Abstract
Undifferentiated embryonal sarcoma of the liver (UESL) is a malignant mesenchymal tumor that occurs typically in children and rarely in adults. Here we describe a case of UESL in a 51-year-old woman who presented with a cystic lesion in the liver. Because it grew slowly, the anterior segment of the liver was resected to check the lesion. Histologically, the lesion looked like a telangiectatic hepatic adenoma. Two years after resection, the tumor recurred, and she died 3 years later due to liver failure. The autopsy revealed that these lesions were UESL with massive sinusoidal invasion, and a review of the case indicated the primary lesion was also UESL. We also confirmed these tumor cells by staining with CD56, alpha-smooth muscle actin (SMA), and adipophilin, suggesting that they have a character similar to that of stellate cells in the space of Disse. The histological result of our patient revealed atypical UESL. Therefore, UESL should be considered when a hepatic lesion with degeneration is seen, even in an adult. In addition, the immunohistochemical appearance of this case implies that UESL is perhaps derived from stellate cells or stellate cells with myofibroblast differentiation in the space of Disse.
Keywords: Adipophilin, adult, hepatic adenoma, liver, stellate cell, undifferentiated embryonal sarcoma
Introduction
Undifferentiated embryonal sarcoma of the liver (UESL) is a malignant mesenchymal tumor first characterized by Stocker and Ishak in 1978 [1]. It is very aggressive and prognosis is poor. UESL typically occurs before the second decade of life and is rare in adults. To the best of our knowledge, only 14 cases of UESL have been reported in patients over 50 years of age [1-3]. Typically, UESL is a solid tumor composed of bizarre spindle cells and polygonal cells loosely arranged in a myxoid matrix. Tumor cells sometimes infiltrate slightly into the adjacent liver parenchyma with entrapped hepatocytes in the invasion front of UESL. Because of the rarity of UESL in adults, it may be misdiagnosed as another type of neoplasm, including some kinds of sarcoma and hepatocellular carcinoma [1,4-6]. Furthermore, due to its primitiveness and the diversity of histological appearance, the origin of UESL remains unknown.
In this paper, we describe a case of UESL in an adult that mimicked hepatic adenoma. The tumor showed massive intrasinusoidal invasion with sinusoid dilatation, and its appearance was similar to that of a hepatocytic lesion. Such histological appearance and immunohistochemical features suggest that UESL has a character resembling that of stellate cells in the liver.
Clinical summary and pathological appearance of the primary lesion
The patient was a 51-year-old Japanese woman who had suffered from multiple sclerosis for 20 years. Liver function tests were slightly abnormal when she was admitted to our hospital for an exacerbation of multiple sclerosis, and her liver was checked by computed tomography (CT). On CT, there was a small cystic lesion (approximately 1cm diameter) in the right lobe of the liver. Laboratory data indicated no hepatitis viral infection. At the age of 53 years, the cystic lesion had grown to 10cm in diameter (Figure 1A). Because it was very difficult to determine whether or not it was malignant by CT, the anterior segment of the liver was resected and pathological analysis was performed.
Figure 1.
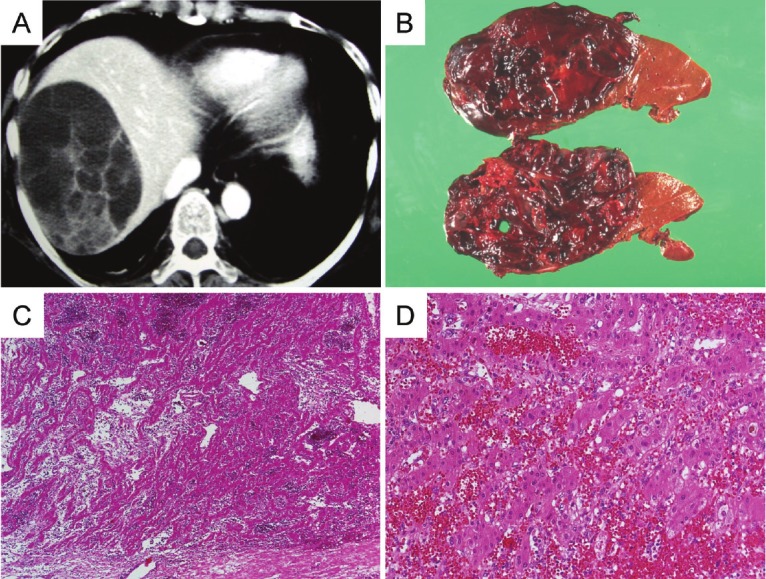
A: Computed tomography scan at 53 years old. There is a cystic lesion (approximately 10 cm in diameter) with septa in the right lobe of the liver. B: Gross features of the primary lesion. The cut surface is reddish and soft, with degeneration. C and D: Microscopic features of the primary lesion. Low power and medium power views showing a fibrous capsule containing hepatocyte cords with sinusoidal dilatation, congestion and hemorrhage.
