Abstract
Kawasaki disease (KD) is the most common cause of multisystem vasculitis in childhood. Although cervical lymphadenitis is one of the major symptoms in KD, lymph node biopsy is rarely performed, because KD is usually diagnosed by clinical symptoms. A cervical lymph node biopsy was taken from a girl aged 1 year and 8 months who had suspected lymphoma, but she was diagnosed with KD after the biopsy. The cervical lymph node specimen was analyzed with multivirus real-time PCR that can detect >160 viruses, and unbiased direct sequencing with a next-generation DNA sequencer to detect potential pathogens in the lymph node. Histologically, focal necrosis with inflammatory cell infiltration, including neutrophils and macrophages, was observed in the marginal zone of the cervical lymph node, which was compatible with the acute phase of KD. Multivirus real-time PCR detected a low copy number of torque teno virus in the sample. Comprehensive direct sequencing of the cervical lymph node biopsy sample sequenced more than 8 million and 3 million reads from DNA and RNA samples, respectively. Bacterial genomes were detected in 0.03% and 1.79% of all reads in DNA and RNA samples, respectively. Although many reads corresponded to genomes of bacterial environmental microorganisms, Streptococcus spp. genome was detected in both DNA (77 reads) and RNA (2,925 reads) samples. Further studies are required to reveal any association of microbial or viral infection with the pathogenesis of KD.
Keywords: Kawasaki disease, lymph node, next-generation sequencer, multivirus real-time PCR, torque teno virus, streptococcus
Introduction
Kawasaki disease (KD) is an acute systemic vasculitis of infancy and early childhood that is characterized by prolonged fever, conjunctivitis, inflammation of the oropharynx, rash, erythematous induration of the distal extremities, and cervical lymphadenopathy [1]. Its etiology remains to be elucidated, and epidemiological studies have shown that it is more frequently observed in winter [2]. The disease occurs worldwide, but its incidence is 10 times higher in Japan than in western countries [3], suggesting that race-specific genotypes might be associated with the disease.
The resemblance of KD to toxic shock syndrome (TSS) suggests a causative role for asyet-uncharacterized infectious agents. TSS is caused by the superantigen (SAg) TSS toxin-1 [4]. SAgs are toxins of microbial or viral origin that cross-link antigen-presenting cells and T cells by simultaneously binding to class II major histocompatibility complex molecules [5-7] and the variable b-chain of T-cell receptors (TCR Vb) [8-10]. Although the etiology of KD is unknown, Lactobacillus cell wall [11], immunization with Bacillus Calmette-Guerin (BCG) [12], or Candida albicans fractions [13,14] induce vasculitis and coronary arteritis in animal models, whereas tumor necrosis factor (TNF)-α has been suggested as necessary for this induction [15]. These observations suggest that, in addition to microbial SAg, infectious agents could be potential candidates for the onset of KD. To date, a number of SAg-producing bacteria [16], including Streptococcus pyogenes, Staphylococcus aureus and Yersinia pseudotuberculosis [17,18], as well as viruses, such as Epstein-Barr virus [19], have been speculated to be the causative agents of KD; however, there is a lack of consistency among reports. These disparate findings suggest that the inflammation observed in KD is not the result of a single agent, but rather from numerous infectious agents in genetically susceptible individuals [20-22].
Cervical lymphadenitis is one of the major symptoms in KD [1,23]. A pathological study demonstrated that lymph node biopsy from KD patients showed focal necrosis with inflammatory cell infiltration [24]. This observation implied that lymphoadenopathy is a symptom of the acute phase of KD, and suggested the association of bacterial or virus infection with the pathogenesis of KD. However, lymph node biopsy is rarely performed in KD patients, because KD is usually diagnosed by clinical symptoms [23].
In the present study, we analyzed a cervical lymph node biopsy from a girl aged 1 year and 8 months who had suspected lymphoma, but she was diagnosed with KD after biopsy. The recently developed next-generation sequencer is a powerful tool for detecting pathogen genomes in clinical samples. Comprehensive direct sequencing without any filtering preparation steps by a next-generation sequencer enables one to detect pathogens in representative and unbiased conditions in a small number of clinical samples [25-28]. The multivirus realtime PCR system developed recently by our group is another powerful tool to detect virus genomes in pathological samples. This system can detect >160 viruses in frozen or formalin-fixed paraffin-embedded (FFPE) tissues based on the techniques of the Taqman real-time PCR system [29]. To identify the potential pathogens in KD patients, the lymph node sample was analyzed with multivirus real-time PCR and comprehensive direct sequencing using a next-generation sequencer.
Materials and methods
Ethics statement
The study protocol was approved by the Institutional Medical Ethics Committee, National Institute of Infectious Diseases, Japan (Approval No. 295), and Niigata City General Hospital. The study was conducted according to the principles of the Declaration of Helsinki.
