Abstract
The mammalian target of rapamycin (mTOR) plays an important role in cell growth, proliferation, and metabolism. Some studies have associated phosphorylated mTOR (p-mTOR) expression with worse outcome in breast cancers. However, the significance of p-mTOR expression specifically in triple negative breast carcinoma (TNBC) is unknown. In this study, p-mTOR expression was evaluated by immunohistochemistry in 172 TNBCs and the result was correlated with clinicopathologic variables and disease outcome. The majority of tumors (72.1%) were p-mTOR positive; p-mTOR expression did not correlate with age, tumor size, grade, lymph node status, or tumor stage. In patients at stage 1 and 2 disease, those with p-mTOR expression had significantly worse overall as well as recurrence-free survival compared to those without p-mTOR expression. p-mTOR expression appears to be an adverse prognostic indicator in early-stage TNBCs. The assessment of p-mTOR expression in these tumors may also help to stratify patients for future target therapy studies.
Keywords: Phosphorylated mammalian target of rapamycin (p-mTOR), triple negative, breast cancer
Introduction
Breast cancer is a heterogeneous disease consisting of distinct groups that are recognized by their specific morphological, immunohistochemical, and molecular characteristics, as well as different biological behaviors and clinical outcomes [1-4]. Triple negative breast cancer (TNBC) is defined by the lack of estrogen receptor (ER) -alpha, progesterone receptor (PR), and human epidermal growth factor receptor 2 (Her2) amplification; it constitutes 10-17% of all breast cancers, affects younger patients, and is generally associated with a more aggressive clinical behavior and worse prognosis [5-7]. The role of targeted therapeutic options is currently unknown for this group of tumors and there is no recommended standard or specific regimen of chemotherapy. A recent study analyzed the gene expression profiles of 587 cases of TNBC and identified 6 TNBC subtypes with putative molecular targets [8], thus demonstrating the heterogeneity of TNBC. A number of studies for TNBC have been conducted in an attempt to identify potential markers, such as Akt [9], PTEN [10] and SRC [11], that may carry implications for prognosis or targeted drug therapies.
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase which regulates cell growth, proliferation, and metabolism in response to growth factors, hormones, nutrients, hypoxia, and energy (ATP) [12]. mTOR is activated by phosphorylation of Ser2448 through the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway [13]. It belongs to the family of phosphatidylinositol kinase-related kinase (PIKK) [14]. Alterations leading to mTOR activation results in increased protein biosynthesis, cell growth, and tumor development [15]. Mutations in the PIK3CA gene, PTEN loss and aberrant activation of AKT are alterations that have been described in breast cancers [16,17]. mTOR controlling cell growth and translation by phosphorylation of two major targets, including ribosomal S6 kinase 1(S6K1) and 4E-BP family, subsequently leads to the translation of mRNA encoding ribosomal proteins and elongation factors and the promotion of ribosome recruitment and initiation of translation, respectively [18,19]. Inhibition of translation initiation through targeting the mTOR-signaling pathway has emerged as a promising therapeutic option. Moreover, it has been shown that mTOR activation caused by either PTEN mutation or AKT overexpression is particularly susceptible to mTOR inhibitors [20,21]. Therefore, various components of these important pathways have gained much attention in recent years, because of their potential to act as attractive targets for therapy and as markers for predicting disease behavior and outcome in a variety of cancers including breast, ovarian, colon, pancreatic, and gastric cancers [13,22-26]. However, the related studies on breast cancer mainly pertain to breast cancers in general. A study on familial and sporadic invasive breast carcinomas showed that the expression of phosphorylated mTOR (p-mTOR), the activated form of mTOR, had a positive association with lymph node status and a negative impact on patients’ overall survival [26]. Another study found that breast cancers with mTOR overexpression had a three times greater risk for disease recurrence [25]. Very limited data is available in the literature on mTOR expression specifically in TNBCs. To date, there are only few studies that shed light on the role of mTOR in TNBC [10,27,28], but no investigation pertaining to the relationship of p-mTOR expression with disease outcome of TNBC has been performed. Our study is the first to assess the expression of p-mTOR in TNBC from a large cohort of 172 patients and to correlate the results with clinicopathologic parameters and disease outcome.