The hepatic lesion was a soft, hemorrhagic mass with degeneration, measuring 12 × 11 × 7cm (Figure 1B). Histologically, the lesion looked as if hepatocytes had proliferated with sinusoidal dilatation and congestion (Figure 1C and 1D). Because there were few atypical cells, the lesion was diagnosed as telangiectatic hepatic adenoma (THA) at that time.
However, when the patient was 55 years old, she developed multiple cystic lesions in the liver and metastatic lesions in the left lung (0.5 cm in diameter) and thoracic spine (5 × 3.5cm). At 56 years old, she died of liver failure due to tumor occupation. An autopsy was performed on the liver.
Pathological findings on autopsy
The liver weighed 2440g. In gross morphology, there were many round, well-circumscribed, but incompletely encapsulated lesions (9cm maximum diameter) containing gelatinous or coagulated blood (Figure 2A). The periphery of these tumors had a solid but soft component demonstrating gray-white, reddish, or green bile.
Figure 2.
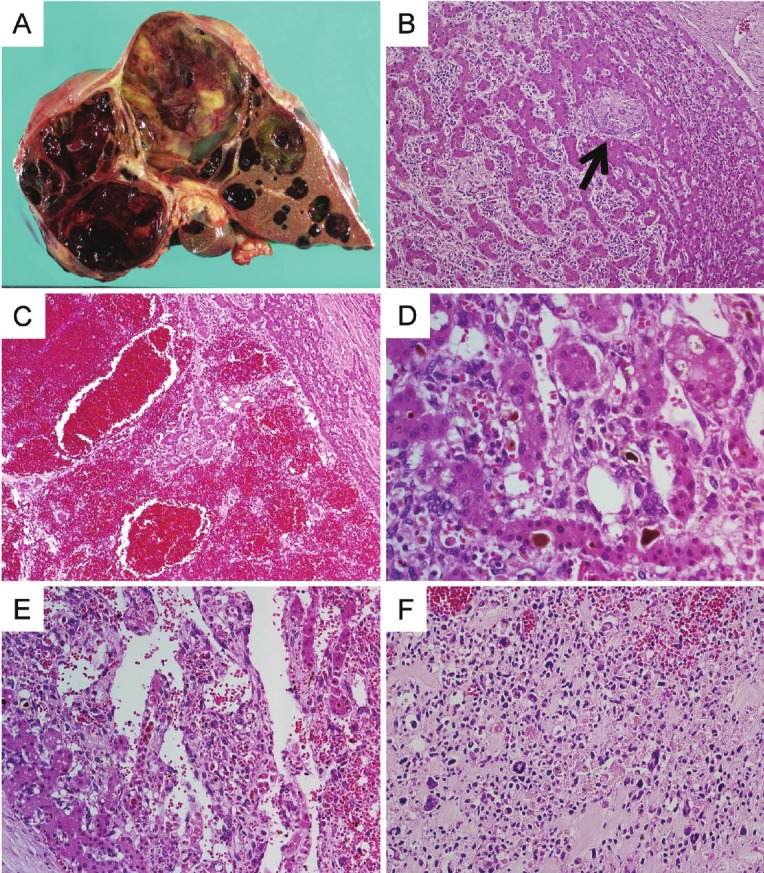
A: Gross features of the liver at autopsy. The cut surface shows multiple lesions with cystic degeneration, the largest measuring 8 × 6cm. B: Low power view showing spindle cell proliferation between hepatocyte cords, with expanding sinusoidal spaces and entrapped bile ducts (arrow). C: Lesions demonstrating congestion and hemorrhage. D: Bizarre spindle cells were seen between hepatocyte cords. Some hepatocytes show bile congestion. E: Dilations of vascular space between tumor cells and pre-existing hepatocytes. F: There are no hepatocytes in the metastatic lesion of the spine.
