Abstract
Aims: Primary peritoneal serous carcinoma (PPSC) is an unusual neoplasm that has not been properly characterized. To better define the clinicopathological and immunohistochemical features of PPSC, we present 6 such cases. Methods: The 6 patients consisted of one man and 5 women, ranging in age from 45 and 75 years. None of the patients had any history or clinical evidence of tumor elsewhere. The immunohistochemical profile was examined using antibodies against β-catenin, E-cadherin, wnt5a, EGFR, VEGF, vimentin, Ki67, and P53. Results: Of all the 6 PPSC cases, 5 cases presented stage IIIC and 1 case presented stage IV. Microscopically, 5 cases were poorly differentiated and 1 was moderately differentiated. All cases showed positive staining for β-catenin, E-cadherin, vimentin, VEGF, P53, and Ki67, 4 cases expressed EGFR, and all cases were consistently negative for wnt5a. Conclusions: We described 6 cases of PPSC with clinicopathological and immunohistochemical features. The findings provide basic knowledge of PPSC.
Keywords: Primary peritoneal serous carcinoma, epithelial ovarian cancer, serous carcinoma
Introduction
Primary peritoneal serous carcinoma (PPSC) is a rare primary malignancy of the peritoneum. Clinically and histopathologically, PPSC is similar to serous ovarian papillary carcinoma, and most scientist are applying the FIGO staging criteria for epithelial ovarian cancer to determine the stage of PPSC [1]. Studies on the molecular pathogenesis suggested that HER-2/neu, p53 [2], Wilm’s tumor suppressor protein (WT1), estrogen and progesterone receptor may be involved in the tumorigenesis of PPSC [3-5].
In most of the reported studies, the emphasis has been on the clinical characteristics of the tumor. However, only a few studies examined the immunohistochemical profiles of PPSC. We herein present 6 cases of PPSC with an emphasis of immunohistochemical features and evaluated their correlation with clinicopathological characteristics.
Materials and methods
This study has been approved by the Sun Yat-Sen University Ethics Committee. Six cases of PPSC were retrieved from the electronic medical records of the Department of Pathology, Sun Yat-Sen University Cancer Center in a period of 20 years (1991–2011). Diagnosis of PPSC was confirmed by the clinical and histologic characteristics, excluding the presence of mesothelioma, ovarian cancer, and occult fallopian tube cancer. Formalin-fixed, paraffin-embedded tissue blocks were available for review and immunohistochemical studies in each case. Immunohistochemical studies for β-catenin (Cell Signaling Technology, USA; 1:100), Wnt5a (Abnova, Taiwan; 1:200), E-cadherin (Invitrogen, USA; 1:100), VEGF (BioGenex, USA; 1:100), EGFR (Invitrogen, USA; 1:200), vimentin (Invitrogen, USA; 1:200), Ki67 (DAKO, Denmark; 1:100), and P53 (Invitrogen, USA; detects mutant p53, 1:200) were performed with concurrent adequate controls. Clinical follow-up information was obtained from the patients’ medical charts.
Immunohistochemistry staining was performed according to standard techniques. All stained slides were separately scored by two pathologists. Both the intensity and percentage of IHC staining were analyzed. The intensity was scored as follows: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining; and the percentage of stained cells was scored as: 0 (0 positive cells), 1 (1-10% positive cells), 2 (11–50% of positive cells), 3 (51-80% of positive cells), or 4 (81-100% of positive cells). A final score was defined by multiplying the percentage of positive cells by the intensity [6]. The labeling index for Ki-67 and P53 were represented by the ratio of positive cells in relation to total cells using Image J software. Approximately 2000 nuclei were counted in 5 randomly selected high-power fields (40X) in each specimen.
Results
Clinical features
The main clinical features of all 6 cases are summarized in Table 1. The patients were 5 women and 1 man aged 45 to 75 years (mean age 59 years) at first surgery. Of all the 6 PPSC cases, 5 (83.3%) was poorly differentiated (grade 2) and 1 (16.7%) was moderately differentiated (grade 3). Surgical stage was IIIC in 5 (83.3%) cases and IV in the remaining 1 (16.7%) case. The main presenting symptoms were related to mass effect and included abdominal swelling, abdominal pain, and pelvic discomfort. The main affected organs included uterus, ovary, omentum, mesentery, colorectum, appendix, and Liver. None of the patients had a previous history or clinical evidence of tumor elsewhere. Follow-up ranged from 1 to 64 months. Four patients developed recurrence and were all alive at the last follow-up. One patient died of cerebral infarction 1 month after surgery. One patient was lost to follow-up.
