Abstract
Background: To evaluate the prognostic implication of cancer stem cell markers in pancreac ductal adenocarcinoma (PDAC), the expression of CD133 and nestin were investigated in a series of PDAC patients in relation to the survival rate. Methods: This series included 42 cases of PDAC patients and evaluated the stem cell markers CD133 and nestin expression detected by immunohistochemistry. The presence of immunopositive tumor cells considering intensity and area was evaluated and interpreted in comparison to the patients’ clinicopathological and survival data. Results: Twenty eight cases (66.7%) showed high CD133 expression. The CD133 expression was mainly identified in the apical border of the tumor cell, but aberrant expression in the cytoplasmic or perinuclear location was also noted. High nestin expression in tumor cells were found in only 2 cases, but high nestin expression along perinuerial or stromal region was found in 15 cases (35.7%). There was no correlation between CD133, nestin expression and gemcitabine resistance. Statistically significant difference was found in patient survival in N stage (p=0.007), and CD133 expression (p= 0.014) in univariate analysis. Nestin expression wan not statistically significant, but it was helpful to identify the perineurial invasion. In Cox-regression hazard model stratified by age and sex for multivariable analysis, AJCC stage and CD133 were independent prognostic factors for overall survival. Conclusions: CD133 expression is upregulated in PDAC that is related to poor prognosis, and treatment targeted the CD133 positive cancer/cancer stem cells might be a promising therapeutic strategy for this patients.
Keywords: Pancreatic ductal adenocarcinoma, cancer stem cell, CD133, nestin, prognosis
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is usually detected at an advanced stage and is considered one of the human cancers with the worst prognosis. The poor prognosis of PDAC is related to a combination of late detection and ineffective treatment regimens. Surgical resection is the only known curative treatment for PDAC, but most patients present in an already an inoperable state [1]. Although the first line of current FDA therapy for unresectable pancreatic cancer is gemcitabine monotherapy, PDAC is known to be notoriously chemo-resistant.
Irrespective of the still-ongoing debate about the cell-of-origin in PDAC, increasing evidence suggests that cells with stemness features, also termed cancer stem cell (CSC), exclusively drive pancreatic tumorigenesis in humans [2].
Cancer stem cells in PDAC have been identified a few years ago and it has been characterized using the surface markers, CD44, CD24, EpCAM, CD133, and CXR4 [3,4]. CSC display resistance to classical cytotoxic agents due to several reasons including remains in a quiescent state [5]. Also, the subpopulation of CD133+CXCR4+ CSCs is related to tumor metastasis in pancreatic cancer [4]. Therefore, the identification and subsequent isolation and characterization of CSC from PDAC suggested this population to be highly responsible for the failure of conventional therapy in pancreatic tumors [2].
The process of isolating putative CSC populations from resected tumors is important, but isolated CSC may lose their properties because they are not interacting with the stromal environment. To achieve comprehensive results about the significance of CSC in human tumor tissues, the immunohistochemical analysis may be considered as a proper tool to recapitulate the whole heterogeneity of the resected tumor and mimic its complex network of relationships.
CD133, also known as Prominin-1 in rodents, is a transmembrane glycoprotein and is originally expressed in a subset of stem cells in the hematopoietic system as well as in a few solid tumors of the brain, prostate, colon, and the genitourinary system [6-11]. CD133 is also overexpressed in PDAC tissues and several hepatobiliary carcinoma cell lines [12,13]. CD133+ cancer cells have a cancer stem/progenitor cells and exhibited resistance to radiation, greater ability of invasion, and metastasis in various gastrointestinal malignancies. Moreover, CD133 mRNA expression is an independent prognostic factor for overall survival, as increased levels were detected in the peripheral blood of cancer patients. A few studies have published conflicting data on the expression of this marker for the evaluation of clinical significance in PDAC [11,14,15]. However, these data have shown a divergence arising from different CD133 antibodies, tumor location, and acquisition of representative tumor sample from patient samples.
Nestin was initially described as a marker for neural stem cell [16]. Nestin-positive cells residing in the adult human pancreas could be differentiated into pancreatic endocrine, exocrine, and hepatic cell phenotypes in vitro [17], and share many phenotypic markers with mesenchymal stem cells derived from the bone marrow [18]. Nestin expression in the pancreas has been detected in exocrine and mesenchymal cells, including stellate cells, pericytes, and endothelial cells [19]. Nestin has also been demonstrated in many kinds of pancreatic neoplasia [7,19,20] and recently included pancreatic intraepithelial neoplasias (PanINs), which are putative precursors of PDAC [21]. However, the biological features, clinical significance of nestin expression and relationship to other stem cell markers such as CD133 in PDAS should be more clearly elucidated.
