Abstract
A peculiar case of bladder carcinoma showing shadow cell differentiation (SCD) in a 72-year-old man is presented. The tumor histologically revealed high grade urothelial carcinoma (UC) and partially contained squamous component with a transition to shadow cell nests, similar to those seen in cutaneous pilomatricoma (PMX). Immunohistochemically, the modes of cell death in the component of SCD were identical to those in PMX. The present case as well as 10 cases of cutaneous PMX showed nuclear expression of beta-catenin, whereas 10 cases of bladder UC with squamous differentiation revealed membranous localization without nuclear expression. These results suggest that nuclear accumulation of beta-catenin may play an important role for SCD in the present case. SCD in extracutaneous tumor is extremely rare and, in the literature, the present case is the second one as for bladder carcinoma.
Keywords: Bladder carcinoma, shadow cell differentiation, pilomatricoma, beta-catenin
Introduction
Shadow cells are specialized form of keratinized cells differentiating toward hair matrix and are characteristically found in cutaneous pilomatricoma (PMX) [1], craniopharyngioma and odontogenic cyst [2]. Shadow cell differentiation (SCD) is also found in gonadal teratomatous tumors [3-7] and in some visceral carcinomas [8-14], on extremely rare occasion. Carcinoma of the urinary tract usually reveals a histological feature of urothelial carcinoma (UC), while some cases partially show squamous and/or glandular differentiation [15]. However, there has been only one case report of bladder carcinoma showing SCD, as far as we know [14]. This paper presents an additional case of UC with SCD. Furthermore, the modes of cell death as well as expression pattern of beta-catenin were compared among the present case, cutaneous PMX and bladder UC with squamous differentiation.
Case Report
Clinical course
A 72-year-old Japanese man noticed asymptomatic gross hematuria and malignant cells were detected in urinary cytology specimens. He visited Suwa Red Cross Hospital (Nagano, Japan) for further examination and treatment of the tumor. Computed tomography (CT) revealed a cauliflower-like mass, approximately 3cm. in diameter, with muscularis propria invasion, on anterior wall of urinary bladder. Transurethral resection (TUR) of the tumor was performed and a histopathological diagnosis of bladder carcinoma was made. Although invasion of carcinoma into muscularis propria was not confirmed histopathologically, he underwent total cystectomy based on CT diagnosis. The residual carcinoma showed intraepithelial spread without wall invasion in the resected bladder. The patient has been well without recurrence of the tumor for one year after operation.
Histopathology
The TUR specimens, resected piece-by-piece, mostly consisted of carcinoma tissue infiltrating into lamina propria of bladder mucosa. Histologically, the tumor formed irregular nests composed of atypical urothelial cells, including a small number of large atypical cells bearing bizarre nucleus, and was diagnosed as high grade UC (Figure 1A). The tumor nests partially contained necrotic foci and was scattered with apoptotic bodies. In addition, parts of carcinoma tissue were intermingled with shadow cells forming nests or scattered among UC nests (Figure 1B). The shadow cells showed the same morphological feature as those in PMX; they preserved cell shape with eosinophilic cytoplasm and ghost-like nucleus. Although UC nests and shadow cell nests mostly revealed abrupt mutual transition, some of the latter contained a small amount of squamous components in the periphery of nests through a small number of “transitional” cells (Figure 1B). In this paper, “transitional” cells do not mean urothelial cells, but the cells similar to those seen between basaloid cell layer and shadow cell nests in PMX.
Figure 1.
