC was determined by measuring the circumference at the level of the symphysis pubis and the greatest gluteal protuberance. The waist-to-hip ratio (WHR) was then calculated by dividing the WC by the HC.
Oral glucose tolerance test
All women underwent a standard OGTT with 75 g of glucose. The measurement for women with PCOS was at original diagnosis of PCOS before they use any therapy for PCOS. After 08:00h overnight fasting, blood samples were drawn for the determination of glucose and insulin before the glucose load, and they were then drawn again at 30, 60, 120, and 180 min (marked as Gx, and Ix, where G is glucose and I is insulin). All participants showed a fasting plasma glucose <7.0 mmol/L.
Laboratory analysis
All laboratory evaluations were performed at 08:00h after an overnight fast during the early follicular phase (days 2-5) of a spontaneous menstrual cycle, except in subjects with amenorrhoea >3 months who were examined randomly. Analysis of high-sensitivity C-reactive protein (hsCRP) was performed using immunonephelometric methods and a BN-II analyzer (Dade Behring, Deerfield, Germany). The inter-and intra-assay coefficients of variation were 4.9% and 6.8%,respectively.Competitive electrochemiluminescence immunoassays on the Elecsys autoanalyzer 2010 (Roche Diagnostics, Indianapolis,IN) were used to quantify serum total testosterone, LH and FSH. SHBG levels were measured by chemiluminescent immunoassay (Elecsys autoanalyzer 2010, Roche Diagnostics) validated for plasma SHBG [19]. The coefficient of variation for SHBG using this methodology was 6%. Free testosterone values were calculated based on total testosterone and SHBG levels according to the method outlined by Vermeulen et al [20] assuming an albumin concentration of 4g/dl (http://www.issam.ch/freetesto.htm). Plasma glucose was determined using the glucose oxidase methodology. Insulin levels were measured by radioimmunoassay (RIA). The intra-assay CV of insulin and steroid hormone assays were 5.5% and <10%, respectively.
Calculations
1) Insulin resistance was calculated by the homeostasis model assessment.
Insulin resistance index (HOMA-IR): fasting insulin (mU/ml) ×fasting plasma glucose (mmol/l)/22.5 [21]. FIRI: fasting insulin (mU/ml) ×fasting plasma glucose (mmol/l)/25 [22]; Suma I was calculated by the sum of fasting insulin, 60min insulin and 120 insulin (mU/ml) [23].
2) The whole body insulin sensitive was calculated by Matsuda index. Matsuda index =10 000/square root of [(fasting glucose x fasting insulin) x (mean glucose x mean insulin during OGTT)] [24]. FGIR: fasting plasma glucose (mg/ dl) / fasting insulin (mU/ml).
3) Islet beta cell function was evaluated by homeostasis model assessment βcell function (HOMA-F): 20×fasting insulin (mU/ml)/(fasting plasma glucose (mmol/l)-3.5) [21] and Insulinogenic index( ΔI30/ΔG30 )(mIU/mmol): (I30-I0)/(G30-G0), which can reflect the early-phase insulin secretion [24].
4) The glucose and insulin response to glucose were also assessed by calculating the area under the curve during OGTT performance for glucose (AUCglu) and insulin (AUCins), using the trapezoidal rule [25].
5) The Deposition index (DI) was calculated to estimate the β-cell response relative to the prevailing insulin sensitivity [26], ie: DI=ΔI30/ΔG30 (mIU/mmol)/HOMA-IR: (I30-I0)/(G30-G0)/ HOMA-IR.
Statistical analysis
All statistical analyses were performed using SPSS version 17.0 (Statistical Package for the Social Sciences, USA). Distributions of continuous variables were tested for normality by use of the Kolmogorov–Smirnov test. Part of results not normally distributed, based on the normal quartile plot, was log-transformed for all statistical analyses and reported back-transformed in their original units. All results were reported as means, or geometric means for log-transformed variables, with 95% CIs. P-values <0.05 were considered significant.
For continuous variables, subgroup means were compared with one-way analysis of variance (ANOVA) testing considering four subgroups of women: lean control, obese control, lean PCOS and obese PCOS. Tukey’s honestly significantly difference (HSD) test was performed. Levene statistic was used for test homogeneity of variance. For nonhomogeneous data, we used the robust Kruskal-Wallis test followed by Mann-Whitney U tests.
The relationships between insulin sensitivity and insulin secretion and hormonal and metabolic variables were evaluated by Spearman’s correlation tests. A forward, stepwise, multiple linear regression analyses were performed.