In this study, CD133 and nestin were selected as markers. Here, we compared the expression patterns and clinical relevance of cancer stem cell markers in pancreatic adenocarcinoma.
Materials and methods
Patients and tumor samples
Formalin fixed and paraffin embedded surgical specimens of pancreatic adenocarcinoma (PDAC) were obtained from 42 cases of previously untreated patients according to the WHO classification. All patients were treated at the same surgical department at Hwasun Chonnam University Hospital, Chonnam, Korea, during the period between 2004 and 2009. No patients had received chemotherapy or radiation therapy before surgery. Recurrence-free survival was assessed radiographically by reviewing CT scans of the chest, abdomen, and pelvis which were obtained every 3 months after resection. Overall survival was ascertained by well documented clinical follow-up in each patient’s medical record. Pathology reports were reviewed for important tumor factors that are known to have prognostic values for disease recurrence and survival.
Immunohistochemistry
Immunohistochemistry was adopted and modified from our previous report [22]. Immunostains were performed by the routine avidin-biotin complex (ABC) method. Briefly, representative paraffin blocks were consecutively cut at 4 micron thickness and the tissue sections were collected on aminopropyltriethoxysilane (APTE)-coated slides. Sections were deparaffinized in xylene, treated with 0.3% hydrogen peroxide in methanol for 20 min to block endogeneous peroxidase activity on Sequenza Rack (Shandon, UK). Pretreatment of the tissues for heatinduced epitope retrieval was done for 3 min in a 125°C pressure cooker with 10 mM citrate buffer of pH 6.0. Antibodies for nestin (dilution 1:200; Millipore, Billerica, MA, USA), and CD133 (dilution 1:100; Abcam, Cambridge, UK) were used for immunohistochemical analysis. The streptavidin-horseradish peroxidase (Dako) detection system was then applied. After treatment with 1% avidin-biotin horseradish peroxidase complex for 1 h at room temperature, the immunoreactivities of all the tissue sections were visualized by chromogen reactions with the tissue sections that were initially treated with 0.02% diaminobenzidine (DAB). The sections were counterstained with hematoxylin and mounted in Universal Mount (Research Genetics. USA). Negative controls were treated in a similar manner with the exception of primary antibodies.
Evaluation of CD133, and nestin
All immunostained slides were evaluated independently by a pathologist (Kim HS) and were repeated two times each. The evaluator was blinded to background information. For immunohistochemical results assessment, reactions in the center of acini for CD133, capillary endothelial cell and peripheral nerve for nestin, which were present in all specimens, were used as internal built-in positive controls. Because the expression area and intensity varied along with the morphological heterogeneity of the tumor, [11] the semi-quantitative summation method was used as we did in our previous experiment [22]. The intensity of CD133 staining was scored on a scale of 0 to 3, in which 0 = negative staining, 1 = weakly positive staining, 2 = moderately positive staining, and 3 = strongly positive staining. The staining intensity of the internal control was considered as moderately positive staining and tumor cell expression intensity was compared with internal controls. The extent of staining was scored as 0 (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%), and 4 (76-100%) according to the percentages of the positively stained areas in relation to the total carcinoma area. The sum of the intensity and the extent score was used as the final staining score (0-7) for each antibodies. Tumors having a mean final staining score > 3 by each antibody summation were considered positive.
Statistical analysis
Statistical analysis was performed using the SPSS software program (version 13.0 for Windows; SPSS INC., Chicago, IL). The relationships between CD133, and nestin expression and categorical variables were compared with the Chi-quare test, or Fisher’s exact probability test, when appropriate. Continuous variables were compared with the Mann-Whitney U test. The strength of association between the CD133, and nestin was assessed by the Spearman rank correlation test. The Kaplan-Meier method was used to estimate survival, and differences were analyzed by the log-rank test. The Cox proportional hazard model was used for multivariate analysis of prognostic factors. P < 0.05 was considered to be significant.