Histologically, most parts of the tumor were degenerated. In the solid component, spindle shaped, polygonal, and multi-nucleated cells were arranged in sheets between hepatocyte cords and bile ducts (Figure 2B). In some lesions, congestion and hemorrhage with dilation of sinusoidal spaces was seen, similar to the primary lesion (Figure 2C). In other lesions, bizarre cells (Figure 2D) or hemangiopericytoma-like structures were also present (Figure 2E). The background liver tissue showed neither hepatitis nor fibrosis.
The metastatic lesion in the thoracic spine was gray-white and solid, and the cut surface exhibited hemorrhage. Histologically, the metastatic lesion contained no hepatocytic cells, but spindle, polygonal, and bizarre cells were seen in an edematous matrix (Figure 2F), suggesting that hepatocytic cells in the primary and recurrent lesions were not tumor cells but pre-existing hepatocytes.
Immunohistochemical analysis revealed that the spindle cells expressed vimentin diffusely (Figure 3A). They also expressed CD56 (neural cell adhesion molecule) at the cell membrane (Figure 3B). Some spindle cells were positive for alpha-smooth muscle actin (SMA) (Figure 3C), muscle-specific actin (HHF-35), and desmin. A few tumor cells were also CD68 positive. In addition, some bizarre cells contained small vesicles stained positively with adipophilin (adipose differentiation-related protein) in the cytoplasm (Figure 3D, arrows). Staining for cytokeratin CAM5.2, cytokeratin AE1/AE3, EMA, hepatocyte-1, S100, CD10, HMB-45, glypican-3, CD31 (Figure 3E), and CD34 was negative.
Figure 3.
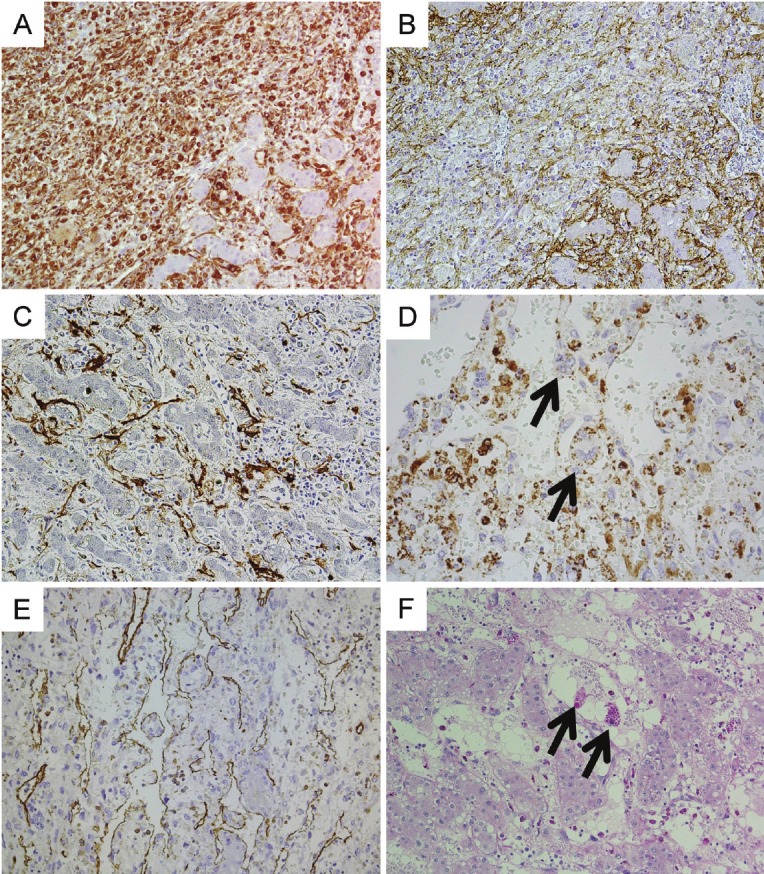
Immunohistochemical features of the hepatic lesion on autopsy (A to E). A: Diffuse reactivity for vimentin in spindle cells. B: CD56 stained on the cell membrane. C: Some tumor cells expressed alpha-smooth muscle actin (SMA). D: Some bizarre tumor cells contain small vesicles stained with adipophilin (arrows). Some small mononuclear macrophages are also stained positively. E: CD31 staining reveals tumor cells located in the space of Disse. Some small mononuclear macrophages are also stained with CD31. F: Diastase treated PAS staining of the primary lesion. There are some giant cells containing diastase-resistant PAS positive globules (arrows) between pre-existing hepatocytes.