Results
Clinical and biochemical patterns of target subjects
The clinical characteristics and biochemical variables for the four groups of women according to PCOS and BMI status are summarized in Table 1. As expected, there were no significant differences in BMI in LC compared with the LP group or in OC compared with the OP group. Furthermore, no significant differences in WC or WHR between control and PCOS women according to BMI status were found. Regarding mean total and free testosterone values were also significantly different between groups (P<0.000 for both). Total testosterone values where highest in the LP and lowest in the OC. Free testosterone values were significantly higher in the OP group compared with the other three groups. Inversely, SHBG levels where highest in the LC group and lowest in the OP group. Furthermore, mean LH values and LH/FSH ratio were also significantly higher in PCOS group than control group. With respect to lipid profile, a significantly higher LDL cholesterol (P=0.018) and lower HDL (P=0.015) cholesterol were found in OP than OC group. Furthermore, mean hsCRP values were significantly higher (P<0.000) in the obese groups (OC and OP) compared with the lean groups (LC and LP). HsCRP values were significantly higher in the PCOS group compared with BMI-matched control groups (P<0.0001 for both). However, significant differences in hsCRP values between OC and LP women were not observed.
Table 1.
Clinical and biochemical variables in women with PCOS and normal control women according to BMI status
| Variable | Lean control | Obese control | Lean PCOS | Obese PCOS | P-value |
|---|---|---|---|---|---|
| n | 91 | 32 | 59 | 78 | N/A |
| Age (yrs) | 27.3 (26.2-28.3) | 27.8 (27.1-28.3) | 25.4 (24.1-26.8) | 26.6 (24.8-28.4) | .067 |
| BMI (kg/m2) | 20.7 (20.3-21.4)a,c | 29.5 (28.2-30.7)b,d | 20.8 (20.3-21.4)a,c | 29.8 (28.9-30.6)b,d | .0001 |
| Waist circumference (cm) | 71.4 (70.3-72.5)a,c | 93.4 (89.5-97.2)b,d | 72.9 (70.9-74.8)a,c | 93.7 (91.4-96.1)b,d | .0001 |
| WHR | 0.80 (0.79-0.81)a,c | 0.90 (0.87-0.92)b,d | 0.81 (0.79-0.83)a,c | 0.89 (0.88-0.91)b,d | .0001 |
| Total cholesterol (mmol/L) | 4.25 (4.06-4.45)c | 4.66 (4.44-4.87)c | 4.59 (4.31-4.87) | 4.90 (4.68-5.11)d | .0001 |
| HDL cholesterol (mmol/L) | 1.57 (1.49-1.65)c,a | 1.40 (1.29-1.51)c,d | 1.52 (1.43-1.61)c | 1.20 (1.15-1.25)b,d,a | .0001 |
| LDL cholesterol (mmol/L) | 2.35 (2.24-2.47)c | 2.64 (2.41-2.86)c | 2.62 (2.45-2.80)c | 3.06 (2.86-3.25)b,d,a | .0001 |
| Triglycerides (mmol/L) | 0.93 (0.80-1.06)c,a | 1.60 (1.30-1.91)d | 1.23 (1.01-1.45) | 1.55 (1.41-1.69)d | .0001 |
| LH (IU/L) | 7.54 (6.20-8.88)b | 7.69 (5.46-9.92)b | 12.70 (10.54-14.87)a,d | 9.92 (8.77-11.08) | .0001 |
| LH/FSH | 0.99 (0.86-1.11)b,c | 0.94 (0.71-1.16)b,d | 1.57 (1.33-1.81)d,c | 1.37 (1.21-1.53)d | .0001 |
| Total testosterone (nmol/L) | 1.80 (1.66-1.93)b,c | 1.64 (1.35-1.93)b,c | 2.72 (2.73-3.05)a,d | 2.63 (2.38-2.88)a,d | .0001 |
| SHBG (nmol/L) | 73.81 (71.65-75.96)a,b,c | 37.73 (32.73-42.74)c,d,b | 44.58 (41.10-48.05)c,d,a | 28.41 (25.88-30.93)a,b,d | .0001 |
| Free testosterone* (pmol/L) | 19.32 (17.81-20.83)b,c | 28.55 (23.69-33.42)b,c | 44.95 (37.83-52.06)a,c,d | 55.94 (49.88-61.99)a,b,d | .0001 |
| hsCRP (mg/L) | 1.08 (0.93-1.23)a,b,c | 3.37 (2.69-4.04)c,d | 2.80 (2.24-3.36)c,d | 4.56 (3.94-5.18)a,b,d | .0001 |
Data are means (95% CI) unless noted.