Results
There were 28 men and 14 women with a mean age of 64.5 years (range, 45-81 years, median, 64 years). Descriptive statistics of the study are listed in Table 1. Patient follow-up information was available in all cases. The median overall survival, with a median Kaplan-Meier estimated follow-up time of 67 months, from diagnosis until death (mOS) was 11.5 months.
Table 1.
Summary of patient population
| Variable | Number (n) | |
|---|---|---|
| Mean, 64.5 | range, 45~81 years | |
| Age (year) | <65 | 19 |
| ≥65 | 23 | |
| Sex | Male | 28 |
| Female | 14 | |
| T stage | T1 | 3 |
| T2 | 7 | |
| T3 | 28 | |
| T4 | 4 | |
| N stage | N0 | 19 |
| N1 | 17 | |
| N2 | 1 | |
| Stage | I | 8 |
| IIA | 15 | |
| IIB | 15 | |
| III | 4 | |
| IV | 0 | |
| Op. type | Distal Pancreatectomy/Whipple Op. | 21 |
| PPPD | 21 |
In the normal pancreas, CD133 is expressed in the centroacinar epithelium and terminal ductal cells as previously reported. [11,23] In our analysis, 28 cases (66.7%) showed high CD133 expression by considering the intensity and areas. We observed two distinct CD133 staining patterns. In 21 cases with well to moderately differentiated PDAC of CD133 positive cases, CD133 was showed apical membranous staining pattern in the malignant glandular epithelial cells (Figure 1A). However, 7 of 11 cases of poorly differentiated PDAC mainly showed perinuclear/cytoplasmic positivity (Figure 1B). Moreover, in two cases of poorly differentiated PDAC, distinct cytoplasmic globules expressing CD133 were observed where mucus had been retained in the cytoplasm. However, the distribution pattern of CD133 positive cells was heterogeneous and patchy.
Figure 1.
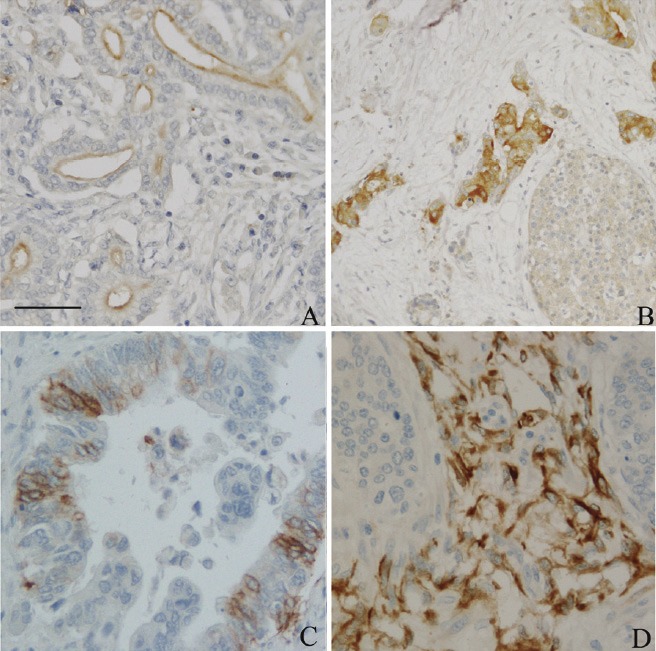
CD133 and nestin expression in human primary pancreatic ductal adenocarcinoma (PDAC) tissues. Paraffin-embedded sections of PDAC with well differentiated (A), and poorly differentiated PDAC (B) were detected immunohistochemical staining with CD133 antibody. Nestin was identified in PDAC tumor glands (C) and stroma (D), respectively. Scale bar in 1A was 50 m.
Nestin expression was easily identified in capillary endothelial cells within the islets in the non-lesional pancreas. Acinar, ductal, and islet cell expressions were not noted. In PDAC, nestin positive cells were identified in all cases of PDAC with various intensity in the positive cell population (Figure 1C). According to our grading system, high nestin expression in our study group was just 4.8% (2 cases). However, in agreement with previous reports, [19] nestin immunoreactivity was easily identified in the nerve bundle with tumor invasion and in the peritumoral small capillary vessels. Interestingly, this stromal nestin positive group had a large bulky immunoreactive region in front of the tumor glands (Figure 1D). This peritumoral/stromal nestin expression was 31.0% (13 of 42 cases) in this study. Also, to compare with CD133 expression patterns, we did not make any significant correlations with nestin expression.