Table 1.
Summary of clinical features
| Case | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Sex | F | F | M | F | F | F |
| Age (Years) | 70 | 53 | 75 | 45 | 59 | 53 |
| FIGO Stage | IIIC | IIIC | IIIC | IIIC | IV | IIIC |
| Grade | 2 | 3 | 3 | 3 | 3 | 3 |
| Affected Organs | Ut, Ov, Co, Om | Ut, Ov, Co | Co, Me | Ut, Ov, Co, Om, Me, Li | Ut, Ov, Co, Om, Me | Ut, Ov, Co, Om, Ap |
| Follow-up | NK | Alive at 9 mo | Dead at 1 mo | Alive at 8 mo | Alive at 64 mo | Alive at 5 mo |
Abbreviations: F, female; M, male; NK, not known. Ut, Uterus; Ov, Ovary; OM, omentum; Me, mesentery; Co, colorectum; Ap, appendix; Li, Liver.
Immunohistochemical features
Immunohistochemical features are shown in Table 2. All the 6 cases were consistently positive for β-catenin (Figure 1A-C) and E-cadherin (Figure 1D), showing strong staining intensity in more than 80% of tumor cells. Most cases were positive for membranous (6/6) and cytoplasmic (5/6) β-catenin staining, while only 1 case showed nuclear staining of β-catenin. All the 6 cases were consistently negative for wnt5a (Figure 1E-F). EGFR was expressed in 4 of 6 cases. Three cases demonstrated more than 80% of positive cells, and the remaining 1 case showed 51% to 80% of positive cells. Staining intensity was classified as weak and moderate in 2 and 2 cases, respectively (Figure 2A-B). VEGF was expressed in all cases, with 3 cases demonstrating more than 80% of positive cells, and 3 cases showing 11% to 50% of positive cells. Staining intensity was classified as moderate and strong in 1 and 5 cases, respectively (Figure 2C). Vimentin was strongly expressed in all the 6 cases, with 5 cases demonstrating 1% to 10% of positive cells, and the remaining 1 case showing 11% to 50% of positive cells (Figure 2D). The immunohistochemical score was defined by multiplying the percentage of positive cells by the intensity to reflect the amount the protein markers expressed by the cancer cells more accurately. Ki-67 and P53 were expressed in all cases, with labeling ranged from 7.5% to 57.5%, and from 16% to 92.9%, respectively (Figure 2E-F).
Table 2.
Summary of immunohistochemical scores and labeling index
| Case | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| β-catenin | 12 | 8 | 12 | 12 | 4 | 8 |
| E-cadherin | 12 | 4 | 12 | 8 | 8 | 12 |
| Wnt5a | 0 | 0 | 0 | 0 | 0 | 0 |
| EGFR | 8 | 0 | 8 | 2 | 4 | 0 |
| VEGF | 6 | 8 | 12 | 12 | 6 | 6 |
| Vimentin | 3 | 6 | 3 | 3 | 3 | 3 |
| P53% | 92.9% | 91.3% | 52.2% | 16.7% | 92.1% | 90.7% |
| Ki67% | 36.3% | 25.9% | 7.5% | 22.6% | 6.2% | 57.5% |
Figure 1.