Materials and methods
A total of 177 patients with TNBC were identified within a period of 4 years. TNBC was defined as invasive breast carcinoma with negative ER, PR, and Her2 immunostain results. Clinicopathologic data, including age at operation, tumor size, tumor grade, lymph node status, TNM stage, follow-up time until death, loss of follow-up, or until December 31, 2009 were recorded and recurrence or metastasis was noted. Hematoxylin and eosin stained tissue sections as well as ER, PR, and Her2 immunostains of the tumors were retrieved from the pathology archives and reviewed by two pathologists (Hsueh S. and Ueng S.H.). Tumor grade was assessed according to the Nottingham combined histologic grading system [29]. ER, PR and Her2 immunostains were reviewed and evaluated according to the recommended guidelines of the College of American Pathologists [30,31]. Only Her2 immunostain negative cases were included. In-situ hybridization for Her2 was not performed. Discrepant results were resolved by consensus review. The clinical stage was defined according to the 2002 American Joint Committee on Cancer criteria [32].
Immunohistochemical detection of phosphomTOR
Formalin-fixed paraffin-embedded tissue blocks of the tumors were retrieved from the archives and one representative tissue block was chosen for each tumor. Immunohistochemical stains for p-mTOR were performed on formalin-fixed, paraffin-embedded sections (5μm thick) using bond polymer detection system and bond automated machine with polymer refine kit. The monoclonal rabbit anti-p-mTOR (Ser2448)( 49F9) antibody ( Cell Signaling, Danvers, MA, USA) was applied at a dilution of 1:100. After deparaffinization, antigen retrieval was performed with ER2 buffer (Leica). Sections were incubated with primary antibodies at room temperature for 60 minutes, followed with polyhorseradish peroxidase (HRP) anti-rabbit IgG reagent to localize the primary antibody, and diaminobenzidine (DAB) was used to visualize the complex. Then the sections were counterstained with hematoxylin, dehydrated, cleared, and mounted. Prostate adenocarcinoma tissue served as the positive control. Negative controls were performed by omission of the primary antibody. Nonneoplastic stroma served as an internal negative control for each slide. Assessment of p-mTOR immunohistochemical stains was carried out independently by two pathologists (Hsueh S. and Ueng S.H.) without prior knowledge of any clinical information during assessment. Discrepant results were resolved by consensus review. Staining was scored as 0 if all cancer cells showed no staining, 1 if staining was faint and present in 5-50% of the tumor cells or if staining was moderate to strong and present in 1-10% of the tumor cells, and 2 if staining was faint in >50% of cells or staining was moderate to strong in >10% of cells. Either nuclear staining or cytoplasmic staining was considered positive. A score of 0 was regarded as negative, score 1 as low expression, and score 2 as high expression; both scores 1 and 2 were regarded as positive in the statistical analysis.
Statistical analysis
Statistical analysis was done using the SPSS statistical program for windows (version 17.0; SPSS, Chicago, USA). The association of p-mTOR expression with clinicopathologic parameters was analyzed using the chi-square test. The Kaplan-Meier method was used to describe the overall survival time and disease free survival time and statistical significance of the difference in survival times was evaluated by the log-rank test. Comparisons were considered significant at P < 0.05.