There were no statistically significant differences between CD133 expression and other clinicopathological variables. There was no statistical significance between high nestin including tumor itself or stromal expression and clinicopathological variables and overall survival (Table 2). However, there was a significant association between high CD133 expression and overall survival by the Kaplan-Meier method (p=0.014, Figure 2). In multivariate analysis using the Cox proportional hazard model after controlling for gender and age, only AJCC stage and CD133 expression retained their significance as independent predictors of survival (Table 3).
Table 2.
Relationship between expression of CD133, nestin, and clinicopathological features
| Variable | No (n). | CD133 | P | Nestin (Tumor) | P | Nestin (Stroma) | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Negative 28 (66.7%) | Positive 14 (33.3%) | Negative 2 (4.8%) | Positive 40 (95.2%) | Negative 15 (35.7%) | Positive 27 (64.3%) | ||||||
| Age (yr) | <65 | 19 | 15 | 4 | 0.11 | 1 | 18 | 0.72 | 6 | 14 | 0.32 |
| ≥65 | 23 | 13 | 10 | 1 | 22 | 9 | 13 | ||||
| Sex | M | 28 | 21 | 7 | 0.1 | 1 | 27 | 0.56 | 9 | 20 | 0.32 |
| F | 14 | 7 | 7 | 1 | 13 | 6 | 7 | ||||
| OP type | DP/WO* | 21 | 13 | 8 | 0.42 | 2 | 19 | 0.08 | 9 | 13 | 0.58 |
| PPPD** | 21 | 15 | 6 | 0 | 21 | 6 | 14 | ||||
| Tumor recurrence | No | 6 | 2 | 4 | 0.06 | 0 | 6 | 0.56 | 3 | 4 | 0.64 |
| Yes | 36 | 26 | 10 | 2 | 34 | 12 | 23 | ||||
| Histologic grade | Well to moderate | 31 | 21 | 10 | 0.31 | 2 | 29 | 0.54 | 12 | 19 | 0.28 |
| Poorly | 11 | 7 | 4 | 0 | 11 | 3 | 8 | ||||
DP/WO: distal pancreatomy/Whipple operation;
PPPD: pylorus preserving pancreatico-duodenectomy.
Figure 2.
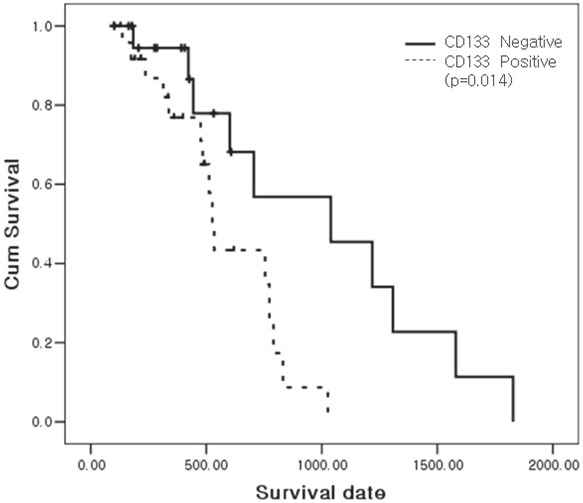
The CD133 expression was correlated significantly with the patients’ overall survival (p=0.014) by the Kaplan-Meier method.
Table 3.
Cox-regression hazard model for multivariable analysis
| Variables | p value | Exp(B) | CI for Exp(B) | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| Age | 0.002 | 1.276 | 1.092 | 1.490 |
| Sex | 0.040 | 0.100 | 0.011 | 0.903 |
| Stage | 0.015 | 1.337 | 0.991 | 1.566 |
| Histologic grade | 0.513 | 0.535 | 0.082 | 3.493 |
| CD133 | 0.022 | 1.445 | 1.012 | 2.214 |
| Nestin | 0.161 | 0.392 | 0.106 | 1.452 |
Discussion
The current knowledge of the biological properties of cancer stem cells (CSCs) is very limited. It is in fact totally unclear whether CSCs are a fraction of cells that are transiently present or actually represent a unique subpopulation of tumor cells that are capable of surviving in hazardous environments for an extended duration [2]. In pancreatic adenocarcinoma (PDAC), CSCs biology has received attention as a possible candidate in relation to prognosis. CD133 and nestin is well established cancer stem cell marker for several human malignant tumors. With respect to PDAC, a few articles have investigated the association of their expressions and clinical importance. We have revealed that the high CD133 expression was directly correlated with clinical outcome in patients with PDAC.