KD patient
A girl aged 1 year and 8 months had prolonged fever and cervical lymphadenopathy. She was subjected to a lymph node biopsy for suspicion of malignant lymphoma; however, histological features were compatible with KD and suggested no malignancy (Figure 1) [24]. After the biopsy, conjunctivitis, skin rash, and “strawberry tongue” were observed in the patient. These symptoms met the diagnostic criteria for KD established by the Japanese Kawasaki Diseases Research Committee [1,23]. The patient was positive for 5 markers (fever, conjunctiva, exanthema, strawberry tongue, and lymphadenopathy) of the 6 KD symptom criteria at day 10 from the appearance of their earliest symptom. Administration of intravenous immunoglobulin and aspirin resulted in rapid decline of fever, and all symptoms disappeared at day 20 from the appearance of the earliest symptom.
Figure 1.
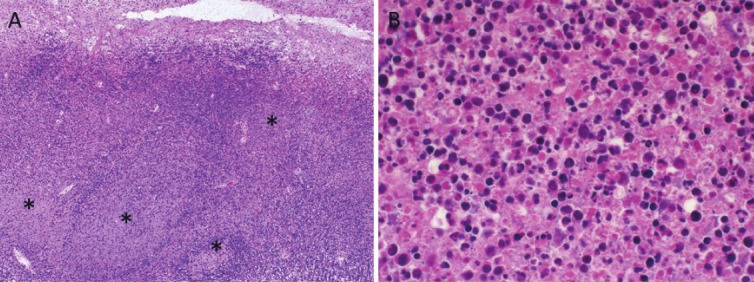
Histopathological investigation of the lymph node sample from KD patient. A. Low-power view of the lymph node. Focal necrosis was observed in the marginal zone of the lymph node (asterisks). B. High-power view of the focal necrosis. Many necrotic ghost cells with neutrophils were found in the focal necrotic area.
Histopathology
Hematoxylin-eosin (HE), periodic acid-Schiff (PAS), Gram, and Giemsa staining was performed on the paraffin sections. In immunohistochemistry, monoclonal or polyclonal antibodies to herpes simplex virus (HSV)-1 and -2 [30], varicella-zoster virus (VZV) [31], human cytomegalovirus (CMV) [32], human herpesvirus 6 (HHV-6) (P101; Millipore, Bedford, MA, USA), Kaposi’s sarcoma-associated herpesvirus (KSHV) [33], and human papillomavirus (HPV) [34] were used as the primary antibodies. The labeled avidin-biotin method was used to detect virus antigens. In situ hybridization was performed to detect EBV-encoded small RNA (EBER) as described previously [35].
Total nucleic acid preparation from specimens
Total DNA and RNA were prepared from a FFPE 7-mm cube of the autopsy lymph node tissue using QIAamp DNA extraction kit for FFPE (Qiagen, Hilden, Germany) and PureLink FFPE RNA isolation kit (Invitrogen, Carlsbad, CA, USA), respectively. Total RNA was treated with DNase (TurboDNase, Ambion, Austin, TX, USA).
Multivirus real-time PCR system
The multivirus real-time PCR system that detects >160 viruses was used as reported previously [29]. Total DNA and RNA (each 1 μg) were applied to the system.
Short-read DNA sequencing
The first-strand cDNA was prepared from ~0.5 μg total RNA using the random priming method with the RT2 First Strand kit (Supper Array Bioscience, Frederick, MD, USA), and double-strand cDNA (ds-cDNA) was synthesized with DNA polymerase I (Escherichia coli, 10 U/μl, Invitrogen) with RNase H (2 U/μl; Invitrogen). The ds-cDNA was purified using a QIAquick PCR Purification Kit (Qiagen). A DNA library was prepared using a Genomic DNA Sample Prep Kit (Illumina, San Diego, CA, USA), and DNA clusters were generated on a slide using a Cluster Generation Kit (version 2) on an Illumina cluster station, according to the manufacturer’s instructions. To obtain ~1.0 × 107 clusters for 1 lane, the general procedure, as described in the standard protocol (Illumina), was performed as follows: template hybridization, isothermal amplification, linearization, blocking, denaturation, and hybridization of the sequencing primer. All sequencing runs for 75-80 mers were performed with the Illumina Genome Analyzer IIx sequencing kit. Fluorescent images were analyzed using the Illumina base-calling pipeline 1.4.0 to obtain FASTQ-formatted sequence data. The detailed sequencing parameters are summarized in Table 2. The short read archives have been deposited in the DNA Data Bank of Japan (DDBJ; accession numbers: DRA000437).
Table 2.