Geometric means.
BMI - body mass index, HDL - high density lipoprotein, hsCRP - high sensitive chronic reactive protein, HOMA-IRhomeostasis model assessment - insulin resistance index, SHBG- Sex hormone-binding globulin; P-value for ANOVA for difference between groups.
- <0.05 compared to obese control;
- <0.05 compared with lean PCOS;
- <0.05 compared with obese PCOS;
- <0.05 compared to lean control.
To convert cholesterol to mg/dL, divide by 0.02586; to convert triglycerides to mg/dL, divide by 0.01129; to covert glucose to mg/dL; divide by 0.05551; to convert total testosterone to ng/ml, divide by 3.467; to convert free testosterone to pg/ml, divide by 3.467.
OGTT, insulin secretion, and Insulin sensitivity of target subjects
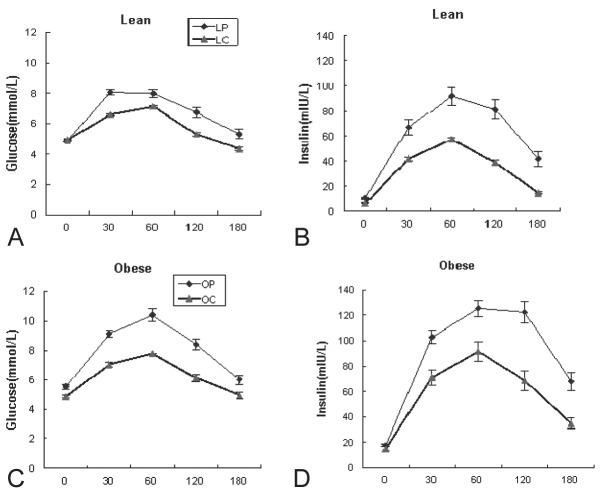
As shown in Figure 1 and Table 2, mean glucose values at 30min (P<0.000), 120 min (P=0.001) and 3h (P=0.027) were significantly higher in LP than in LC group during the OGTT (Figure 1A). Meanwhile, mean insulin values at five points were significantly higher in LP than in LC group (Figure 1C). There were significant difference between LP and LC groups concerning AUCgluc(P=0.03) and AUCins(P<0.000). However, mean fasting glucose (P=.001) and mean glucose values at 30min, 60min and 120min (P<.000 for all) were significantly higher in OP than in OC group (Figure 1B). Mean insulin values at 30min (P<.000), 60min (P=.002), 120min (P<.000) and 180 min (P=.001) but not fasting (P=0.244) , were significantly higher in OP than in OC group (Figure 1D). There were significant difference between OP and OC groups concerning AUCgluc and AUCins (P<0.000 for both).
Figure 1.
Glucose and insulin concentrations (mean ± SE) during oral tolerance test (OGTT) in the subjects of the study (polycystic ovarian syndrome, ♦; controls,▲). **P< 0.01,*P< 0.05; P-value for ANOVA for difference compared with BMI-matched control. LC- lean control women (n=91); LP - lean PCOS women (n=59), OC - obese control women (n=32); OP - obese PCOS women (n=78).
Table 2.
Differences in Insulin Resistance, Insulin sensitivity and beta cell function between PCOS and normal control women according to BMI status
| Index | Lean control (n=91) | Obese control (n=32) | Lean PCOS (n=59) | Obese PCOS(n=78) | P-value |
|---|---|---|---|---|---|
| ΔI30/ΔG30 # | 24.61 (21.68-27.55)b | 27.86 (21.36-34.36)b | 20.83 (15.79-25.88)a,c,d | 28.21 (23.46-32.97)b | .014 |
| HOMA-F* | 117.54 (90.53-144.54)c | 262.54 (199.76-325.32)c | 183.74 (149.22-218.25) | 213.39 (180.45-246.32)a,d | .000 |
| HOMA-IR* | 1.38 (1.24-1.52)a,b,c | 3.27 (2.56-3.98)c,d | 2.36 (1.91-2.81)d,c | 4.5 (3.79-5.22)b,a,d | .0001 |
| FIRI* | 1.24 (1.12-1.37)a,b,c | 2.94 (2.30-3.58)d | 2.56 (1.61-3.52)d,c | 4.05 (3.41-4.70)b,a,d | .000 |
| Suma I# | 102.57 (96.05-109.09)a,b,c | 175.08 (144.29-205.86)c,d | 188.93 (155.33-212.53)d,c | 265.29 (236.57-294.02)b,a,d | .000 |
| Matsuda Index* | 151.81 (141.4-162.22)a,b,c | 86.22 (65.67-106.78)d,c | 95.53 (81.89-109.17)d,c | 50.59 (44.94-56.24)b,d,a | .000 |
Data are means (95% CI) unless noted.