The expression of CD133 along the apical and intraluminal borders was easily observed in well to moderately differentiated PDAC with a varying degree. This finding was similar with previous reports [11,14], and we agreed that CD133 has a role in regulating the formation of lumina and ducts. Immervoll et al. [11] described that cytoplasmic CD133 staining was seen in less than 1% of the malignant epithelial cells and in some non-epithelial malignancies, cytoplasmic positivity was observed either diffusely in the cytoplasm of the majority of tumor cells or as discrete, perinuclear dots in a few tumor cells. This result could be adopted with the localization CD133 was influencing cell polarity, forming lumina, and growth pattern of tumor cells [11,23]. However, in this study, we observed 3 cases of poorly differentiated, and 2 cases of moderately differentiated PDAC which showed aberrant localization of the CD133 expression in the perinuclear or cytoplasm which was poorly differentiated in the PDAC group. To clarify this high aberrant CD133 localization in our study, various in vitro assays should be performed in further evaluation.
CD133 expression, which is also expressed by normal neural stem cells, has been proposed to identify cells at the top of the hierarchy formed by tumor stem cells and their more differentiated progeny, and to be therefore the paramount of pancreatic cancer stem cells. The AC133/AC141 epitopes bound by the widely used monoclonal CD133 antibodies are glycosylated and regulated independently by the much more widespread expression of their mRNAs [24]. The differences between the studies could be due to the differences between antibodies with the antigen retrieval method and the histological techniques [11]. In fact, since most commercially available CD133 antibodies were designed to detect the glycosylated AC133 epitope which was present on their surface, immunohistochemistry using AC133 to detect CD133 is not completely concordant [12,25,26]. These results suggest that further researches should include mRNA expression pattern with protein expression to clarify the significance of CD133 in PDAC.
In our study, CD133 expression was increased in PDAC and it had a statistical significance for univariable (p=0.014) and Cox-regression hazard multivariable models (p=0.022). Actually, the human pancreatic carcinoma cell line with higher level of mRNA expression for CD133 has high resistance to gemcitabine treatment [27,28] via abnormal activation of the Sonic hedgehog (Shh) pathway [29] and mTOR signaling [30]. Although tumor recurrence group showed large populations of high CD133 expression (72.2%), we could not reach a statistical significance between CD133 and gemcitabine resistance in this study. Considering these previous reports and the present data, CD133 may have a potential clinical marker to predict the PDAC patient outcome, but the correlation of CD133 expression and gemcitabine treatment resistance in human PDAC sample may need further investigation with a large population.
The roles of nestin in PDAC have known to include being a potential target for a targettherapy correlated with tumor metastasis, nerve invasion, and one of the CSCs markers in some tumor types [20]. Recently, a combined detection of Nestin/CD133 co-expression was found to possibly benefit in the prediction of aggressive nature of this tumor [31].
Nestin immunoreactivity in PDAC was reported with a wide range. In this study, nestin positive cells were identified in all cases, but high nestin expression group in the tumor gland was very low as detected by Ohike et al. [32] than other previously reported studies [19,20]. These results could be open to conflict. These previous studies just have counted as positive immunoreactivity revealed in tumor cell cytoplasm whether it was strong or not. However, because nestin expression pattern showed heterogeneity in staining intensity and positive cell distribution, we had to adopt the semiquantitative summation method for proper evaluation of the nestin expression pattern such as high and low expression groups. Although Lenz et al. avoided the inclusion of an assessment of staining intensity into the statistical analysis, we have performed the basic scoring for nestin expression and compared to internal controls such as capillary endothelial cells. In contrast to other reports [33], we were able to obtain reproducible and sufficient results from our previous report [22] and applied in this study.
Recently, nestin was found to play important roles in pancreatic cancer cell migration, invasion and metastasis by selectively modulating the expression of actin and cell adhesion molecules in an in vitro study [34]. However, in the present study, we did not find any significant correlations between high nestin expression group and certain clinicopathological findings. Stromal high nestin expression groups had a positive correlation with histological types (well to moderate vs. poorly differentiated group) and N stage, but did not reach a statistical significance (p=0.096, and 0.11, respectively). In fact, nestin expression did not prove to be a valuable prognostic factor in several previous reports [20] but simply correlated with cancer cell nerve invasion, and cancer-positive surgical margins in PDAC [19]. Therefore, further studies are needed to determine why strong and abundant nestin positive stromal cells are accumulated around tumor glands.