Results
Of the 177 TNBCs that underwent p-mTOR immunostaining, 5 yielded suboptimal results due to excessive tissue fragmentation and were, therefore, excluded in the final analyses of 172 tumors. The 172 patients were all female. The median age at the time of diagnosis was 47.0 yr (range, 29-79 yr). All underwent surgical resection of tumor with axillary lymph node dissection. None of the patients received preoperative chemotherapy or preoperative radiotherapy. Follow-up time ranged from 5.75 to 119.28 months (mean 68.84 months). Review of the available histologic and immunostained slides confirmed that all of the tumors were negative for ER, PR, and Her2 by immunohistochemistry. One hundred and fifty seven (91.3%) tumors were invasive ductal carcinomas of no special type. Invasive carcinomas with special features included 5 (2.9%) invasive ductal carcinomas with prominent apocrine features, 4 (2.3%) medullary carcinomas, 4 (2.3%) metaplastic carcinomas, 1 (0.6%) invasive mixed ductal and lobular carcinoma, and 1 (0.6%) adenoid cystic carcinoma. Clinicopathologic characteristics are shown in Table 1. There were 65 (37.8%) patients of age less than 45 years and 107 (62.2%) patients who were 45 years or older. Tumor size varied from 0.8 to 17.0 cm (median 2.5); 47 tumors were 2 cm or less in size and 125 tumors were larger than 2 cm. There were 11 (6.4 %) grade 2 tumors, 161 (93.6%) grade 3 tumors, and no grade 1 tumors. Eighty (46.5%) patients had lymph node metastasis while 92 (53.3%) had no metastatic nodes. There were 125 (72.7%) early-stage (defined as stages 1 and 2) tumors and 47 (27.3%) advanced-stage (defined as stages 3 and 4) tumors. Adjuvant chemotherapy was given to most patients except postmenopausal women with tumor size less than 1 cm and without lymph node metastasis. Adjuvant radiotherapy was given to patients who received breast-conserving surgery, whose tumors were larger than 5 cm, or who had more than ten positive axillary nodes. Disease-free survival varied between 2.76 and 119.28 months (median 72.72, mean 62.66), and overall survival ranged from 5.75 to 119.28 months (median 76.87, mean 68.84).
Table 1.
Clinicopathologic characteristics of 172 patients with TNBC
| Variables | No. of cases (%) |
|---|---|
| Age | |
| ≤ 45 | 65 (37.8) |
| >45 | 107 (62.2) |
|
| |
| Tumor size | |
| ≤ 2 cm | 47 (27.3) |
| >2 cm | 125 (72.7) |
|
| |
| Tumor grade | |
| 1 | 0 (0) |
| 2 | 11 (6.4) |
| 3 | 161 (93.6) |
|
| |
| Lymph node status | |
| Negative | 92 (53.5) |
| Positive | 80 (46.5) |
|
| |
| Tumor stage | |
| 1 and 2 | 125 (72.7) |
| 3 and 4 | 47 (27.3) |
|
| |
| p-mTOR | |
| Negative | 48 (27.9) |
| Positive | 124 (72.1) |
| Cytoplasmic positive only | 100 (58.1) |
| Cytoplasmic and nuclear positive | 24 (14.0) |
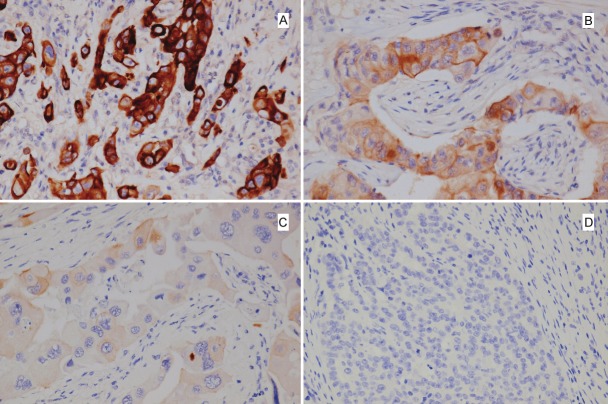
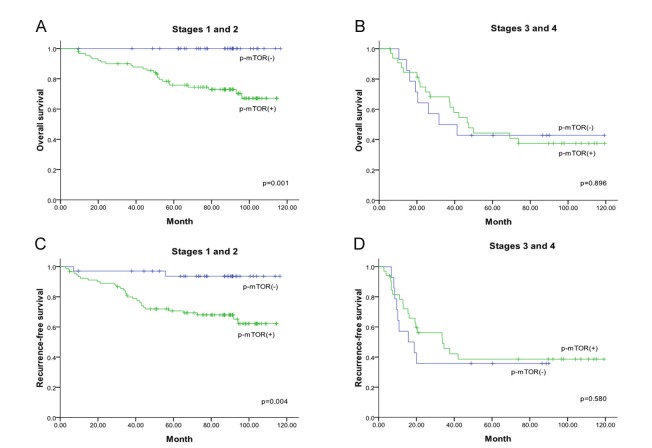