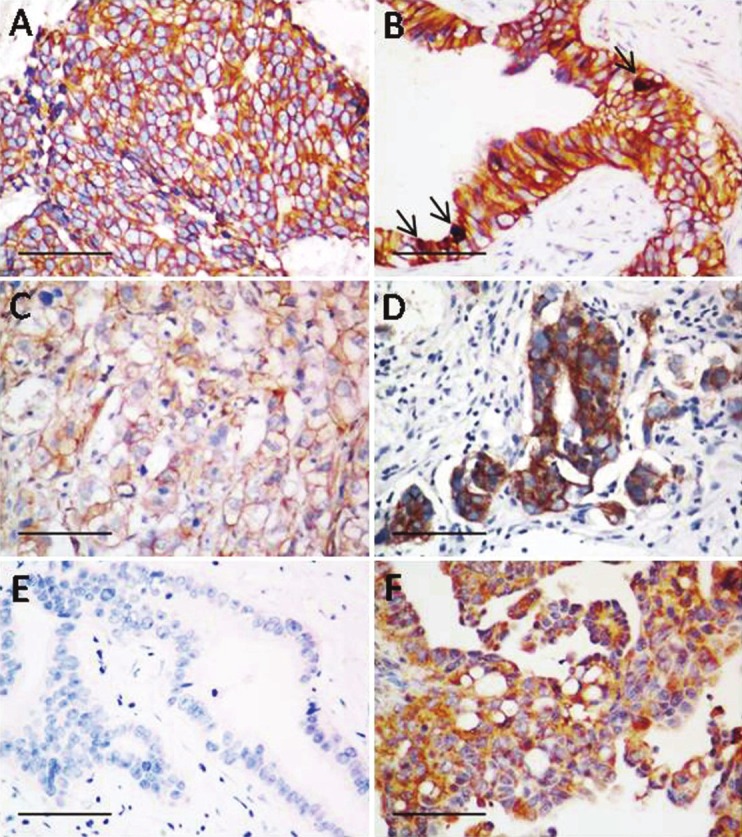
Representative immunostaining of wnt signaling molecules (β-catenin, Wnt5a, and E-cadherin) in PPSC. A: Membranous and cytoplasmic β-catenin staining; B: Membranous β-catenin staining; C: Nuclear β-catenin staining (arrow); D: E-cadherin immunostaining of PPSC; E: wnt5a staining is absent in PPSC; F: wnt5a immunostaining in ovarian cancer as a control. Bars 100 μm.
Figure 2.
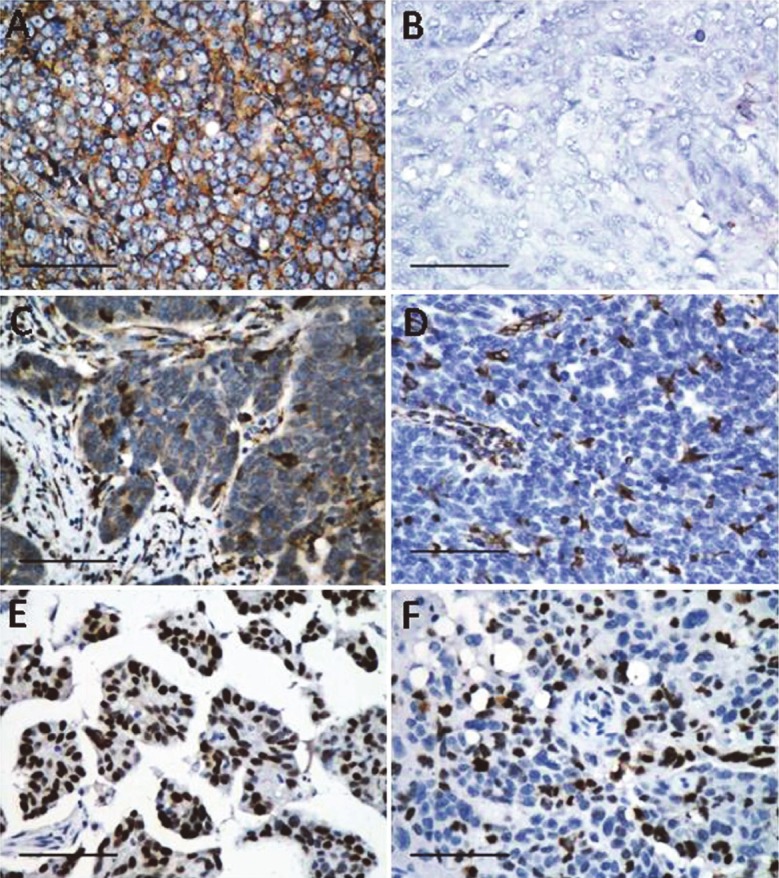
Representative immunostaining of positive EGFR (A), negative EGFR (B) VEGF (C), vimentin (D), mutant P53 (E), and Ki-67 (F) in PPSC. Bars 100μm.