Geometric means. P-value for ANOVA for difference between groups.
- Due to non-homogeneity variance, Kruskal-Wallis test followed by Mann-Whitney U test was used instead of ANOVA.
- <0.05 compared to obese control;
- <0.05 compared with lean PCOS;
- <0.05 compared with obese PCOS;
- <0.05 compared to lean control.
BMI - body mass index; ΔI30/ΔG30-insulinogenic index; HOMA-F - homeostasis model assessment β cell function; HOMA-IR - homeostasis model assessment insulin resistance index; FIRI – fasting insulin × fasting glucose /25; Suma I – sums of insulin (0min, 60min and 120min).
Regarding the indexes of IR, HOMA-IR was higher in PCOS women than those of BMI matched control subjects (P<0.001). Significant different also were found in other IR indexes (Suma I (P=.000) and FIRI (P<.05)) between LC and LP group. However, significant different was found in SumaI (P=0.000) between OC and OP group. As to the indexes of IS, Matsuda index (P<0.000) was significantly lower in PCOS women than those of BMI matched subjects.
With respect to insulin secretion, there was significant difference in early phase insulin secretion detected by insulinogenic index (P=0.009) between LP and LC groups, the same trend also was found between LP and OC groups (P=.013), but no significant difference was found between OP subjects and OC or LC subjects (P=0.028).Meanwhile, there was significant difference between OP and OC (P=.045) or LC (P=.000) groups concerning βF estimated by HOMA-F. However, there was no significant difference in HOMA-F between LC and LP groups (P>0.05).
DIs derived from fasting and stimulated state parameters of target subjects
As shown in Table 3, the DIs were calculated to estimate the β-cell response relative to the prevailing insulin sensitivity. All of the combinations of products insulin secretion × IS used followed a hyperbolic relationship. DI values were calculated parameters of insulin secretion (ΔI30/ΔG30 and HOMA-F ) combination parameters of IS(1/HOMA-IR, Matsuda index, 1/I120, 1/SumaI and so on). When combinations of ΔI30/ΔG30 and the stimulated state parameters of insulin sensitivity (1/I120, 1/SumaI and Matsuda index) which derived from OGTT were evaluated, significantly lower values of all DIs were found in PCOS than those in BMI-matched control subjects (all P<0.05). However, when combinations of HOMA-F and the fasting state parameters of insulin secretion (1/HOMA-IR, 1/I0 and 1/FIRI) were evaluated, only significantly lower values of DI were found in OP than in LC or LP subjects (all P<.01), with no significant difference between LP and LC groups (all P>0.05). When combinations of ΔI30/ΔG30 and 1/HOMAIR, significantly lower values of DI was found in LP than those in LC subjects (P=.004), but with no significant difference between OP and OC subjects (P=0.999).
Table 3.
Differences in DI between PCOS and normal control women according to BMI status
| Insulin | Control | PCOS | P-value | ||
|---|---|---|---|---|---|
|
|
|
|
|||
| Secretion Sensitivity | Lean (n=91) | Obese(n=32) | Lean (n=59) | Obese (n=78) | |
| ΔI30/ΔG30× 1/HOMA-IR | 19.71 (17.07-22.34)a,b,c | 10.18 (8.01-12.36)d | 12.42 (8.12-16.71)d | 9.8 (7.21-12.39)d | .000 |
| ΔI30/ΔG30× Matsuda | 3099.47 (2680.89-3518.06)b,c | 2897.93 (239.21-3476.64)c | 1851.16 (1236.38-2465.95)d | 1443.32 (1094.23-1792.4)a,d | .000 |
| ΔI30/ΔG30× 1/Suma I | 0.22 (0.20-0.25)b,c | 0.22 (0.18-0.27)b,c | 0.14 (0.09-0.19)a,d | 0.12 (0.09-0.14)a,d | .000 |
| ΔI30/ΔG30× 1/I120 | 0.74 (0.61-0.86)b,c | 0.62 (0.44-0.56)b,c | 0.34 (0.23-0.44)a,d | 0.30 (0.23-0.36)a,d | .000 |
| HOMA-F × 1/HOMA-IR* | 104.44 (81-127.89)c | 74.74 (53.75-95.73) | 93.24 (69.61-116.88)c | 57.73 (49.83-65.63)b,d | .003 |
| HOMA-F × 1/I0* | 20.45 (16.72-24.19)c | 15.64 (12.25-19.04) | 18.66 (14.89-22.42)c | 12.75 (11.40-14.10)b,d | .002 |
| HOMA-F × 1/FIRI* | 116.09 (90.02-142.15)c | 83.05 (59.68-106.43) | 100.58 (74.35-126.81)c | 64.13 (55.36-72.89)b,d | .004 |
DIs were calculated by the combinations of products Insulin secretion and insulin sensitivity. Data are DI means (95% CI).