Information of sequencing reads for the specimens
| Sample | Total sequencing reads | Human | Archaea | Bacteria | Virus | Not assigned | No hits |
|---|---|---|---|---|---|---|---|
| DNA | 8,151,996 | 99.6% | 0.0001% | 0.03% | 0.0000% | 0.02% | 0.36% |
| RNA | 3,644,872 | 77.4% | 0.0047% | 1.79% | 0.0006% | 0.11% | 20.67% |
Homology search analysis
All of the obtained DNA sequence reads were aligned to a reference sequence of human genomic DNA, followed by quality trimming to remove low-quality reads, and further excluding reads with similarity to ambiguous human sequences by a BLASTN search with a cutoff value of e-8. The remaining sequence reads were investigated using a BLASTN search against a nucleotide database. The results of the BLASTN search were analyzed and visualized using MEGAN v4.2.6 [36] with the following parameters: minimum support, 1 hit; minimum score 50 for the genus level.
Results
Histopathological findings
HE stain showed focal and severe necrosis mainly in the lymphoid follicle in the marginal zone of the lymph node (Figure 1). Lymph node structure was destroyed by necrosis, and no intact germinal center was found. The necrotic area contained many necrotic ghost cells with hemorrhage and infiltration of neutrophils, eosinophils and macrophages. Apoptotic cells were also frequently found around the necrotic area. The lymph node capsule was thickened with inflammatory cell infiltration. These observations were compatible with the acute phase of KD [24]. No bacteria or fungi were detected by PAS, Gram and Giemsa staining. HSV-1, CMV, HHV-6, VZV, KSHV and HPV antigens were not detected by immunohistochemistry, and EBER was negative by in situ hybridization.
Multivirus real-time PCR
To detect viruses in the lymph node sample, we used the multivirus real-time PCR system, which can detect >160 DNA and RNA viruses (Table 1). RNA extracted from the lymph nodes was negative for all RNA viruses, while internal control RNA (hGAPDH mRNA) was detected. The multivirus real-time PCR system revealed that 30 ng DNA extracted from the lymph node contained 14 copies of torque teno virus (TTV). All other DNA viruses examined in the system were negative.
Table 1.
Results of multivirus real-time PCR
| Results | |
|---|---|
| DNA viruses | |
| Polyomavirus: JC virus, BK virus, Simian virus 40 | - |
| Papillomaviruses: HPV 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52,56, 58, 59, 66, 68, 73 | - |
| Parvoviruses: adeno-associated virus 1, 2, 3 and 5; parvovirus B19; human bocavirus; adenovirus A-F | - |
| Herpes viruses: HHV 1-8, B virus | - |
| Poxviruses: variola virus, monkey pox virus, molluscum contagiosum virus | - |
| Anellovirus: TTV | + |
| Hepadnavirus: Hepatitis B virus | - |
| Others: mimivirus | |
| RNA viruses | |
| Filoviruses: Ebola virus, Marburg virus | - |
| Bunyaviruses: Crimean-Congo hemorrhagic fever virus, hemorrhagic fever with renal syndrome virus (Hantaan, Dovrava, Puumala, and Seoul), Rift valley fever virus, sin nombre virus | - |
| Arenaviruses: Lassa virus, Junin, Guanarito, Machupo and Sabia viruses | - |
| Togaviruses: equine encephalitis virus (Venezuelan, Eastern, and Western), Sindbis virus, Mayaro virus, Getah virus, Chikungunya virus, rubella virus | - |
| Enteroviruses: enterovirus 68 and 71; poliovirus 1-3; coxsackievirus A2-A6, A8-A10, A16, A21, A24, B1-B6; echovirus 5, 6, 7, 9, 11, 13-18, 25, 30; parechovirus 1 and 3; rhinovirus A and B; rotavirus; reovirus 1-4; Melaka virus; Colorado tick borne fever virus | - |
| Flaviviruses: dengue virus 1 and 2; Japanese encephalitis virus; Murray Valley encephalitis virus; St. Louis encephalitis virus; West Nile virus; Tick-borne encephalitis virus; yellow fever virus | - |
| Orthomyxoviruses: influenza virus A-C and H5N1 | - |
| Paramyxoviruses: parainfluenza virus 1-3; Hendra virus; mumps virus; measles virus; Sendai virus; RS virus A and B; metapneumovirus; Nipah virus | - |
| Rabdoviruses: rabies virus, lyssavirus 5 and 6, Chandipura virus, Duvenhage virus | - |
| Coronaviruses: coronavirus OC43, 229E and NL63; SARS virus | - |
| Caliciviruses: sapovirus, Norwalk-like virus 1 and 2 | - |
| Hepatitis viruses: hepatitis A virus, hepatitis C virus, hepatitis D virus, hepatitis E virus, GB virus | - |
| Retroviruses: human immunodeficiency virus 1, human T cell leukemia virus 1 and 2 | - |
| Others: astrovirus, Borna disease virus | - |
Sequencing reads by next-generation sequencer
To determine the potential pathogens in the patient, direct sequencing was performed on the DNA or ds-cDNA from total RNA extracted from an FFPE sample of the lymph node biopsy. The Illumina Genome Analyzer IIx sequencing system produced several million 75-mer reads from the DNA or ds-cDNA libraries (Table 2). 99.59% and 77.43% of the sequences from the DNA and RNA samples were originated from a possible human source, respectively (Table 2 and Figure 2). The remaining reads were further analyzed using a BLASTN search against non-redundant databases, revealing potential bacterial and viral sequences (Figure 2). Regarding the bacterial hits, genus classification was determined from the results of a BLASTN search (Figure 3). The most abundant bacteria in both DNA and RNA samples were from the genera Methylotenera, Cytophaga, Flavobacterium and Polynucleobacter, which are well-known environmental microorganisms, but our other sequencing trials with clinical specimens suggested that these bacteria could be the result of contamination through the handling of FFPE samples, because these species were always detected (data not shown). In addition, Escherichia and Propionibacterium spp. were frequently detected as possible contaminants. Sequencing of the lymph node showed that Streptococcus spp. was present, but it could not be confirmed as the specific pathogen related to the onset of KD (Figure 3; panel Lymph-RNA). No virus genome was detected by the direct sequencing.