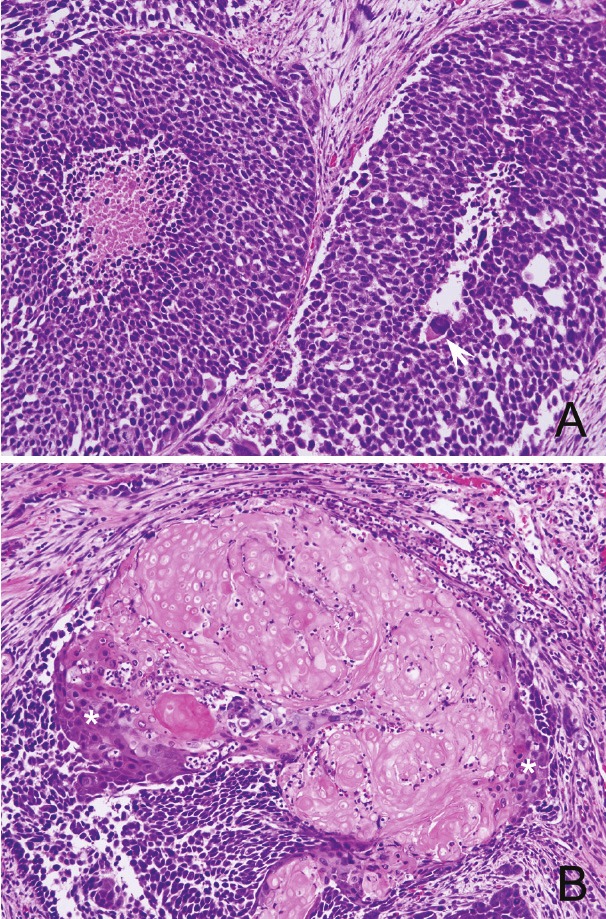
Histology of the bladder tumor (H.E. stain). A: A feature of high-grade urothelial carcinoma with intermingled large atypical cells with bizarre nucleus (arrow). B: Shadow cell nests with peripheral squamous component (asterisks) through “transitional” cells.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections of bladder tumor were stained for single-stranded DNA (ssDNA), gamma-H2AX, cleaved caspase-3, cleaved lamin A, caspase-14, uroplakin, involcurin and beta-catenin in order to examine the modes of cell death/keratinization in SCD, as previously described [16], and to characterize SCD. Shadow cell nests were negative for gamma-H2AX, ssDNA, caspase-14, cleaved caspase-3 or cleaved lamin A, while “transitional” cells were reactive for gamma-H2AX (Figure 2B) and ssDNA (Figure 2C). Cleaved caspase-3 and cleaved lamin A were positive in some apoptotic bodies, but were not in the “transitional” cells.
Figure 2.
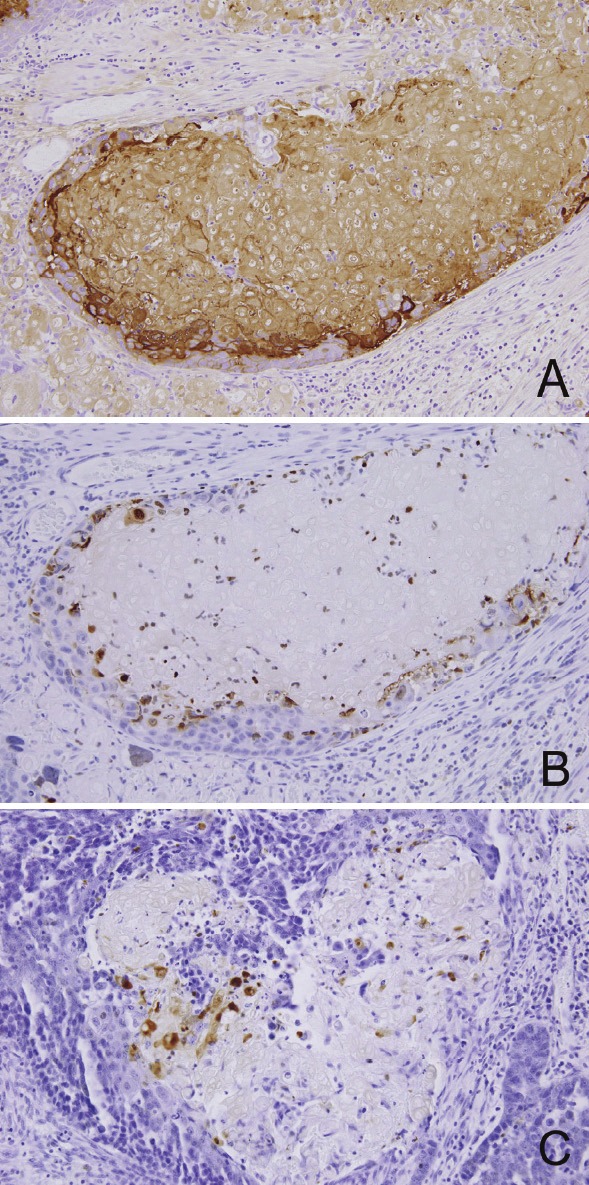
Immunohistochemistry of the bladder tumor. (A) Shadow cell nests as well as peripheral squamous component and “transitional” cells are positive for involcurin. (B)(C) “Transitional” cells in the area of shadow cell differentiation are immunoreactive for gamma-H2AX (B) and single-stranded DNA (C).