Log transformed prior to analysis.
- <0.05 compared to obese control;
- <0.05 compared with lean PCOS;
- <0.05 compared with obese PCOS;
- <0.05 compared to lean control.
HOMA-F - homeostasis model assessment - β cell function, ΔI/ΔG30-0 - insulinogenic index, HOMA-IR - homeostasis model assessment -insulin resistance index, FIRI- fasting insulin ×fasting glucose /25, Suma I -sums of insulin (0min, 60min and 120min), I0- fasting Insulin, I120- Insulin at 120min.
Correlation between metabolic parameters and Matsuda index and DI
As shown in Table 4, both DI and Matsuda index had inverse linear relationships with BMI, WC, WHR, free testosterone, triglycerides, total and LDL cholesterol levels (P<0.001 for all) according to all women (n=260). Furthermore, strong positive linear correlation were observed between SHBG levels and both DI and Matsuda index (P<0.001 for both). Strong positive linear correlation was observed between Matsuda index and HDL (P<0.001).However, no correlation was observed between DI and HDL (P=0.09). Finally, Significant inverse linear relationships were also demonstrated between DI and hsCRP and LH/FSH (P<.001 for all), and significant inverse linear relationship was observed between Matsuda index and hsCRP (P<0.001).
Table 4.
Correlation between metabolic parameters and Matsuda index and DI in normal control and PCOS women
| Variable | Matsuda index* | DI(insulinogenic index×1/HOMA-IR)* | ||
|---|---|---|---|---|
|
| ||||
| Correlation | P-value | Correlation | P-value | |
| BMI | -0.598 | .0001 | -0.244 | .0001 |
| WC | -0.414 | .0001 | -0.236 | .0001 |
| WHR | -0.203 | .0001 | -0.203 | .0001 |
| SHBG* | 0.502 | .0001 | 0.424 | .0001 |
| Free testosterone* | -0.429 | .001 | -0.28 | .0001 |
| LH/FSH | -0.12 | 053 | -0.151 | .0001 |
| Total cholesterol | -0.13 | .0001 | -0.213 | .001 |
| HDL cholesterol | 0.422 | .0001 | 0.106 | .096 |
| LDL cholesterol | -0.244 | .001 | -0.25 | .001 |
| Triglycerides* | -0.28 | .001 | -0.298 | .0001 |
| hsCRP* | -0.414 | .0001 | -0.308 | .0001 |
Log transformed prior to analysis.
DI- disposition index, BMI - body mass index, WC- Waist circumference, WHR - waist-to-hip ratio, HDL - high density lipoprotein, LDL - low density lipoprotein, HOMA-IR - homeostasis model assessment - insulin resistance index, hsCRP-high sensitive chronic reactive protein; SHBG - sex hormone binding globulin.
Determinants of DI (insulinogenic index × 1/HOMA-IR)
Using forward, stepwise, linear regression analysis, with DI as the dependent variable and age, free testosterone, hsCRP, PCOS status and BMI as potential independent variables. Considering these variables, only BMI and PCOS status were selected as an independent variable for the final model predicting DI. In the final model for HOMA-IR, the adjusted R2 was 0.091 (P =0.008), and the regression coefficients (β) were -0.021 (P=0.000) for BMI and 0.167 (P=0.008) for PCOS status. Thus, BMI and PCOS status were all independent predictors of DI as calculated by ΔI30/ΔG30 ×1/HOMA-IR.
Discussion
The main finding of this study is the demonstration that impaired βF, as estimated by DI, was an important feature found in women with PCOS. This primary defect in insulin secretion, as estimated by insulinogenic index in lean women with PCOS, is perhaps a more important indicator than those of obese women with PCOS in China. In addition, the decreased DI was related to increased hsCRP and free testosterone in both normal and PCOS women, but independent of both the overall and visceral adiposity. Lastly, our data suggested that insulin resistance in PCOS women is at least partially intrinsic to the syndrome.