Figure 2.
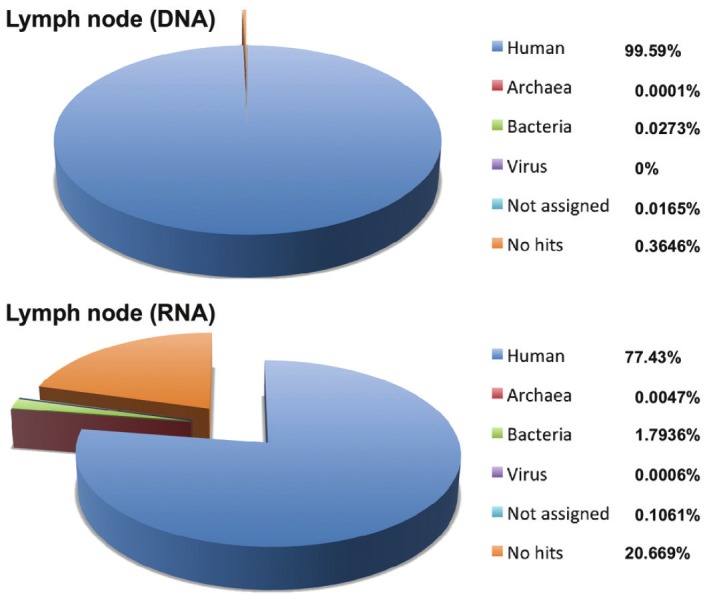
Pie chart of the homology search results for all of the obtained reads from the lymph node biopsy sample. Percentage reads are shown in parentheses.
Figure 3.
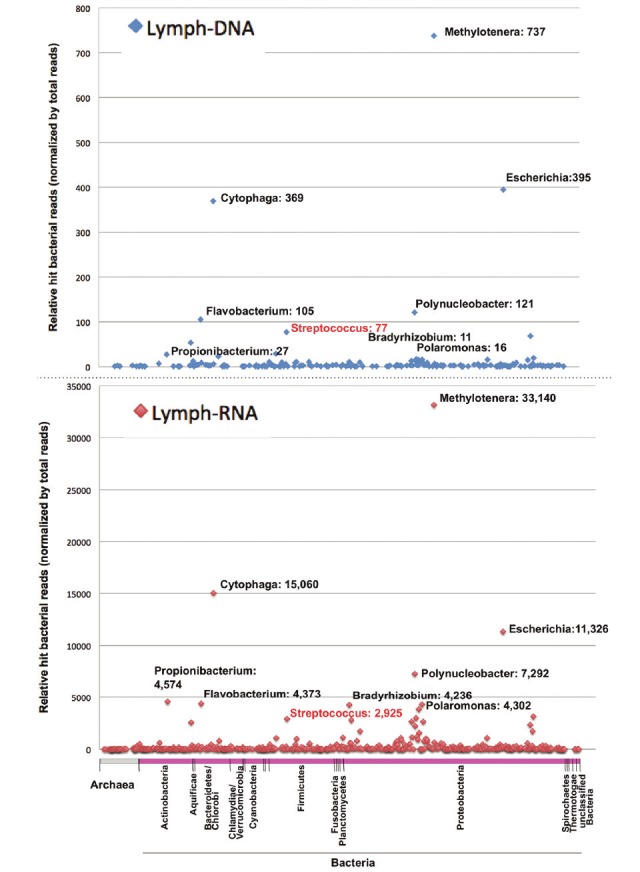
Dot plot of the short read numbers for archaea and all bacterial genera from the lymph node biopsy.The sequencing reads from lymph node DNA or RNA samples were analyzed by BLASTN homology search with athreshold score of 50.0, and the similarity results were classified into 11 and 653 genera of archaea and bacteria,respectively. The phylum taxonomy of the bacteria is shown below the dot plot. Notable genera and the correspondingnumber of sequencing reads are shown beside each dot.