In conclusion, our results show that high CD133 expression is associated with prognostically worse pathological variables in PDACs, and tumor recurrence group after gemcitabine treatment show higher CD133 expression. More specific CD133 repressive or inhibitory drugs should be considered in therapeutic interventions to eliminate the cancer stem cells.
Acknowledgement
This study was supported by a grant from the Chonnam National University Hospital Research Institute (Kim HS, CRI09019-1), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Kim HS, 2009-0074754), and Chonnam National University Research Institute of Medical Sciences (Kim JC, 2009).
References
- 1.Goedegebuure P, Mitchem JB, Porembka MR, Tan MC, Belt BA, Wang-Gillam A, Gillanders WE, Hawkins WG, Linehan DC. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets. 2011;11:734–751. doi: 10.2174/156800911796191024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balic A, Dorado J, Alonso-Gomez M, Heeschen C. Stem cells as the root of pancreatic ductal adenocarcinoma. Exp Cell Res. 2011 doi: 10.1016/j.yexcr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 4.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Lonardo E, Hermann PC, Heeschen C. Pancreatic cancer stem cells - update and future perspectives. Mol Oncol. 2010;4:431–442. doi: 10.1016/j.molonc.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 7.Kim KJ, Lee KH, Kim HS, Moon KS, Jung TY, Jung S, Lee MC. The presence of stem cell marker-expressing cells is not prognostically significant in glioblastomas. Neuropathology. 2011;31:494–502. doi: 10.1111/j.1440-1789.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- 8.Missol-Kolka E, Karbanova J, Janich P, Haase M, Fargeas CA, Huttner WB, Corbeil D. Prominin-1 (CD133) is not restricted to stem cells located in the basal compartment of murine and human prostate. Prostate. 2011;71:254–267. doi: 10.1002/pros.21239. [DOI] [PubMed] [Google Scholar]
- 9.Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151–2162. doi: 10.1053/j.gastro.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 10.Liu WH, Li R, Dou KF. Convenient and efficient enrichment of the CD133+ liver cells from rat fetal liver cells as a source of liver stem/progenitor cells. Stem Cell Rev. 2011;7:94–102. doi: 10.1007/s12015-010-9119-4. [DOI] [PubMed] [Google Scholar]
- 11.Immervoll H, Hoem D, Sakariassen PO, Steffensen OJ, Molven A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olempska M, Eisenach PA, Ammerpohl O, Ungefroren H, Fandrich F, Kalthoff H. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat Dis Int. 2007;6:92–97. [PubMed] [Google Scholar]
- 13.Moriyama T, Ohuchida K, Mizumoto K, Cui L, Ikenaga N, Sato N, Tanaka M. Enhanced cell migration and invasion of CD133+ pancreatic cancer cells cocultured with pancreatic stromal cells. Cancer. 2010;116:3357–3368. doi: 10.1002/cncr.25121. [DOI] [PubMed] [Google Scholar]
- 14.Maeda S, Shinchi H, Kurahara H, Mataki Y, Maemura K, Sato M, Natsugoe S, Aikou T, Takao S. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer. 2008;98:1389–1397. doi: 10.1038/sj.bjc.6604307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vizio B, Mauri FA, Prati A, Trivedi P, Giacobino A, Novarino A, Satolli MA, Ciuffreda L, Camandona M, Gasparri G, Bellone G. Comparative evaluation of cancer stem cell markers in normal pancreas. Oncol Rep. 2012;27:69–76. doi: 10.3892/or.2011.1461. [DOI] [PubMed] [Google Scholar]
- 16.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 17.Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Hong TP, Hu J, Liu YN, Wu YH, Li LS. Nestin-positive progenitor cells isolated from human fetal pancreas have phenotypic markers identical to mesenchymal stem cells. World J Gastroenterol. 2005;11:2906–2911. doi: 10.3748/wjg.v11.i19.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamoto M, Ishiwata T, Cho K, Uchida E, Korc M, Naito Z, Tajiri T. Nestin expression correlates with nerve and retroperitoneal tissue invasion in pancreatic cancer. Hum Pathol. 2009;40:189–198. doi: 10.1016/j.humpath.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenz J, Karasek P, Jarkovsky J, Muckova K, Dite P, Kala Z, Veselska R, Hermanova M. Clinicopathological correlations of nestin expression in surgically resectable pancreatic cancer including an analysis of perineural invasion. J Gastrointestin Liver Dis. 2011;20:389–396. [PubMed] [Google Scholar]