Discussion
The origin of PPSC has not been well characterized. It was thought to arise from the mesothelium of the peritoneum in early studies [7], or from the coelomic epithelium lining the abdominal cavity responding to oncogenic stimulus [8]. More recent data suggested that the fallopian tube may be another source of PPSC [9]. Molecular studies have been inconclusive in illustrating the tumorigenesis of PPSC. Carlson et al. [10] discovered that fimbria is the source of nearly one half of PPSCs by comparing the p53 mutation in both peritoneal and tubal lesions. Schorge et al. [11] described BRCA1 mutations in 48% of patients with PPSC, of which 89% p53 mutations were observed, which is consistent to our observation that almost all of the PPSC patients express a high level of mutant p53.
There have been many studies demonstrating the clinical and biological similarities between PPSC and epithelial ovarian cancer, as well as some differences. Dubernard et al. [12] compared PPSC and epithelial ovarian cancer in tumor histologic subtype, tumor stage, tumor grade, residual disease, and age. They concluded that the overall survival of patients with PPSC is similar to that of epithelial ovarian cancer group, and that the management of these two diseases should not be different. Choi et al. [13] reported that patients with PPSC have higher levels of CA-125, more omental involvement, and less effect on response to chemotherapy than that with epithelial ovarian cancer. Histologically, the differences of PPSC and epithelial ovarian cancer are currently indistinguishable [4,14]. Because of the similarity in histological profile, nearly 10% of epithelial ovarian cancer diagnosed were reclassified as PPSC [15]. In addition, the similar histology and close clinical relationship of PPSC and epithelial ovarian cancer indicates that they may develop from the same origin [16].
To date, there have been few comprehensive immunohistochemical studies of PPSC. Von Riedenauer et al. [4] showed positive estrogen receptor (ER), cytokeratin 7 (CK7), Wilm’s tumor suppressor gene (WT1), and cancer antigen 125 (CA 125) staining in PPSC. Chen et al. [17], who examined 32 patients of PPSC, showed that the samples were positive for HER-2/neu (34.4%), p53 (71.9%), bcl-2 (9.4%), and nm23-H1 (100%). Barnetson et al. [14], who investigated 14 cases of PPSC, have shown strong expression of Ber-EP4 (86%), Mesothelin (71%), MOC31 (71%), CA 125 (79%), and ER (86%).
Based on the presence of the histologic features of PPSC, we made an attempt at discovering some potential molecular markers that could help understanding the molecular mechanisms of PPSC tumorigenesis. We investigated the expression of key molecules of wnt signaling (β-catenin, Wnt5a, and E-cadherin), which have been proved to be involved in ovarian tumorigenesis [18], in PPSC. As the result, we found that the tumor cells were strong positive for β-catenin and E-cadherin, but consistently negative for Wnt5a. It has been proposed that the nuclear localization of β-catenin was a prognostic marker in a number of human cancers. Verghese et al. [19] described that the fibroblasts with nuclear β-catenin in tumors is a good prognostic indicator for breast cancer. Liu et al. [20] reported that β-catenin was positively correlated with the Karnofsky performance scale (KPS) score and World Health Organization (WHO) grades of human gliomas. Kildal et al. [21] demonstrated that nuclear β-catenin localization was positively correlated associated with good prognostic outcome in patients with ovarian cancer, and that higher nuclear β-catenin expression was observed in grade 1 (16%) and 2 (24%) than in grade 3 (6%) ovarian carcinomas. The present study concurs with those previous studies in that nuclear β-catenin expression was uncommon in high grade tumors.
It has been shown that the expression of E-cadherin was significantly positively correlated with overall survival of ovarian carcinoma, probably through suppressing tumor invasion and metastasis [22,23]; yet, there have been reports demonstrating that E-cadherin may facilitate the ovarian tumor cells aggregation, adherence and invasion to the peritoneum, which results in coelomic metastasis of ovarian cancer [24,25]. Our data may explain the correlation of multiple organs involvement in PPSC and the cell migration and invasion-promoting effects of E-cadherin. Moreover, some other studies assessed the correlation of nuclear β-catenin expression and the loss of E-cadherin in tumor invasion [26,27]. However, no such reverse association between nuclear β-catenin and E-cadherin expression was found in this study. This could be because of the small sample size, or the intrinsic tumor heterogeneity.