There was heterogeneous expression of p-mTOR in the nuclei and/or cytoplasm of the tumor cells. Forty eight tumors (27.9%) were negative for p-mTOR while 124 (72.1%) tumors showed variable positive staining for p-mTOR in mild, moderate to strong intensity (Figure 1A, B, and C). P-mTOR was located exclusively in the cytoplasm in 100 (58.1%) samples and it was also located in the nucleus in 24 (14.0%). There were 69 (40.1%) p-mTOR score 1 tumors and 55 (32.0%) p-mTOR score 2 tumors. Nuclear and cytoplasmic p-mTOR expression did not correlate with the clinicopathologic variables investigated (Table 2). In patients who had early-stage (stages 1and 2) tumors, those with p-mTOR positivity showed worse overall survival (P=0.001) as well as worse recurrence-free survival (P=0.004) compared to those without p-mTOR positivity (Figure 2). For the early-stage group, the 5-year overall survival in p-mTOR positive patients and negative patients was 76.9% as opposed to 100%, and the 5-year recurrence-free survival in p-mTOR positive patients and negative patients was 71.4% as opposed to 94.1%. This difference was not observed in advanced-stage (stages 3 and 4) patients.
Figure 1.
p-mTOR expression in triple negative breast carcinoma with strong (A) , moderate (B), weak (C), and negative immunoreactivity (D).
Table 2.
Association of p-mTOR with clinicopathologic variables
| Variables | Negative | Positive | Cytoplasmic positive only | Nuclear and cytoplasmic positive | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. (%) | No. (%) | P value | No. (%) | P value | No. (%) | P value | |
| Age | NS | NS | NS | ||||
| ≤ 45 yr | 20 (30.8) | 45 (69.2) | 36 (55.4) | 9 (13.8) | |||
| >45 yr | 28 (26.2) | 79 (73.8) | 64 (59.8) | 15 (14.0) | |||
| Tumor size | NS | NS | NS | ||||
| ≤ 2 cm | 13 (27.7) | 34 (72.3) | 23 (48.9) | 11 (23.4) | |||
| >2 cm | 35 (28.0) | 90 (72.0) | 77 (61.6) | 13 (10.4) | |||
| Tumor grade | NS | NS | NS | ||||
| 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 2 | 1 (9.1) | 10 (90.9) | 6 (54.5) | 4 (36.4) | |||
| 3 | 47 (29.2) | 114 (70.8) | 94 (58.4) | 20 (12.4) | |||
| Lymph node | NS | NS | NS | ||||
| Negative | 27 (29.3) | 65 (70.7) | 52 (56.5) | 13 (14.1) | |||
| Positive | 21 (26.3) | 59 (73.8) | 48 (60) | 11 (13.8) | |||
| Tumor stage | NS | NS | NS | ||||
| 1 and 2 | 34 (27.2) | 91 (72.8) | 74 (59.2) | 17 (13.6) | |||
| 3 and 4 | 14 (29.8) | 33 (70.2) | 26 (55.3) | 7 (14.9) | |||
NS: not significant.
Figure 2.
Kaplan-Meier survival curves for triple negative breast cancer patients according to p-mTOR positivity (green color line) and p-mTOR negativity (blue color line). Overall survival for early-stage patients (A), and advanced-stage patients (B). Recurrence-free survival for early-stage patients (C), and advanced-stage patients (D).
Discussion
The only option of treatment for TNBCs is chemotherapy, however, there is no targeted regimen for the time being. Although TNBCs have been reported to respond to neoadjuvant chemotherapy [33,34], the survival of patients with these tumors remains poor [34,35]. Clearly, there is a need for targeted therapies for TNBC but the outline of molecular events involved in the growth of these tumors is still obscure.