The shadow cells as well as squamous component (cells with preserved nucleus) in the periphery of the nests showed immunoreactivity for involcurin (Figure 2A). No components were reactive for uroplakin. As for beta-catenin immunostaining, intense nuclear and cytoplasmic expression was observed in many UC cells, squamous component and some “transitional” cells (Figure 3A), whereas membranous expression was focally present. For comparison, cases of cutaneous PMX and bladder UC with squamous differentiation (10 cases in each) were retrieved from our database file and were stained for beta-catenin. All cases of PMX showed intense nuclear and cytoplasmic expression of beta-catenin in basaloid cells (Figure 3B). On the other hand, all cases of bladder UC with squamous differentiation did not reveal nuclear expression, but showed membranous localization in both components (Figure 3C).
Figure 3.
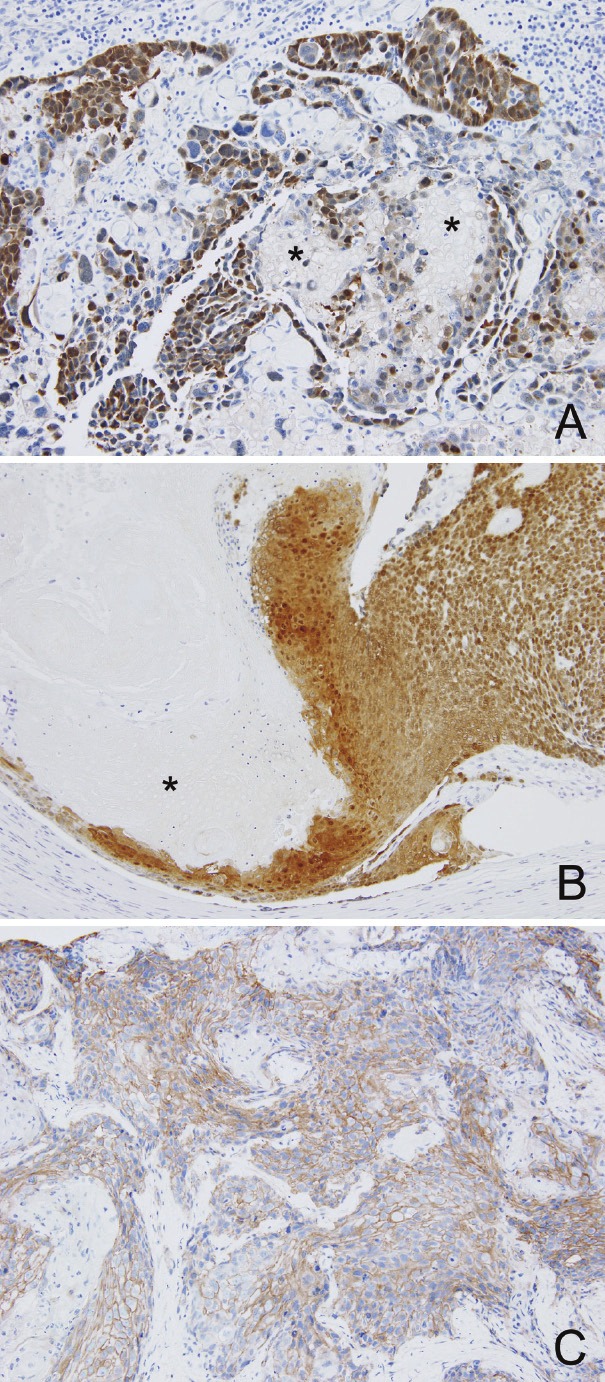
Immunohistochemistry for beta-catenin. (A) (B) Nuclear and cytoplasmic localization in urothelial, squamous and “transitional” cells in the present bladder tumor (A) and in basaloid cells in cutaneous pilomatricoma (B). Shadow cells (asterisks) are negative in both tumors. (C) Membranous localization in the squamous component of bladder carcinoma (different case from the present one).