Wnt5a was found to be highly expressed in high-grade ovarian carcinoma [28] and several malignancies such as stomach, prostate, melanoma, and breast [29]. It may be useful in predicting the prognosis and chemosensitivity to anticancer drugs in ovarian cancers. Kurayoshi et al. [30] suggested that Wnt-5a is correlated with tumor aggressiveness by stimulating cell migration and invasion. However, wnt5a expression was negative in all the present cases, which implies that wnt5a could represent a potential new marker to distinguish epithelial ovarian cancer and PPSC.
We also investigated the expression of VEGF, EGFR, vimentin, and P53, which play critical roles in serous carcinoma carcinogenesis. It is well known that VEGF contribute to tumor angiogenesis and progression, and that it promotes ascites accumulation in ovarian cancers [24]. VEGF expression was all positive in the present cases, as expected. The EGFR and vimentin expression was 67% (4/6) and 33% (2/6), respectively. EGFR has been reported to be expressed in ovarian cancers and is associated with poor prognosis [31]. Vimentin is a member of the intermediate filament protein family. In addition, it represents a potential new marker for epithelial-mesenchymal transition (EMT) [32]. The mean P53 labeling index was 82%, suggesting that P53 mutations are frequent in PPSC. The mean Ki-67 labeling index in the present study was 30%, indicating moderate proliferative activity of PPSC. These immunohistochemical data may provide basic knowledge of PPSC.
PPSC predominantly affects postmenopausal women. In our series, five cases also occurred in postmenopausal women, while only one of our patients was a 75-year-old man. Interestingly, the Ki67 labeling index in this male patient was only 7.5%, which was significantly lower than that in female patients. With regards to other markers, there were no significant difference between the male case and the remaining female cases. Further studies are needed to better understand the underlying links between different sexual immunoprofiles and clinical characteristics.
Conclusion
In summary, we have described the clinicopathologic and immunohistochemical findings in 6 patients with PPSC. We studied the molecular changes occurring in PPSC and analyzed their potential roles in tumorigenesis, which may enable the discovery of biomarkers and targeted therapeutic agents. In addition, we found that wnt5a may be useful in the differential diagnosis of PPSC.
References
- 1.Liu Q, Lin JX, Shi QL, Wu B, Ma HH, Sun GQ. Primary peritoneal serous papillary carcinoma: a clinical and pathological study. Pathol Oncol Res. 2011;17:713–719. doi: 10.1007/s12253-011-9375-x. [DOI] [PubMed] [Google Scholar]
- 2.Chivukula M, Niemeier LA, Edwards R, Nikiforova M, Mantha G, McManus K, Carter G. Carcinomas of Distal Fallopian Tube and Their Association with Tubal Intraepithelial Carcinoma: Do They Share a Common “Precursor” Lesion? Loss of Heterozygosity and Immunohistochemical Analysis Using PAX 2, WT-1, and P53 Markers. ISRN Obstet Gynecol. 2011;2011:858647. doi: 10.5402/2011/858647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakao M, Oguri T, Maeno K, Ota C, Takakuwa O, Iwashima Y, Miyazaki M, Kutsuna T, Nakamura A, Sato S, Ueda R. Endobronchial metastasis from primary papillary serous carcinoma of the peritoneum. Intern Med. 2009;48:1165–1168. doi: 10.2169/internalmedicine.48.2140. [DOI] [PubMed] [Google Scholar]