Our study suggests that TNBC patients may benefit from mTOR inhibitors since the majority of TNBCs (72.1%) showed positive immunostaining for p-mTOR. No significant relationship was found between p-mTOR expression and clinicopathologic variables, however, p-mTOR expression was found to correlate with overall survival and disease-free survival in early-stage (stages 1 and 2) TNBCs. In the early-stage group, patients with tumors negative for p-mTOR had significantly better survival than those with tumors positive for p-mTOR. It was noted that survival time for patients with early-stage TNBCs did not differ significantly between the groups of high p-mTOR expression (score 2) and low expression (score 1); and that both high and low p-mTOR positive TNBCs showed significantly worse survival compared to p-mTOR negative TNBCs. Likewise, the survival time did not differ significantly between tumors with exclusive cytoplasmic expression and those with both cytoplasmic and nuclear expression.
Our results are in agreement with some previous studies of p-mTOR expression in breast carcinomas in general [25,26,36]. Zhou et al. found that higher levels of p-mTOR expression was associated with a poor disease free survival in 165 breast carcinomas; they also discovered that p-mTOR was positively associated with Her2 overexpression [36]. In another study that evaluated protein expression in 145 invasive breast cancers and 140 ductal carcinomas in-situ (DCIS) by immunohistochemistry on tissue microarray, the Akt pathway was found to be activated early in the in-situ stage, and cancers with mTOR overexpression showed a three times greater risk for disease recurrence [25]. Bakarakos et al. detected p-mTOR expression by immunohistochemistry and imaging analysis in 44.2% of 215 invasive breast carcinomas and found that p-mTOR showed a positive association with lymph node status (P = 0.010) and had a negative impact on patients’ overall survival (P = 0.016) [26]. It appears that activation of the mTOR pathway is related to a more aggressive phenotype in both TNBC and non-TNBC breast cancers.
It is already known that aberrant activation of the PI3K/Akt/mTOR pathway is involved in the oncogenesis and progression of breast cancer [23-26], and in-vitro, in-vivo, as well as preclinical studies of drug therapies targeting constituents of this particular pathway have shown significant benefit for certain subsets of breast cancer [20,23,37,38]. Most published data regarding p-mTOR in breast carcinomas have been derived from studies of breast cancers in general. With respect to TNBCs, there is scant information in the literature concerning the p-mTOR status in these tumors. One study of basal-like breast carcinoma (BLBC) found the PI3K pathway to be activated and up-regulated to a higher extent in 13 BLBCs compared with 11 Her2 positive tumors, shown by a significantly increased activation of the downstream targets Akt and mTOR by western blotting [10]. Another study regarding the PI3K pathway in TNBC utilized immunohistochemistry and immunoblotting to demonstrate the higher level of pAkt and the significantly greater ratio of pAkt : Akt in TNBCs compared with other groups of breast cancers (ER+ and Her2-, ER+ and Her2+, and ER- and Her2+), though the study was limited to a small number of cases with only 8 TNBCs in a total of 44 samples of breast cancer, and p-mTOR status was not studied [9]. In a more recent study, Walsh et al. investigated mTOR and p-mTOR immunohistochemical staining in tissue microarrays of 89 TNBCs and 99 non-TNBCs. They found that mTOR was expressed in a similar proportion of patients with TNBCs and non-TNBCs (53% vs 41%). In the total population of TNBCs and non-TNBCs, mTOR was found to be more frequently detected in high grade (grade III) than in low grade (grade I and II) cancers (p 0.009). Nuclear p-mTOR was detected more frequently in the TNBC than non-TNBC samples, therefore, the authors suggested that mTOR may play a more important role in the development of TNBC compared to non-TNBC [27].Our p-mTOR immunostaining results also show that a considerable number of tumors had nuclear positivity, albeit constituting a smaller proportion in comparison with tumors showing exclusive cytoplasmic staining. In the study by Walsh et al., staining intensity was not taken into consideration, and comparable results were obtained irrespective of the cut-off point chosen for the staining extent of positive tumor cells, including 10%, 20% and 50%. Similarly, though both staining intensity and extent were included in our scoring system, no statistic difference was observed regardless of various cut-off points used to stratify the tumors into high and low p-mTOR expression groups. Thus, we divided the tumors into positive and negative groups in the statistical analysis.