Discussion
Various types of pathological keratinization, such as squamous eddies, cancer pearls or dyskeratosis are frequently observed in the squamous proliferative lesions. SCD is also a specialized form of keratinization toward hair matrix [1], while it is extremely rare to find SCD in the tumors except for PMX, craniopharyngioma and odontogenic cyst [2]. According to review of the literature, extracutaneous tumors showing SCD are divided into two categories: (a) gonadal teratoma and extragonadal dermoid cyst containing squamous components [3-7] and (b) carcinoma containing squamous components (i.e., squamous cell carcinoma, adenosquamous carcinoma, adenoacanthoma (adenocarcinoma with squamous metaplasia) or other types of carcinoma with squamous metaplasia) [8-14]. There have been 12 reported cases in the latter category (Table 1) and the primary sites include ovary [8,9], uterus [10], colon [10,11], lung [12], gallbladder [13] and urinary bladder [14]. Most of these reported cases contained squamous component, suggesting that shadow cell nests are derived from squamous components as a variant of keratinization. Zamecnik et al. [13] also mentioned that SCD was frequently found in endometrial adenosquamous carcinoma. As for bladder carcinoma showing SCD, only one case has been described so far [14]; the present case is the second one as far as we know. The tumor in our case also contained a small amount of squamous component, which was confirmed by positive immunoreaction for involcurin, in the periphery of shadow cell nests. Morphologically, it could be regarded as a variant of UC with squamous differentiation.
Table 1.
Reported cases of tumor with shadow cell differentiation (except for primary cutaneous & odontogenic tumors, craniopharyngioma and teratoma/dermoid cyst)
| Primary site | Age/Sex | Histological type* | Authors [ref.] | Year |
|---|---|---|---|---|
| Ovary | 48/F | ASC | Fang et al. [8] | 1996 |
| 31/F | endometrioid AC (skin metastasis) | Lalich et al. [9] | 2010 | |
| Uterus | 40/F | AC with squamous differentiation | Zamecnik et al. [10] | 1995 |
| 53/F | “ | “ | ||
| 46/F | “ | “ | ||
| 27/F | AH with squamous metaplasia | “ | ||
| Colon | 63/M | AC with squamous differentiation | “ | |
| 58/M | ASC | “ | ||
| 65/M | AC with squamous metaplasia | Nakayama et al. [11] | 1997 | |
| Lung | 76/M | SCC | Garcia-Escudero et al. [12] | 2002 |
| Gallbladder | 67/F | small cell carcinoma | Zamecnik et al. [13] | 1998 |
| Bladder | 75/M | UC with squamous metaplasia | Zamecnik et al. [14] | 1996 |
| 72/M | UC with squamous differentiation | Nakamura (present case) | 2012 |
AC: adenocarcinoma, ASC: adenosquamous carcinoma, AH: adenomatous hyperplasia, SCC: squamous cell carcinoma, UC: urothelial carcinoma.