Discussion
Using a next-generation DNA sequencer and multivirus real-time PCR system, we detected possible pathogens from a cervical lymph node biopsy specimen, which is a tissue that is more likely to be associated with pathogens responsible for the pathogenesis of KD. Direct sequencing from DNA or RNA revealed that abundant environmental bacteria were unexpectedly identified, but variable bacterial genera were identified in the lymph node. Although we could not definitively identify the responsible pathogen for the lymphadenopathy, the presence of Streptococcus spp. in the lymph node suggests the association of streptococcal infection with the lymph node lesion in a KD patient.
Pathological findings in the lymph node from this patient, such as focal necrosis with abundant apoptotic cells, are compatible with previous findings in the lymph nodes of KD patients [24]. Focal necrosis with abundant apoptotic cells and severe inflammatory cell infiltration are frequently observed in any bacterial or viral infection, non-specifically. However, any specific pathogen has not been identified in the lymph node of KD patients so far. These previous findings suggest that etiological pathogens are already excluded by inflammation in the necrotic lesion, and some bacterial toxins are associated with formation of necrotic lesions in the lymph node. In such necrotic lesions, it is not easy to detect the pathogens. Another reason why the pathogen has not been identified in the lymph node of KD patients is that lymph node biopsy is not usually performed in such patients because the disease can be diagnosed from clinical symptoms. Thus, the lymph node sample in the present study is important for investigation of KD pathogenesis.
The next-generation sequencer identified Streptococcus spp. in the lymph node as one of the probable suspected pathogens for the pathogenesis of KD. Although detailed species classification could not be performed in our study, some oral Streptococcus spp. such as Streptococcus salivarius, Streptococcus thermophilus, Streptococcus pneumoniae, Streptococcus mitis, Streptococcus sanguinis and Streptococcus dysgalactiae are known to induce platelet activation [37]. In fact, increased platelet count is one of the significant parameters detected at the onset of KD [38,39]. Streptococcal platelet activation is dependent on the species and strain. Some S. sanguinis strains produce platelet-aggregation-associated protein [40], the adhesion of S. pneumoniae to platelets occurs as a consequence of platelet activation by thrombin [41], and although still controversial, S. mitis stimulates platelet aggregation in a strain-dependent manner [42]. In this study, we were unable to identify S. pyogenes using bacterial culture and comprehensive detection; however, it has been reported that the SAg genes of group A streptococci might be transferred among themselves [43]. It has been reported that the Streptococcus spp. isolated from KD patients induce expansion of T-cell populations expressing Vβ2 [44]. Thus, it is possible that an uncharacterized SAg gene could be transferred to the identified Streptococcus spp. Indeed, multiple SAgs have been identified in stool samples from KD patients [45]; however, this finding remains to be confirmed.
The multivirus real-time PCR revealed the absence of viruses, except TTV, in the lymph node from our patient. TTV was originally identified as an etiological agent of hepatitis [46,47]. However, TTV has been detected frequently in various tissues and even from healthy individuals [48,49]. Thus, TTV is thought to be a ubiquitous virus that is not associated with any specific diseases. TTV has been detected frequently even in samples from other children by the multivirus real-time PCR system (data not shown), therefore, it should not be thought of as an etiological agent of KD. However, increased copy numbers of TTV have been found in some disease tissues, suggesting that TTV is activated under certain conditions in some diseases [50-52]. Further studies are required to clarify the association of TTV infection and KD pathogenesis in the lymph node.
Finally, this study used multivirus real-time PCR and unbiased direct sequencing to identify possible causative agents of KD, and demonstrated the presence of TTV and Streptococcus spp. in the lymph node of a KD patient. It remains to be elucidated which species of streptococci are closely related to the etiology of KD. We are currently trying to identify additional specific isolates from KD patients, and whole genome sequencing of these isolates may identify a crucial virulence factor for KD pathogenesis, such as unique SAgs and platelet-activating factors.