In summary, immunohistochemical detection of p-mTOR was performed on 172 cases of TNBC, of which 72.1% were found to be p-mTOR positive. Furthermore, p-mTOR expression in early stage TNBCs correlated with worse overall survival and recurrence-free survival. Thus, our findings suggest that p-mTOR may serve as a potential marker for prediction of prognosis and as an attractive target for anticancer therapy in TNBC patients. However, further studies are warranted to clarify the role of p-mTOR in TNBC and the possible clinical benefit of mTOR inhibitors in patients with TNBCs.
Acknowledgements
This study was supported by grants from the Ministry of Education, Taiwan, ROC (EMRPD1A0391, to C. H.). The authors thank the Linko Chang Gung Memorial Hospital Tissue bank for providing access to the tissue archive.
References
- 1.Perou C, Sorlie T, Eisen M, Rijn M, Jeffrey S, Rees C, Pollack J, Ross D, Johnsen H, Akslen L, Fluge O, Pergamenschikov A, Williams C, Zhu S, Lonning P, Borresen-Dale A, Brown P, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorlie T. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proceedings of the National Academy of Sciences. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis-Filho JS, Westbury C, Pierga JY. The impact of expression profiling on prognostic and predictive testing in breast cancer. J Clin Pathol. 2006;59:225–231. doi: 10.1136/jcp.2005.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, Harris L, Hait W, Toppmeyer D. Locoregional Relapse and Distant Metastasis in Conservatively Managed Triple Negative Early-Stage Breast Cancer. Journal of Clinical Oncology. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clinical Cancer Research. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 7.Reis-Filho JS, Tutt ANJ. Triple negative tumours: a critical review. Histopathology. 2007;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. Journal of Clinical Investigation. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umemura S, Yoshida S, Ohta Y, Naito K, Osamura RY, Tokuda Y. Increased phosphorylation of Akt in triple-negative breast cancers. Cancer Science. 2007;98:1889–1892. doi: 10.1111/j.1349-7006.2007.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marty B, Maire V, Gravier E, Rigaill G, Vincent-Salomon A, Kappler M, Lebigot I, Djelti F, Tourdès A, Gestraud P, Hupé P, Barillot E, Cruzalegui F, Tucker GC, Stern M-H, Thiery J-P, Hickman JA, Dubois T. Frequent PTEN genomic alterations and activated phosphatidylinositol 3-kinase pathway in basal-like breast cancer cells. Breast Cancer Research. 2008;10:R101. doi: 10.1186/bcr2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, Slamon DJ. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/”triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–326. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 12.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 13.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 14.Cutler NS, Heitman J, Cardenas ME. TOR kinase homologs function in a signal transduction pathway that is conserved from yeast to mammals. Mol Cell Endocrinol. 1999;155:135–142. doi: 10.1016/s0303-7207(99)00121-5. [DOI] [PubMed] [Google Scholar]
- 15.Seeliger H, Guba M, Kleespies A, Jauch KW, Bruns CJ. Role of mTOR in solid tumor systems: a therapeutical target against primary tumor growth, metastases, and angiogenesis. Cancer Metastasis Rev. 2007;26:611–621. doi: 10.1007/s10555-007-9077-8. [DOI] [PubMed] [Google Scholar]
- 16.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JKV, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High Frequency of Mutations of the PIK3CA Gene in Human Cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Tenorio G, Alkhori L, Olsson B, Waltersson MA, Nordenskjold B, Rutqvist LE, Skoog L, Stal O. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- 18.Proud CG. Role of mTOR signalling in the control of translation initiation and elongation by nutrients. Curr Top Microbiol Immunol. 2004;279:215–244. doi: 10.1007/978-3-642-18930-2_13. [DOI] [PubMed] [Google Scholar]
- 19.Gera JF, Mellinghoff IK, Shi Y, Rettig MB, Tran C, Hsu JH, Sawyers CL, Lichtenstein AK. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279:2737–2746. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- 20.Noh WC. Determinants of Rapamycin Sensitivity in Breast Cancer Cells. Clinical Cancer Research. 2004;10:1013–1023. doi: 10.1158/1078-0432.ccr-03-0043. [DOI] [PubMed] [Google Scholar]