The modes of cell death in extracutaneous tumors showing SCD have not been examined so far. We previously examined expression pattern of apoptosis-related markers in various types of keratinization and clarified that SCD in PMX is not identical, although partly similar, to apoptosis [16]; i.e., transitional cells showed DNA double strand breaks without caspase-3 activation or nuclear fragmentation. The mode of cell death in SCD in PMX may be “apoptosis-like programmed cell death” after classification by Leist and Jaattela [17]. In the present case also, immunoreaction for gamma-H2AX and ssDNA in the “transitional” cells suggested DNA double strand breaks, although morphology and other immunohistochemical results were different from apoptosis. The patterns of immunoreaction in the areas showing SCD in the current bladder tumor was same as those in PMX [16], indicating that the modes of cell death in these two are identical.
In the present case, nuclear localization of beta-catenin was observed in many UC cells, squamous component and some “transitional” cells. Beta-catenin is a dual-functional protein; it not only acts as a submembranous component of the cadherin-mediated cell adhesion system, but also accumulates to nucleus and acts as a key component in the Wnt signal transduction pathway [18]. This pathway plays an important role in tumorigenesis including formation of hair follicle-related tumors. The majority of cases of cutaneous PMX show nuclear accumulation of beta-catenin [19,20] with frequent mutation in CTNNB1 gene encoding beta-catenin [20,21]. As for bladder carcinoma, on the other hand, nuclear expression of beta-catenin or CTNNB1 gene mutation is infrequently observed in UC [22,23], but beta-catenin has not been investigated in UC with squamous differentiation. We compared the present case, cutaneous PMX and bladder UC with squamous differentiation, and found that beta-catenin accumulated to nucleus of viable tumor cells in the present case and cutaneous PMX, but did not in conventional bladder UC with squamous differentiation. These results suggest that nuclear accumulation of beta-catenin in the present case plays a significant role in cellular proliferation and differentiation via the Wnt signaling pathway, as in PMX [19-21]. The present case may be different from UC with squamous differentiation from a standpoint of molecular abnormalities. More cases should be examined further in order to clarify the mechanism of SCD in the tumors other than PMX.
Acknowledgement
The author thanks Dr. Y Sakai for his co-operation, and Ms. M Morozumi, Mr. Y Hanami, Ms. K Akahane, Mr. S Hokibara and Mr. M Shimomura for their excellent technical assistance.
References
- 1.Nakamura T. A reappraisal on the modes of cell death in pilomatricoma. J Cutan Pathol. 1999;26:125–129. doi: 10.1111/j.1600-0560.1999.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 2.Hassanein AM, Glanz SM, Kessler HP, Eskin TA, Lin C. Beta-catenin is expressed aberrantly in tumors expressing shadow cells. Pilomatricoma, craniopharyngioma and calcifying odontogenic cyst. Am J Clin Pathol. 2003;120:732–736. doi: 10.1309/EALE-G7LD-6W71-67PX. [DOI] [PubMed] [Google Scholar]
- 3.Minkowitz G, Lee M, Minkowitz S. Pilomatricoma of the testicle. An ossifying testicular tumor with hair matrix differentiation. Arch Pathol Lab Med. 1995;119:96–99. [PubMed] [Google Scholar]
- 4.Zamecnik M, Mukensnabl P, Curik R, Michal M. Shadow cell differentiation in testicular teratomas. A report of two cases. Ces-slov Patol. 2005;41:102–106. [PubMed] [Google Scholar]