Acknowledgment
This work was supported by a grant from the NPO Japan Kawasaki Disease Research Center (2008, to MK and TM) and a grant-in-aid for Scientific Research (C) and Challenging Exploratory Research from the Japan Society for the Promotion of Science (to MK, Grant No. 23590527 and to HK Grant No. 24659212). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2.Nakamura Y, Yashiro M, Uehara R, Sadakane A, Chihara I, Aoyama Y, Kotani K, Yanagawa H. Epidemiologic features of Kawasaki disease in Japan: results of the 2007-2008 nationwide survey. J Epidemiol. 2010;20:302–307. doi: 10.2188/jea.JE20090180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taubert KA, Rowley AH, Shulman ST. Seven-year national survey of Kawasaki disease and acute rheumatic fever. Pediatr Infect Dis J. 1994;13:704–708. doi: 10.1097/00006454-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bergdoll MS, Crass BA, Reiser RF, Robbins RN, Davis JP. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981;1:1017–1021. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- 5.Fischer T, Wiegmann K, Pfizenmaier K. N-glycosidase treatment of Colo 205 cells interferes with hIFN-gamma induced HLA-DR expression. Lymphokine Res. 1989;8:305–309. [PubMed] [Google Scholar]
- 6.Fraser JD. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989;339:221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- 7.Dellabona P, Peccoud J, Kappler J, Marrack P, Benoist C, Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990;62:1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- 8.White J, Herman A, Pullen AM, Kubo R, Kappler JW, Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- 9.Choi YW, Herman A, DiGiusto D, Wade T, Marrack P, Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990;346:471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- 10.Kappler J, Kotzin B, Herron L, Gelfand EW, Bigler RD, Boylston A, Carrel S, Posnett DN, Choi Y, Marrack P. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989;244:811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 11.Duong TT, Silverman ED, Bissessar MV, Yeung RS. Superantigenic activity is responsible for induction of coronary arteritis in mice: an animal model of Kawasaki disease. Int Immunol. 2003;15:79–89. doi: 10.1093/intimm/dxg007. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T, Yamamura J, Sato H, Kakinuma H, Takahashi H. Vasculitis induced by immunization with Bacillus Calmette-Guerin followed by atypical mycobacterium antigen: a new mouse model for Kawasaki disease. FEMS Immunol Med Microbiol. 2007;49:391–397. doi: 10.1111/j.1574-695X.2007.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagi-Miura N, Shingo Y, Adachi Y, Ishida-Okawara A, Oharaseki T, Takahashi K, Naoe S, Suzuki K, Ohno N. Induction of coronary arteritis with administration of CAWS (Candida albicans water-soluble fraction) depending on mouse strains. Immunopharmacol Immunotoxicol. 2004;26:527–543. doi: 10.1081/iph-200042295. [DOI] [PubMed] [Google Scholar]
- 14.Ohno N. Murine model of Kawasaki disease induced by mannoprotein-beta-glucan complex, CAWS, obtained from Candida albicans. Jpn J Infect Dis. 2004;57:S9–10. [PubMed] [Google Scholar]
- 15.Hui-Yuen JS, Duong TT, Yeung RS. TNF-alpha is necessary for induction of coronary artery inflammation and aneurysm formation in an animal model of Kawasaki disease. J Immunol. 2006;176:6294–6301. doi: 10.4049/jimmunol.176.10.6294. [DOI] [PubMed] [Google Scholar]
- 16.Matsubara K, Fukaya T. The role of superantigens of group A Streptococcus and Staphylococcus aureus in Kawasaki disease. Curr Opin Infect Dis. 2007;20:298–303. doi: 10.1097/QCO.0b013e3280964d8c. [DOI] [PubMed] [Google Scholar]
- 17.Konishi N, Baba K, Abe J, Maruko T, Waki K, Takeda N, Tanaka M. A case of Kawasaki disease with coronary artery aneurysms documenting Yersinia pseudotuberculosis infection. Acta Paediatr. 1997;86:661–664. doi: 10.1111/j.1651-2227.1997.tb08952.x. [DOI] [PubMed] [Google Scholar]
- 18.Abe J, Onimaru M, Matsumoto S, Noma S, Baba K, Ito Y, Kohsaka T, Takeda T. Clinical role for a superantigen in Yersinia pseudotuberculosis infection. J Clin Invest. 1997;99:1823–1830. doi: 10.1172/JCI119349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuta H, Matsumoto S, Osato T. Kawasaki disease and Epstein-Barr virus. Acta Paediatr Jpn. 1991;33:765–770. doi: 10.1111/j.1442-200x.1991.tb02606.x. [DOI] [PubMed] [Google Scholar]