- 21.Yu K, Toral-Barza L, Discafani C, Zhang WG, Skotnicki J, Frost P, Gibbons JJ. mTOR, a novel target in breast cancer: the effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer. 2001;8:249–258. doi: 10.1677/erc.0.0080249. [DOI] [PubMed] [Google Scholar]
- 22.O’Reilly KE. mTOR Inhibition Induces Upstream Receptor Tyrosine Kinase Signaling and Activates Akt. Cancer Research. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinonen H, Nieminen A, Saarela M, Kallioniemi A, Klefström J, Hautaniemi S, Monni O. Deciphering downstream gene targets of PI3K/mTOR/p70S6K pathway in breast cancer. BMC Genomics. 2008;9:348. doi: 10.1186/1471-2164-9-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dillon R, White D, Muller W. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 2007;26:1338–1345. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- 25.Bose S, Chandran S, Mirocha JM, Bose N. The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol. 2005;19:238–245. doi: 10.1038/modpathol.3800525. [DOI] [PubMed] [Google Scholar]
- 26.Bakarakos P, Theohari I, Nomikos A, Mylona E, Papadimitriou C, Dimopoulos A-M, Nakopoulou L. Immunohistochemical study of PTEN and phosphorylated mTOR proteins in familial and sporadic invasive breast carcinomas. Histopathology. 2010;56:876–882. doi: 10.1111/j.1365-2559.2010.03570.x. [DOI] [PubMed] [Google Scholar]
- 27.Walsh S, Flanagan L, Quinn C, Evoy D, McDermott EW, Pierce A, Duffy MJ. mTOR in breast cancer: Differential expression in triple-negative and non-triple-negative tumors. The Breast. 2012;21:178–182. doi: 10.1016/j.breast.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima H, Ishikawa Y, Furuya M, Sano T, Ohno Y, Horiguchi J, Oyama T. Protein expression, gene amplification, and mutational analysis of EGFR in triple-negative breast cancer. Breast Cancer. 2012 doi: 10.1007/s12282-012-0354-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Elston CW, Ellis IO, Pinder SE. Pathological prognostic factors in breast cancer. Crit Rev Oncol Hematol. 1999;31:209–223. doi: 10.1016/s1040-8428(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 30.Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FCG, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer (Unabridged Version) Archives of Pathology & Laboratory Medicine. 2010;134:e48–e72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 31.Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Archives of Pathology & Laboratory Medicine. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 32.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, Hughes LL, Hutter RVP, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL. Revision of the American Joint Committee on Cancer Staging System for Breast Cancer. Journal of Clinical Oncology. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 33.Rouzier R. Breast Cancer Molecular Subtypes Respond Differently to Preoperative Chemotherapy. Clinical Cancer Research. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 34.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The Triple Negative Paradox: Primary Tumor Chemosensitivity of Breast Cancer Subtypes. Clinical Cancer Research. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 35.Reis-Filho J, Tutt A. Triple negative tumours: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X. Activation of the Akt/Mammalian Target of Rapamycin/4E-BP1 Pathway by ErbB2 Overexpression Predicts Tumor Progression in Breast Cancers. Clinical Cancer Research. 2004;10:6779–6788. doi: 10.1158/1078-0432.CCR-04-0112. [DOI] [PubMed] [Google Scholar]
- 37.Mondesire WH. Targeting Mammalian Target of Rapamycin Synergistically Enhances Chemotherapy-Induced Cytotoxicity in Breast Cancer Cells. Clinical Cancer Research. 2004;10:7031–7042. doi: 10.1158/1078-0432.CCR-04-0361. [DOI] [PubMed] [Google Scholar]
- 38.Zeng Q, Yang Z, Gao Y-J, Yuan H, Cui K, Shi Y, Wang H, Huang X, Wong STC, Wang Y, Kesari S, Ji R-R, Xu X. Treating triple-negative breast cancer by a combination of rapamycin and cyclophosphamide: An in vivo bioluminescence imaging study. European Journal of Cancer. 2010;46:1132–1143. doi: 10.1016/j.ejca.2010.01.014. [DOI] [PubMed] [Google Scholar]