Findings from previous studies on βF based on IR or insulin secretion in women with PCOS were wide spread and conflicting. The issue on whether impaired βF posed as a primary defect in women with PCOS was also unsettled. Based on the differences in insulin sensitivity, we now have a better understanding on how the β cells regulate in terms of insulin activation, consistent with a classic feedback loop. The DI has emerged as an important integrated measure of β cell compensation by evaluating insulin secretion in the context of prevailing IS in vivo. Furthermore, the product of IS and βF almost all follows a hyperbolic function in NGT or IGT state [27,28]. Recent clinical reports suggest that DI, as calculated by the product of ΔI30/ΔG30 and 1/I0 was a better predictor for diabetes than eitherΔI30/ΔG30 or 1/fasting insulin alone in subjects with NGT or IFG/IGT [28]. Therefore, we speculated that subtle changes in either of these two inherently connected variables (IR and insulin secretion) would be more pronounced and informative when using a DI as their product.
In this study we demonstrated that all DI values, derived from the stimulated state during OGTT, were significantly lower in PCOS than those in both BMI matched control subjects. These findings suggest that the defect in beta cell compensation for ambient IR, particularly in the stimulated state, had already existed in both lean and obese PCOS subjects. It should be noted that this defect is independent of both overall and visceral adiposity. On the other hand, all DI values in the fasting state were found to be significantly lower in OP than those in LC or LP. It should be pointed out that no significant difference in DI values was found between LP and LC. One possible explanation is the fact that, if we only detected DI derived from the fasting state, we may have omitted the defect of βF in LP. In general our results are in agreement with the work of Ehrmann [6] . In addition, O’Meara and coworkers reported that women with functional ovarian hyperandrogenism have significantly higher basal insulin secretory rates and attenuated secretory responses to meals and suggested that these secretory patterns resemble those of noninsulin-dependent diabetes mellitus more than they do those of simple obesity [29].
In terms of combinations of early stage insulin secretion and1/HOMA-IR, significant lower values of DI were found in LP than those in LC. Mean insulinogenic index values, a stimulated insulin secretion evaluation derived from OGTTs, was also significantly lower in LP than those of LC and OC. These findings clearly indicate that the impairment of insulin secretion in the early stage of disturbed glucose metabolism in PCOS women operated differently from women without PCOS in China. It may also suggest that the primary defect of beta-cell function is the primary pathological mechanism in LC. Further, this defect was not caused by obesity or fat distribution, but rather attributed to PCOS itself. These findings are in part in line with the evidence in literature, showing the early phase of defective insulin secretion and delayed hyperinsulinemia in women with PCOS [5,6]. In contrast, Holte and coworkers reported that the early insulin response to glucose was increased in women with PCOS, not accounted for by insulin resistance, closely associated to the increased androgenicity, and present also at low-normal BMI [8].
While there are ample evidence with regard to insulin resistance in patients with PCOS, data on the βF/insulin secretion are rather limited. Adding to the confusion were the conflicting reports on the change of insulin secretion in patients with PCOS from different laboratories, with results ranging from decreased insulin secretion in PCOS [6], to increased insulin secretion in PCOS [9]. Findings from our study seemed not to support the data from two laboratories [8,9]. The discrepancies may come from a number of possibilities. For instance, the insulin secretion was assessed at different stages (basal vs. post-absorptive) [29]. In addition, few studies made attempts to quantify the insulin secretion with the prevailing levels of IR, which might be another factor in compounding the issue [30]. Even among studies which reportedly made the adjustments, conflicting data prevailed with regard to insulin secretion in PCOS from various laboratories [5,8,31]. Further, the different glucose metabolism state in women with PCOS should be taken into consideration in some studies. In a separate study, NGT adolescents with PCOS had increased insulin secretion whereas those with IGT had decreased insulin secretion [7]. Finally, it cannot be ignored that PCOS women with diabetes family history or a history of gestational diabetes might be easy to find impaired βF [32]. At the same time in different stages of PCOS the heterogeneity must be discerned.