- 20.Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, Nakamura Y, Yanagawa H, Wakui K, Fukushima Y, Kishi F, Hamamoto K, Terai M, Sato Y, Ouchi K, Saji T, Nariai A, Kaburagi Y, Yoshikawa T, Suzuki K, Tanaka T, Nagai T, Cho H, Fujino A, Sekine A, Nakamichi R, Tsunoda T, Kawasaki T, Hata A. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40:35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onouchi Y, Ozaki K, Buns JC, Shimizu C, Hamada H, Honda T, Terai M, Honda A, Takeuchi T, Shibuta S, Suenaga T, Suzuki H, Higashi K, Yasukawa K, Suzuki Y, Sasago K, Kemmotsu Y, Takatsuki S, Saji T, Yoshikawa T, Nagai T, Hamamoto K, Kishi F, Ouchi K, Sato Y, Newburger JW, Baker AL, Shulman ST, Rowley AH, Yashiro M, Nakamura Y, Wakui K, Fukushima Y, Fujino A, Tsunoda T, Kawasaki T, Hata A, Tanaka T. Common variants in CASP3 confer susceptibility to Kawasaki disease. Hum Mol Genet. 2010;19:2898–2906. doi: 10.1093/hmg/ddq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeung RS. Kawasaki disease: update on pathogenesis. Curr Opin Rheumatol. 2010;22:551–560. doi: 10.1097/BOR.0b013e32833cf051. [DOI] [PubMed] [Google Scholar]
- 23.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 24.Giesker DW, Pastuszak WT, Forouhar FA, Krause PJ, Hine P. Lymph node biopsy for early diagnosis in Kawasaki disease. Am J Surg Pathol. 1982;6:493–501. doi: 10.1097/00000478-198209000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura S, Yang CS, Sakon N, Ueda M, Tougan T, Yamashita A, Goto N, Takahashi K, Yasunaga T, Ikuta K, Mizutani T, Okamoto Y, Tagami M, Morita R, Maeda N, Kawai J, Hayashizaki Y, Nagai Y, Horii T, Iida T, Nakaya T. Direct metagenomic detection of viral pathogens in nasal and fecal specimens using an unbiased high-throughput sequencing approach. PLoS One. 2009;4:e4219. doi: 10.1371/journal.pone.0004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroda M, Katano H, Nakajima N, Tobiume M, Ainai A, Sekizuka T, Hasegawa H, Tashiro M, Sasaki Y, Arakawa Y, Hata S, Watanabe M, Sata T. Characterization of quasispecies of pandemic 2009 influenza A virus (A/H1N1/2009) by de novo sequencing using a next-generation DNA sequencer. PLoS One. 2010;5:e10256. doi: 10.1371/journal.pone.0010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willner D, Furlan M, Haynes M, Schmieder R, Angly FE, Silva J, Tammadoni S, Nosrat B, Conrad D, Rohwer F. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS One. 2009;4:e7370. doi: 10.1371/journal.pone.0007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pallen MJ, Loman NJ, Penn CW. High-throughput sequencing and clinical microbiology: progress, opportunities and challenges. Curr Opin Microbiol. 2010;13:625–631. doi: 10.1016/j.mib.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Katano H, Kano M, Nakamura T, Kanno T, Asanuma H, Sata T. A novel real-time PCR system for simultaneous detection of human viruses in clinical samples from patients with uncertain diagnoses. J Med Virol. 2011;83:322–330. doi: 10.1002/jmv.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashiwase M, Sata T, Yamauchi Y, Minoda H, Usui N, Iwasaki T, Kurata T, Usui M. Progressive outer retinal necrosis caused by herpes simplex virus type 1 in a patient with acquired immunodeficiency syndrome. Ophthalmology. 2000;107:790–794. doi: 10.1016/s0161-6420(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 31.Muraki R, Baba T, Iwasaki T, Sata T, Kurata T. Immunohistochemical study of skin lesions in herpes zoster. Virchows Arch A Pathol Anat Histopathol. 1992;420:71–76. doi: 10.1007/BF01605987. [DOI] [PubMed] [Google Scholar]
- 32.Maeda A, Sata T, Sato Y, Kurata T. A comparative study of congenital and postnatally acquired human cytomegalovirus infection in infants: lack of expression of viral immediate early protein in congenital cases. Virchows Arch. 1994;424:121–128. doi: 10.1007/BF00193490. [DOI] [PubMed] [Google Scholar]
- 33.Katano H, Sato Y, Kurata T, Mori S, Sata T. High expression of HHV-8-encoded ORF73 protein in spindle-shaped cells of Kaposi’s sarcoma. Am J Pathol. 1999;155:47–52. doi: 10.1016/S0002-9440(10)65097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwasaki T, Sata T, Sugase M, Sato Y, Kurata T, Suzuki K, Ohmoto H, Iwamoto S, Matsukura T. Detection of capsid antigen of human papillomavirus (HPV) in benign lesions of female genital tract using anti-HPV monoclonal antibody. J Pathol. 1992;168:293–300. doi: 10.1002/path.1711680309. [DOI] [PubMed] [Google Scholar]