Diastase-treated periodic acid-Schiff (PAS) staining revealed giant cells containing PAS-positive spherical globules, commonly seen in UESL [1,7]. On the basis of these findings, we diagnosed the hepatic lesion on autopsy as UESL with massive sinusoidal invasion and hepatocyte entrapment.
On review of the primary lesion, a few atypical cells with diastase resistant PAS-positive globules were seen between hepatocyte cords (Figure 3F), and CD56 positive cells were seen in dilated sinusoidal spaces. We also compared the immunohistochemical profiles, including adipophilin, of the primary lesion, the recurrent hepatic lesion and the metastatic lesion. The results indicated that these tumors were of the same origin (data not shown).
Discussion
In children in the first and second decades of life, UESL is the third most common malignant tumor in the liver. However, UESL is extremely rare in adulthood [1-3]. Moreover, UESL shows an enormous variety of histological features; for example, epithelioid appearance, spindle cells with herringbone arrangement, hemangiopericytoma-like structure, rhabdomyosarcomatoid or liposarcoma-like appearance. 4Due to such scarcity and diversity of histological features, UESL in adults tends to be misdiagnosed [1].
In the present case, autopsy revealed that the recurrent lesions contained mainly two types of cells--atypical spindle cells and hepatocytic cells. Both types of cells were also seen in the primary lesion. In this case, the most important point to diagnose was whether or not the hepatocytic cells in these lesions were a tumor component. If only the hepatocytic cells had been tumor cells, the primary lesion would have been a hepatic adenoma because the hepatocytic cells were only slightly atypical and did not invade adjacent parenchyma. Indeed, we initially diagnosed the primary lesion as THA because there were many hepatocytes with sinusoidal dilatation and few atypical cells. However, the tumor had recurrent and metastatic lesions, indicating malignancy, and the metastatic lesions outside the liver did not contain hepatocytic cells. Moreover, there were some portal areas with bile ducts between hepatocytic cells in the primary and recurrent lesions (Figure 2B). Therefore, we concluded that these hepatocytic cells were not tumor cells but pre-existing hepatocytes. It was also suggested that only spindle cells infiltrating into sinusoids were tumor components.
Although UESL exhibits aggressive behavior, UESL usually shows expansive growth with an incomplete, fibrous capsule, or only slight invasion [4,8,9]. Levy et al. reported a series of UESL, in which only 3 of 24 cases showed entrapment of hepatocytes in the tumor periphery [9]. UESL with both massive sinusoidal invasion and hepatocyte entrapment, as in the present case, has not been reported yet. This is why THA is an uncommon candidate for differential diagnose of UESL.
The origin and the direction of differentiation of UESL remain unclear. Recently, two groups reported CD56 expression in UESL. Pérez-Gómez et al. reported that UESL expressed CD56 diffusely in eight cases in children [6], and Li at al. reported diffuse immunostaining for CD56 in UESLs in four adults [5]. In both reports, membranous staining for CD56 was observed in every examined UESL case. On the other hand, Nishio et al. showed that UESL showed myofibroblastic differentiation by immunohistochemistry and electron microscopy [8]. In the present case, tumor cells express CD56 (Figure 3B), desmin, and alpha-SMA (Figure 3C), which is consistent with other reports. Moreover, adipophilin expression was detected in some tumor cells (Figure 3D), showing that UESL in this case contains intracytoplasmic fat droplets, as reported by Agaram et al [10]. In liver tissue, it is known that adipophilin is expressed in not only hepatocytes with steatosis but also stellate cells [11,12]. Hepatic stellate cells are known as vitamin A storage cells located in the space of Disse, and they are sometimes differentiated into myofibroblasts. In the present case, tumor cells are located mainly in the subendothelial space, a similar situation to stellate cells. It is also known that hepatic stellate cells express CD56 [13] and alpha-SMA [11] to some degree. These findings suggest that parts of the tumor cells exhibited a character similar to that of stellate cells in the space of Disse. This insight is consistent with the tumor cell behavior in the present case, where for example, tumor cells showed massive sinusoidal invasion. Although there are no other reports on adipophilin staining in UESL, we consider it a valuable means of verifying adipophilin expression in cases of UESL. Presently UESL is a diagnosis of exclusion. However, such immunohistochemical profiling including CD56 and adipophilin will be useful for UESL.
In conclusion, when a hepatic lesion with sinusoidal dilation is found, not only hepatocytic neoplasm like THA, but also the invasion of UESL should be considered. To prevent misdiagnosis, careful analysis is required and immunohistochemical profiling including CD56 and adipophilin may be helpful.