- 21.Carriere C, Seeley ES, Goetze T, Longnecker DS, Korc M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc Natl Acad Sci USA. 2007;104:4437–4442. doi: 10.1073/pnas.0701117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HS, Youm HR, Lee JS, Min KW, Chung JH, Park CS. Correlation between cyclooxygenase-2 and tumor angiogenesis in non-small cell lung cancer. Lung Cancer. 2003;42:163–170. doi: 10.1016/s0169-5002(03)00290-3. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu K, Itoh T, Shimizu M, Ku Y, Hori Y. CD133 expression pattern distinguishes intraductal papillary mucinous neoplasms from ductal adenocarcinomas of the pancreas. Pancreas. 2009;38:e207–214. doi: 10.1097/MPA.0b013e3181bb5037. [DOI] [PubMed] [Google Scholar]
- 24.Schittenhelm J, Simon P, Harter PN, Zachskorn C, Schlaszus H, Rottger F, Winkels M, Weller M, Meyermann R, Mittelbronn M. CD133 expression is associated with small round blue cell tumour morphology in human central nervous system neoplasms. Histopathology. 2011;58:739–749. doi: 10.1111/j.1365-2559.2011.03801.x. [DOI] [PubMed] [Google Scholar]
- 25.Corbeil D, Roper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2:82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- 26.Han CW, Min BW, Kim Y, Jeong EH, Park CS, Woo YJ, Kim HS, Lee MC. Immunohistochemical analysis of developmental neural antigen expression in the balloon cells of focal cortical dysplasia. J Clin Neurosci. 2011;18:114–118. doi: 10.1016/j.jocn.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Wang YH, Li F, Luo B, Wang XH, Sun HC, Liu S, Cui YQ, Xu XX. A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma. 2009;56:371–378. doi: 10.4149/neo_2009_05_371. [DOI] [PubMed] [Google Scholar]
- 28.Hu G, Li F, Ouyang K, Xie F, Tang X, Wang K, Han S, Jiang Z, Zhu M, Wen D, Qin X, Zhang L. Intrinsic gemcitabine resistance in a novel pancreatic cancer cell line. Int J Onco1. 2011 doi: 10.3892/ijo.2011.1254. [DOI] [PubMed] [Google Scholar]
- 29.Mueller MT, Hermann PC, Witthauer J, Rubio-Viqueira B, Leicht SF, Huber S, Ellwart JW, Mustafa M, Bartenstein P, D’Haese JG, Schoenberg MH, Berger F, Jauch KW, Hidalgo M, Heeschen C. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–1113. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 30.Ng SSW, Tsao MS, Chow S, Hedley DW. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60:5451–5455. [PubMed] [Google Scholar]
- 31.Zhang M, Song T, Yang L, Chen R, Wu L, Yang Z, Fang J. Nestin and CD133: valuable stem cell-specific markers for determining clinical outcome of glioma patients. J Exp Clin Cancer Res. 2008;27:85. doi: 10.1186/1756-9966-27-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohike N, Sato M, Hisayuki T, Imataka H, Sato S, Wada Y, Saito K, Takahashi M, Tajiri T, Kunimura T, Morohoshi T. Immunohistochemical analysis of nestin and c-kit and their significance in pancreatic tumors. Pathol Int. 2007;57:589–593. doi: 10.1111/j.1440-1827.2007.02143.x. [DOI] [PubMed] [Google Scholar]
- 33.Zlobec I, Terracciano L, Jass JR, Lugli A. Value of staining intensity in the interpretation of immunohistochemistry for tumor markers in colorectal cancer. Virchows Arch. 2007;451:763–769. doi: 10.1007/s00428-007-0466-8. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda Y, Naito Z, Kawahara K, Nakazawa N, Korc M, Ishiwata T. Nestin is a novel target for suppressing pancreatic cancer cell migration, invasion and metastasis. Cancer Biol Ther. 2011;11:512–523. doi: 10.4161/cbt.11.5.14673. [DOI] [PMC free article] [PubMed] [Google Scholar]