- 35.Howe JG, Steitz JA. Localization of Epstein-Barr virus-encoded small RNAs by in situ hybridization. Proc Natl Acad Sci U S A. 1986;83:9006–9010. doi: 10.1073/pnas.83.23.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17:377–386. doi: 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNicol A, Israels SJ. Mechanisms of oral bacteria-induced platelet activation. Can J Physiol Pharmacol. 2010;88:510–524. doi: 10.1139/y10-029. [DOI] [PubMed] [Google Scholar]
- 38.Chaiyarak K, Durongpisitkul K, Atta T, Soongswang J, Laohaprasitiporn D, Nana A. Clinical manifestations of Kawasaki disease: what are the significant parameters? Asian Pac J Allergy Immunol. 2009;27:131–136. [PubMed] [Google Scholar]
- 39.Xiu-Yu S, Jia-Yu H, Qiang H, Shu-Hui D. Platelet count and erythrocyte sedimentation rate are good predictors of Kawasaki disease: ROC analysis. J Clin Lab Anal. 2010;24:385–388. doi: 10.1002/jcla.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erickson PR, Herzberg MC. The Streptococcus sanguis platelet aggregation-associated protein. Identification and characterization of the minimal platelet-interactive domain. J Biol Chem. 1993;268:1646–1649. [PubMed] [Google Scholar]
- 41.Niemann S, Kehrel BE, Heilmann C, Rennemeier C, Peters G, Hammerschmidt S. Pneumococcal association to platelets is mediated by soluble fibrin and supported by thrombospondin-1. Thromb Haemost. 2009;102:735–742. doi: 10.1160/TH09-01-0049. [DOI] [PubMed] [Google Scholar]
- 42.Ohkuni H, Todome Y, Mizuse M, Ohtani N, Suzuki H, Igarashi H, Hashimoto Y, Ezaki T, Harada K, Imada Y, et al. Biologically active extracellular products of oral viridans streptococci and the aetiology of Kawasaki disease. J Med Microbiol. 1993;39:352–362. doi: 10.1099/00222615-39-5-352. [DOI] [PubMed] [Google Scholar]
- 43.Vojtek I, Pirzada ZA, Henriques-Normark B, Mastny M, Janapatla RP, Charpentier E. Lysogenic transfer of group A Streptococcus superantigen gene among Streptococci. J Infect Dis. 2008;197:225–234. doi: 10.1086/524687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagata S, Yamashiro Y, Ohtsuka Y, Shimizu T, Sakurai Y, Misawa S, Ito T. Heat shock proteins and superantigenic properties of bacteria from the gastrointestinal tract of patients with Kawasaki disease. Immunology. 2009;128:511–520. doi: 10.1111/j.1365-2567.2009.03135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suenaga T, Suzuki H, Shibuta S, Takeuchi T, Yoshikawa N. Detection of multiple superantigen genes in stools of patients with Kawasaki disease. J Pediatr. 2009;155:266–270. doi: 10.1016/j.jpeds.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Naoumov NV, Petrova EP, Thomas MG, Williams R. Presence of a newly described human DNA virus (TTV) in patients with liver disease. Lancet. 1998;352:195–197. doi: 10.1016/S0140-6736(98)04069-0. [DOI] [PubMed] [Google Scholar]
- 47.Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241:92–97. doi: 10.1006/bbrc.1997.7765. [DOI] [PubMed] [Google Scholar]
- 48.Matsubara H, Michitaka K, Horiike N, Yano M, Akbar SM, Torisu M, Onji M. Existence of TT virus DNA in extracellular body fluids from normal healthy Japanese subjects. Intervirology. 2000;43:16–19. doi: 10.1159/000025018. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh SY, Wu YH, Ho YP, Tsao KC, Yeh CT, Liaw YF. High prevalence of TT virus infection in healthy children and adults and in patients with liver disease in Taiwan. J Clin Microbiol. 1999;37:1829–1831. doi: 10.1128/jcm.37.6.1829-1831.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurihara C, Ishiyama N, Nishiyama Y, Katayama K, Miura S. Changes of DNA titer and sequence variance of TT virus in hepatic disorders. Hepatol Res. 2001;19:212–224. doi: 10.1016/s1386-6346(00)00106-6. [DOI] [PubMed] [Google Scholar]
- 51.Thom K, Petrik J. Progression towards AIDS leads to increased Torque teno virus and Torque teno minivirus titers in tissues of HIV infected individuals. J Med Virol. 2007;79:1–7. doi: 10.1002/jmv.20756. [DOI] [PubMed] [Google Scholar]
- 52.Zhong S, Yeo W, Tang MW, Lin XR, Mo F, Ho WM, Hui P, Johnson PJ. Gross elevation of TT virus genome load in the peripheral blood mononuclear cells of cancer patients. Ann N Y Acad Sci. 2001;945:84–92. doi: 10.1111/j.1749-6632.2001.tb03868.x. [DOI] [PubMed] [Google Scholar]