In present study, our results also demonstrate the level of hsCRP, a marker of low-grade chronic inflammation and a potent predictor of cardiovascular event, was significantly higher in women with PCOS in comparison to those of BMI-matched normal women. Interestingly, the levels of DI as calculated by insulinogenic index ×1/HOMA-IR displayed a strong inverse linear relationship with the level of hsCRP in PCOS and control women. In the light of recent evidence in korea women demonstrating that there were close relationships between hsCRP and 2h postprandial insulin level in the lean patients with PCOS [33]. Furthermore, they also showed hsCRP level correlated with other metabolic risk, elevated serum hsCRP levels may represent a plausible explanation for the observed differences in DI between lean PCOS and BMI-matched normal women. However, given the cross-sectional design of our study, causality cannot be established. Additionally, in a forward stepwise, linear regression analysis performed to determine predictors of DI in PCOS and normal women, hsCRP was no longer an independent predictor of DI after controlling for PCOS status and BMI.
Furthermore, we found that decreased DI was associated with increased free testosterone and decreased SHBG in all women, and this association was independent of both overall adiposity and visceral adiposity. However, considering the forward stepwise, linear regression analysis performed to determine predictors of DI in PCOS and normal women, free testosterone and SHBG were no longer an independent predictor of DI.
Several limitations of our study should be considered. In present study, we based the diagnosis of PCOS on the Rotterdam diagnostic criteria, and thus represent a more heterogenous group of women. Hence, appropriately large sample sizes will be needed. Although OGTT is less precise than intravenous tests, may be increasing variability of DIx values. Nevertheless, OGTT is much more physiological than intravenous one, particularly because glucose sensors widespread through gastrointestinal tract may actively participate in insulin secretion and action [34]. Additionally, this study was performed in Asia, thus, we cannot generalize the conclusions to other ethnic populations.
In conclusion, we have demonstrated that impaired βF estimated by DI as another characteristic feature was found in lean and obese PCOS in china. Decreased insulin secretion is probably connected to the pathogenesis of PCOS. Further investigation is necessary to better understand the importance of insulin secretion in PCOS. These findings imply that early screening and intervention for PCOS would be therapeutic for Chinese women.
Acknowledgments
We thank all of the women who participated in the study. We also gratefully acknowledge the assistance of the nursing staff and physicians at Shanghai Renji Hospital and technical assistants who performed the biochemical analyses.
Financial support
This work was supported by the Natural Science Foundation of Shanghai, China (12ZR1417800 to T.T, 2012), Shanghai Science and Technology Development Fund (08411953000 to T.T, 2008).
Conflict of interest statement
The authors have no financial or conflicts of interest to disclose.
References
- 1.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–4011. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 2.Hu W, Hu WH, Qiao J, Wang LN, Zhao CY. Characteristics of abnormal glucose tolerance in patients with polycystic ovary syndrome. Zhonghua Yi Xue Za Zhi. 2009;89:2053–2055. [PubMed] [Google Scholar]
- 3.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90:3236–3242. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implication for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 5.Dunaif A, Finegood DT. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:942–947. doi: 10.1210/jcem.81.3.8772555. [DOI] [PubMed] [Google Scholar]
- 6.Ehrmann DA, Sturis J, Byrne M, Karrison T, Rosenfield RL, Polonsky K. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1995;96:520–527. doi: 10.1172/JCI118064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and ß-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86:66–71. doi: 10.1210/jcem.86.1.7123. [DOI] [PubMed] [Google Scholar]
- 8.Holte J, Bergh T, Berne C, Berglund L, Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab. 1994;78:1052–1058. doi: 10.1210/jcem.78.5.8175959. [DOI] [PubMed] [Google Scholar]
- 9.Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Tapanainen JS. Insulin sensitivity, insulin secretion, and metabolic and hormonal parameters in healthy women and women with polycystic ovarian syndrome. Hum Reprod. 2000;15:1266–1274. doi: 10.1093/humrep/15.6.1266. [DOI] [PubMed] [Google Scholar]
- 10.Gennarelli G, Rovei V, Novi RF, Holte J, Bongioanni F, Revelli A, Pacini G, Cavallo-Perin P, Massobrio M. Preserved insulin sensitivity and beta-cell activity, but decreased glucose effectiveness in normal-weight women with the polycystic ovary syndrome. Clin Endocrinol Metab. 2005;90:3381–3386. doi: 10.1210/jc.2004-1973. [DOI] [PubMed] [Google Scholar]