- 5.Ulbright TM, Srigley JR. Dermoid cyst of the testis: a study of five postpubertal cases, including a pilomatrixoma-like variant, with evidence supporting its separate classification from mature testicular teratoma. Am J Surg Pathol. 2001;25:788–793. doi: 10.1097/00000478-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Alfsen GC, Strom EH. Pilomatrixoma of the ovary: a rare variant of mature teratoma. Histopathology. 1998;32:182–183. doi: 10.1046/j.1365-2559.1998.0285c.x. [DOI] [PubMed] [Google Scholar]
- 7.Hitchkock MG, Ellington KS, Friedman AH, Provenzaie JM, McLendon RE. Shadow cells in an intracranial dermoid cyst. Arch Pathol Lab Med. 1995;119:371–373. [PubMed] [Google Scholar]
- 8.Fang J, Keh P, Katz L, Rao MS. Pilomatrixoma-like endometrioid adenosquamous carcinoma of the ovary with neuroendocrine differentiation. Gynecol Oncol. 1996;61:291–293. doi: 10.1006/gyno.1996.0142. [DOI] [PubMed] [Google Scholar]
- 9.Lalich D, Tawfik O, Chapman J, Fraga G. Cutaneous metastasis of ovarian carcinoma with shadow cells mimicking a primary pilomatrical neoplasm. Am J Dermatopathol. 2010;32:500–504. doi: 10.1097/DAD.0b013e3181c6dfc1. [DOI] [PubMed] [Google Scholar]
- 10.Zamecnik M, Michal M. Shadow cell differentiation in tumours of the colon and uterus. Zentralbl Pathol. 1995;140:421–426. [PubMed] [Google Scholar]
- 11.Nakayama H, Kimura A, Okumichi T, Miyazaki E, Kajihara H, Enzan H. Metaplastic shadow cells in rectal adenocarcinoma: Report of a case with immunohistochemical study. Jpn $lxfS1$ 1997;27:427–432. doi: 10.1093/jjco/27.6.427. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Escudero A, Navarro-Bustos G, Jurado-Escamez P, Rios-Martin J, Gonzalez-Campora R. Primary squamous cell carcinoma of the lung with pilomatricoma-like features. Histopathology. 2002;40:201–202. doi: 10.1046/j.1365-2559.2002.1179b.x. [DOI] [PubMed] [Google Scholar]
- 13.Zamecnik M, Michal M, Mukensnabl P. Pilomatrixoma-like visceral carcinomas. Histopathology. 1998;33:395. doi: 10.1046/j.1365-2559.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- 14.Zamecnik M, Michal M, Mukensnabl P. Shadow cells in extracutaneous locations. Arch Pathol Lab Med. 1996;120:426–428. [PubMed] [Google Scholar]
- 15.Eble JN, Sauter G, Epstein JE, Sesterhenn IA. World Health Organization Classification of Tumors. Lyon: IARC Press; 2004. Tumors of the Urinary System and Male Genital Organs. [Google Scholar]
- 16.Nakamura T. Comparative immunohistochemical analyses on the modes of cell death/keratinization in epidermal cyst, trichilemmal cyst and pilomatricoma. Am J Dermatopathol. 2011;33:78–83. doi: 10.1097/DAD.0b013e3181e3aec1. [DOI] [PubMed] [Google Scholar]
- 17.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 18.Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Kim YS, Shin DH, Choi JS, Kim K-H. The immunohistochemical patterns of beta-catenin expression in pilomatricoma. Ann Dermatol. 2010;22:284–289. doi: 10.5021/ad.2010.22.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Bueno G, Gamallo C, Perez-Gallego L, Contreras F, Falacios J. Beta-catenin expression in pilomatricomas. Relationship with beta-catenin gene mutations and comparison with beta-catenin expression in normal hair follicles. Br J Dermatol. 2001;145:576–581. doi: 10.1046/j.1365-2133.2001.04455.x. [DOI] [PubMed] [Google Scholar]
- 21.Xia J, Urabe K, Moroi Y, Koga T, Duan H, Li Y, Furue M. Beta-catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J Dermatol Sci. 2006;41:67–75. doi: 10.1016/j.jdermsci.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Kanai Y, Saito A, Kondo Y, Hirohashi S. Aberrant expression of beta-catenin and mutation of exon 3 of the beta-catenin gene in renal and urothelial carcinomas. Pathol Int. 2000;50:945–952. doi: 10.1046/j.1440-1827.2000.01139.x. [DOI] [PubMed] [Google Scholar]
- 23.Stoehr R, Krieg RC, Knuechel R, Hofstaedter F, Pilarsky C, Zaak D, Schmitt R, Hartmann A. No evidence for involvement of beta-catenin and APC in urothelial carcinomas. Int J Oncol. 2002;20:905–911. [PubMed] [Google Scholar]