- 4.von Riedenauer WB, Janjua SA, Kwon DS, Zhang Z, Velanovich V. Immunohistochemical identification of primary peritoneal serous cystadenocarcinoma mimicking advanced colorectal carcinoma: a case report. J Med Case Reports. 2007;1:150. doi: 10.1186/1752-1947-1-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross SN, Cocco E, Bellone S, Anagnostou VK, Brower SL, Richter CE, Siegel ER, Schwartz PE, Rutherford TJ, Santin AD. Differential sensitivity to platinum-based chemotherapy in primary uterine serous papillary carcinoma cell lines with high vs low HER-2/neu expression in vitro. Am J Obstet Gynecol. 2010;203:162.e1–8. doi: 10.1016/j.ajog.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han CP, Kok LF, Wang PH, Wu TS, Tyan YS, Cheng YW, Lee MY, Yang SF. Scoring of p16(INK4a) immunohistochemistry based on independent nuclear staining alone can sufficiently distinguish between endocervical and endometrial adenocarcinomas in a tissue microarray study. Mod Pathol. 2009;22:797–806. doi: 10.1038/modpathol.2009.31. [DOI] [PubMed] [Google Scholar]
- 7.Raju U, Fine G, Greenawald KA, Ohorodnik JM. Primary papillary serous neoplasia of the peritoneum: a clinicopathologic and ultrastructural study of eight cases. Hum Pathol. 1989;20:426–436. doi: 10.1016/0046-8177(89)90006-3. [DOI] [PubMed] [Google Scholar]
- 8.Truong LD, Maccato ML, Awalt H, Cagle PT, Schwartz MR, Kaplan AL. Serous surface carcinoma of the peritoneum: a clinicopathologic study of 22 cases. Hum Pathol. 1990;21:99–110. doi: 10.1016/0046-8177(90)90081-f. [DOI] [PubMed] [Google Scholar]
- 9.Seidman JD, Zhao P, Yemelyanova A. “Primary peritoneal” high-grade serous carcinoma is very likely metastatic from serous tubal intraepithelial carcinoma: assessing the new paradigm of ovarian and pelvic serous carcinogenesis and its implications for screening for ovarian cancer. Gynecol Oncol. 2011;120:470–473. doi: 10.1016/j.ygyno.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Carlson JW, Miron A, Jarboe EA, Parast MM, Hirsch MS, Lee Y, Muto MG, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J. Clin. Oncol. 2008;26:4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schorge JO, Muto MG, Lee SJ, Huang LW, Welch WR, Bell DA, Keung EZ, Berkowitz RS, Mok SC. BRCA1-related papillary serous carcinoma of the peritoneum has a unique molecular pathogenesis. Cancer Res. 2000;60:1361–1364. [PubMed] [Google Scholar]
- 12.Dubernard G, Morice P, Rey A, Camatte S, Fourchotte V, Thoury A, Pomel C, Pautier P, Lhomme C, Duvillard P, Castaigne D. Prognosis of stage III or IV primary peritoneal serous papillary carcinoma. Eur J Surg Oncol. 2004;30:976–981. doi: 10.1016/j.ejso.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Choi CH, Kim TJ, Kim WY, Ahn GH, Lee JW, Kim BG, Lee JH, Bae DS. Papillary serous carcinoma in ovaries of normal size: a clinicopathologic study of 20 cases and comparison with extraovarian peritoneal papillary serous carcinoma. Gynecol Oncol. 2007;105:762–768. doi: 10.1016/j.ygyno.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Barnetson RJ, Burnett RA, Downie I, Harper CM, Roberts F. Immunohistochemical analysis of peritoneal mesothelioma and primary and secondary serous carcinoma of the peritoneum: antibodies to estrogen and progesterone receptors are useful. Am J Clin Pathol. 2006;125:67–76. [PubMed] [Google Scholar]
- 15.Steinhagen PR, Sehouli J. The involvement of retroperitoneal lymph nodes in primary serous-papillary peritoneal carcinoma. a systematic review of the literature. Anticancer Res. 2011;31:1387–1394. [PubMed] [Google Scholar]
- 16.Schmeler KM, Sun CC, Malpica A, Deavers MT, Bodurka DC, Gershenson DM. Low-grade serous primary peritoneal carcinoma. Gynecol Oncol. 2011;121:482–486. doi: 10.1016/j.ygyno.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Chen LM, Yamada SD, Fu YS, Baldwin RL, Karlan BY. Molecular similarities between primary peritoneal and primary ovarian carcinomas. Int J Gynecol Cancer. 2003;13:749–755. doi: 10.1111/j.1525-1438.2003.13605.x. [DOI] [PubMed] [Google Scholar]