References
- 1.Pachera S, Nishio H, Takahashi Y, Yokoyama Y, Oda K, Ebata T, Igami T, Nagino M. Undifferentiated embryonal sarcoma of the liver: case report and literature survey. J Hepatobiliary Pancreat Surg. 2008;15:536–544. doi: 10.1007/s00534-007-1265-y. [DOI] [PubMed] [Google Scholar]
- 2.Gasljevic G, Lamovec J, Jancar J. Undifferentiated (embryonal) liver sarcoma: synchronous and metachronous occurrence with neoplasms other than mesenchymal liver hamartoma. Ann Diagn Pathol. 2011;15:250–256. doi: 10.1016/j.anndiagpath.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Tucker SM, Cooper K, Brownschidle S, Wilcox R. Embryonal (undifferentiated) sarcoma of the liver with peripheral angiosarcoma differentiation arising in a mesenchymal hamartoma in an adult patient. Int J Surg Pathol. 2012;20:297–300. doi: 10.1177/1066896911424899. [DOI] [PubMed] [Google Scholar]
- 4.Zheng JM, Tao X, Xu AM, Chen XF, Wu MC, Zhang SH. Primary and recurrent embryonal sarcoma of the liver: clinicopathological and immunohistochemical analysis. Histopathology. 2007;51:195–203. doi: 10.1111/j.1365-2559.2007.02746.x. [DOI] [PubMed] [Google Scholar]
- 5.Li XW, Gong SJ, Song WH, Zhu JJ, Pan CH, Wu MC, Xu AM. Undifferentiated liver embryonal sarcoma in adults: a report of four cases and literature review. World J Gastroenterol. 2010;16:4725–4732. doi: 10.3748/wjg.v16.i37.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Gomez RM, Soria-Cespedes D, de Leon-Bojorge B, Ortiz-Hidalgo C. Diffuse membranous immunoreactivity of CD56 and paranuclear dot-like staining pattern of cytokeratins AE1/3, CAM5.2, and OSCAR in undifferentiated (embryonal) sarcoma of the liver. Appl Immunohistochem Mol Morphol. 2010;18:195–198. doi: 10.1097/PAI.0b013e3181bb2493. [DOI] [PubMed] [Google Scholar]
- 7.Scudiere JR, Jakate S. A 51-year-old woman with a liver mass. Undifferentiated embryonal sarcoma of the liver. Arch Pathol Lab Med. 2006;130:e24–26. doi: 10.5858/2006-130-e24-AYWWAL. [DOI] [PubMed] [Google Scholar]
- 8.Nishio J, Iwasaki H, Sakashita N, Haraoka S, Isayama T, Naito M, Miyayama H, Yamashita Y, Kikuchi M. Undifferentiated (embryonal) sarcoma of the liver in middle-aged adults: smooth muscle differentiation determined by immunohistochemistry and electron microscopy. Hum Pathol. 2003;34:246–252. doi: 10.1053/hupa.2003.44. [DOI] [PubMed] [Google Scholar]
- 9.Levy M, Trivedi A, Zhang J, Miles L, Mattis AN, Kim GE, Lassman C, Anders RA, Misdraji J, Yerian LM, Xu H, Dhall D, Wang HL. Expression of glypican-3 in undifferentiated embryonal sarcoma and mesenchymal hamartoma of the liver. Hum Pathol. 2012;43:695–701. doi: 10.1016/j.humpath.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agaram NP, Baren A, Antonescu CR. Pediatric and adult hepatic embryonal sarcoma: a comparative ultrastructural study with morphologic correlations. Ultrastruct Pathol. 2006;30:403–408. doi: 10.1080/01913120600854699. [DOI] [PubMed] [Google Scholar]
- 11.Lee TF, Mak KM, Rackovsky O, Lin YL, Kwong AJ, Loke JC, Friedman SL. Downregulation of hepatic stellate cell activation by retinol and palmitate mediated by adipose differentiation-related protein (ADRP) J Cell Physiol. 2010;223:648–657. doi: 10.1002/jcp.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straub BK, Stoeffel P, Heid H, Zimbelmann R, Schirmacher P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008;47:1936–1946. doi: 10.1002/hep.22268. [DOI] [PubMed] [Google Scholar]
- 13.Loo CK, Wu XJ. Origin of stellate cells from submesothelial cells in a developing human liver. Liver Int. 2008;28:1437–1445. doi: 10.1111/j.1478-3231.2008.01788.x. [DOI] [PubMed] [Google Scholar]