- 11.Torréns JI, Skurnick J, Davidow AL, Korenman SG, Santoro N, Soto-Greene M, Lasser N, Weiss G. Ethnic differences in insulin sensitivity and beta-cell function in premenopausal or early perimenopausal women without diabetes: the Study of Women’s Health Across the Nation (SWAN) Diabetes Care. 2004;27:354–361. doi: 10.2337/diacare.27.2.354. [DOI] [PubMed] [Google Scholar]
- 12.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J. Prevalence of Diabetes among Men and Women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 13.Ko GT, Chan JC, Cockram CS, Woo J. Prediction of hypertension, diabetes, dyslipidaemia or albuminuria using simple anthropometric indexes in Hong Kong Chinese. Int J Obes Relat Metab Disord. 1999;23:1136–1142. doi: 10.1038/sj.ijo.0801043. [DOI] [PubMed] [Google Scholar]
- 14.Dunaif A, Xia J, Book CB, Schenker E, Tang Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle: A potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest. 1995;96:801–810. doi: 10.1172/JCI118126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization Western Pacific Region, International Association for the Study of Obesity and International Obesity Task Force 2000. Steering Committee. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Melbourne, Australia: Health Communications Australia Pty Limited; 2000. Assessment diagnosis; pp. 15–21. [Google Scholar]
- 17.Lane DE. Polycystic ovary syndrome and its differential diagnosis. Obstet Gynecol Surv. 2006;61:125–135. doi: 10.1097/01.ogx.0000197817.93201.04. [DOI] [PubMed] [Google Scholar]
- 18.Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, Morris DV, Price J, Jacobs HS. Multifollicular ovaries: clinical and endocrine features and response to pusatile gonadotropin releasing hormone. Lancet. 1985;2:1375–1379. doi: 10.1016/s0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- 19.Reynders M, Anckaert E, Schiettecatte J, Smitz J. Evaluation of a new automated electrochemiluminescent sex hormone-binding globulin (SHBG) immunoassay. Clin Chem Lab Med. 2005;43:86–89. doi: 10.1515/CCLM.2005.013. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Duncan MH, Singh BM, Wise PH, Carter G, Alaghband-Zadeh J. A simple measure of insulin resistance. Lancet. 1995;346:120–121. doi: 10.1016/s0140-6736(95)92143-5. [DOI] [PubMed] [Google Scholar]
- 23.Rosolova H, Mayer O Jr, Simon J, Sefrna F. Detection of risk of insulin resistance in the population. Cas Lek Cesk. 1998;137:80–83. [PubMed] [Google Scholar]
- 24.Seltzer HS, Allen EW, Herron AL Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1996;746:323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drivsholm T, Hansen T, Urhammer SA, Palacios RT, Volund A, Borch-Johnsen K, Pedersen OB. Assessment of insulin insensitivity and beta cell function from an oral glucose tolerance test (Abstract) Diabetologia. 1999;42:185. [Google Scholar]
- 26.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte D Jr. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects: evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 27.Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity. 2008;16:1901–1907. doi: 10.1038/oby.2008.307. [DOI] [PubMed] [Google Scholar]
- 28.Utzschneider KM, Prigeon RL, Tong J, Gerchman F, Carr DB, Zraika S, Udayasankar J, Montgomery B, Mari A, Kahn SE. Within-subject variability of measures of β cell function derived from a 2h OGTT: implications for research studies. Diabetologia. 2007;50:2516–2525. doi: 10.1007/s00125-007-0819-5. [DOI] [PubMed] [Google Scholar]
- 29.O’Meara NM, Blackman JD, Ehrmann DA, Barnes RB, Jaspan JB, Rosenfield RL, Polonsky KS. Defects in beta-cell function in functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1993;76:1241–1247. doi: 10.1210/jcem.76.5.8496316. [DOI] [PubMed] [Google Scholar]
- 30.Bergman RN, Finegood DT, Kahn SE. The evolution of β-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest. 2002;32:35–45. doi: 10.1046/j.1365-2362.32.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 31.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, Mc-Neely MJ, Fujimoto WY, Kahn SE. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–341. doi: 10.2337/dc08-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koivunen RM, Juutinen J, Vauhkonen I, Morin-Papunen LC, Ruokonen A, Tapanainen JS. Metabolic and steroidogenic alterations related to increased frequency of polycystic ovaries in women with a history of gestational diabetes. J Clin Endocrinol Metab. 2001;86:2591–2599. doi: 10.1210/jcem.86.6.7612. [DOI] [PubMed] [Google Scholar]
- 33.Kim JW, Han JE, Kim YS, Won HJ, Yoon TK, Lee WS. High sensitivity C-reactive protein and its relationship with impaired glucose regulation in lean patients with polycystic ovary syndrome. Gynecol Endocrinol. 2012;28:259–263. doi: 10.3109/09513590.2011.613967. [DOI] [PubMed] [Google Scholar]
- 34.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]