- 18.Schmid S, Bieber M, Zhang F, Zhang M, He B, Jablons D, Teng NN. Wnt and hedgehog gene pathway expression in serous ovarian cancer. Int J Gynecol Cancer. 2011;21:975–980. doi: 10.1097/IGC.0b013e31821caa6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verghese ET, Shenoy H, Cookson VJ, Green CA, Howarth J, Partanen RH, Pollock S, Waterworth A, Speirs V, Hughes TA, Hanby AM. Epithelial-mesenchymal interactions in breast cancer: evidence for a role of nuclear localized beta-catenin in carcinoma-associated fibroblasts. Histopathology. 2011;59:609–618. doi: 10.1111/j.1365-2559.2011.03917.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Tu Y, Sun X, Jiang J, Jin X, Bo X, Li Z, Bian A, Wang X, Liu D, Wang Z, Ding L. Wnt/beta-Catenin pathway in human glioma: expression pattern and clinical/prognostic correlations. Clin Exp Med. 2011;11:105–112. doi: 10.1007/s10238-010-0110-9. [DOI] [PubMed] [Google Scholar]
- 21.Kildal W, Risberg B, Abeler VM, Kristensen GB, Sudbo J, Nesland JM, Danielsen HE. beta-catenin expression, DNA ploidy and clinicopathological features in ovarian cancer: a study in 253 patients. Eur J Cancer. 2005;41:1127–1134. doi: 10.1016/j.ejca.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Quattrocchi L, Green AR, Martin S, Durrant L, Deen S. The cadherin switch in ovarian high-grade serous carcinoma is associated with disease progression. Virchows Arch. 2011;459:21–29. doi: 10.1007/s00428-011-1082-1. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Cipollone J, Maines-Bandiera S, Tan C, Karsan A, Auersperg N, Roskelley CD. The morphogenic function of E-cadherin-mediated adherens junctions in epithelial ovarian carcinoma formation and progression. Differentiation. 2008;76:193–205. doi: 10.1111/j.1432-0436.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- 24.Liao X, Siu MK, Au CW, Wong ES, Chan HY, Ip PP, Ngan HY, Cheung AN. Aberrant activation of hedgehog signaling pathway in ovarian cancers: effect on prognosis, cell invasion and differentiation. Carcinogenesis. 2009;30:131–140. doi: 10.1093/carcin/bgn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 26.Lugli A, Zlobec I, Minoo P, Baker K, Tornillo L, Terracciano L, Jass JR. Prognostic significance of the wnt signalling pathway molecules APC, beta-catenin and E-cadherin in colorectal cancer: a tissue microarray-based analysis. Histopathology. 2007;50:453–464. doi: 10.1111/j.1365-2559.2007.02620.x. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida S, Furukawa N, Haruta S, Tanase Y, Kanayama S, Noguchi T, Sakata M, Yamada Y, Oi H, Kobayashi H. Expression profiles of genes involved in poor prognosis of epithelial ovarian carcinoma: a review. Int J Gynecol Cancer. 2009;19:992–997. doi: 10.1111/IGC.0b013e3181aaa93a. [DOI] [PubMed] [Google Scholar]
- 28.Peng C, Zhang X, Yu H, Wu D, Zheng J. Wnt5a as a predictor in poor clinical outcome of patients and a mediator in chemoresistance of ovarian cancer. Int J Gynecol Cancer. 2011;21:280–288. doi: 10.1097/IGC.0b013e31820aaadb. [DOI] [PubMed] [Google Scholar]
- 29.Badiglian Filho L, Oshima CT, De Oliveira Lima F, De Oliveira Costa H, De Sousa Damiao R, Gomes TS, Goncalves WJ. Canonical and noncanonical Wnt pathway: a comparison among normal ovary, benign ovarian tumor and ovarian cancer. Oncol Rep. 2009;21:313–320. [PubMed] [Google Scholar]
- 30.Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–10448. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 31.Noske A, Schwabe M, Weichert W, Darb-Esfahani S, Buckendahl AC, Sehouli J, Braicu EI, Budczies J, Dietel M, Denkert C. An intracellular targeted antibody detects EGFR as an independent prognostic factor in ovarian carcinomas. BMC Cancer. 2011;11:294. doi: 10.1186/1471-2407-11-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


