Abstract
Double-negative T cells (DNTCs; ie, CD3+CD4–CD8– T cells) play a role in limiting chronic immune activation. GB virus C (GBV-C) infection is associated with reduced T-cell activation in human immunodeficiency virus (HIV)–infected individuals. T-cell activation and DNTCs were measured in HIV-infected subjects with a nondetectable HIV load. GBV-C–viremic subjects had significantly reduced CD4+ and CD8+ T-cell activation (P = .003 and .034, respectively) and significantly increased DNTCs (P = .038), compared with nonviremic subjects. GBV-C load correlated with DNTC percentage (P = .004). Thus, GBV-C infection is associated with an increase in DNTCs, which may contribute to reduced immune activation during HIV infection.
GB virus C (GBV-C) is a common human flavivirus that, because of shared modes of transmission, has a high prevalence (up to 42%) among human immunodeficiency virus (HIV)–infected individuals [1]. Although GBV-C infection is not convincingly associated with any human disease, several studies and a meta-analysis involving 1294 subjects found an association between persistent GBV-C infection and prolonged survival in HIV-infected individuals [2]. Consistent with these findings, GBV-C load is inversely related to HIV load in coinfected individuals [3]. Additionally, both GBV-C infection and expression of 2 GBV-C proteins inhibit HIV replication in human CD4+ T cells [4]. GBV-C is lymphotropic and replicates in T and B cells [5]. Thus, GBV-C infection may alter lymphocyte functions, which could potentially interfere with HIV infection [4].
HIV infection is associated with chronic immune activation, which contributes to HIV replication and AIDS progression [6, 7]. Immune activation correlates with HIV load and HIV disease progression [8]. Effective combination antiretroviral therapy (ART) reduces T-cell activation but not to levels observed in HIV-uninfected people [9]. GBV-C and HIV coinfection is associated with significantly lower levels of T-cell activation and with reduced CD4+ T-cell expansion in response to therapeutic interleukin 2 infusion in vivo and in vitro [10–12]. The mechanisms by which GBV-C infection might lower immune activation and proliferation are not understood. However, these effects may limit HIV infection and potentially slow disease progression.
Double-negative (ie, CD3+CD4−CD8−) T cells play an important role in reducing immune activation and in maintaining immune homeostasis during infection, inflammation, and autoimmunity [13]. Recently, the level of double-negative T cells was shown to inversely correlate with T-cell activation and proliferation in subjects with acute HIV infection [14]. In addition, double-negative T cells were associated with the secretion of the immunosuppressive cytokines interleukin 10 and transforming growth factor β, which may contribute to the reduced activation. Since GBV-C coinfection is associated with lower T-cell activation and proliferation in HIV-viremic subjects, we examined HIV-infected subjects with nondetectable levels of HIV RNA in plasma who were receiving combination ART, to determine whether there is an association between GBV-C viremia, double-negative T cells, and T-cell activation.
METHODS
Subjects
Asymptomatic HIV-positive individuals attending the University of Iowa HIV Clinic who were receiving ART and had HIV loads below the limit of detection (<48 copies/mL) for >6 months were invited to participate. The average duration of HIV load suppression was 60 months in subjects with GBV-C viremia and 72 months in those without GBV-C viremia [10]. Subjects were selected who had previously been tested for GBV-C viremia, to provide comparable numbers of people with HIV and GBV-C coinfection and people with HIV monoinfection. The clinical characteristics of the cohort was previously described [10]. This study was approved by the University of Iowa Institutional Review Board, and all subjects provided written consent.
Peripheral Blood Mononuclear Cell (PBMC) Isolation and Flow Cytometry
PBMCs were isolated by Ficoll-Hypaque (Pharmacia Biotech) density-gradient centrifugation within 4 hours of blood collection and were evaluated by flow cytometry, using the following antibodies as directed by the manufacturer (Becton Dickinson Biosciences; BD): CD3 (Pacific Blue), CD4 (APC-H7), CD8 (FITC), and CD38 (PerpCp-Cy5.5). The LIVE/DEAD fixable aqua dead cell stain kit (Invitrogen) was used to determine cell viability. Cells were incubated with antibodies on ice for 1 hour, washed 3 times with phosphate-buffered saline, and fixed in 2% paraformaldehyde (Polysciences). Data were acquired using a BD LSRII flow cytometer, and CompBeads (BD) were used for compensation. At least 20 000 live CD3+ events were collected. Data analysis was performed using FlowJo software (Tree Star).
Statistical Analysis
Statistics analyses were performed using GraphPad software V4.0 (GraphPad Software). Comparisons between the 2 groups were performed using Mann–Whitney U tests. Correlation between GBV-C load and double-negative T cells was evaluated by linear regression analysis and Spearman's rho. P values of <.05 were considered statistically significant.
RESULTS
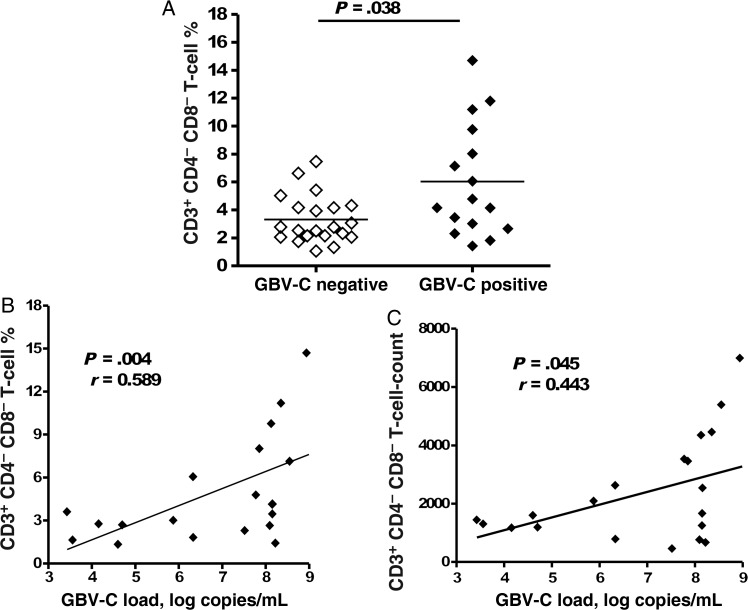
Forty-two HIV-infected subjects who were effectively treated with combination ART were evaluated. A total of 21 subjects had GBV-C viremia, with an average GBV-C load of 1.6 × 108 genome copies/mL. There were no statistically significant differences between the GBV-C–positive and GBV-C–negative groups with respect to age (46.5 vs 47.7 years), female sex (1 vs 3 subjects), race (2 black and 19 white subjects vs 3 black and 18 white subjects), nadir CD4+ T-cell count (236 vs 192 cells/mm3), percentage of CD4+ T cells (29.3% vs 32%), duration of HIV suppression (64 vs 76 months), or mode of HIV transmission. Since GBV-C viremia is associated with lower T-cell activation and because double-negative T cells play an important role in suppressing immune activation, we compared the percentage of total CD3+ cells that lacked both CD4 and CD8 surface expression (ie, double-negative T cells) in subjects with and subjects without GBV-C viremia. The percentage of double-negative T cells was significantly higher among subjects with GBV-C viremia (median, 4.47%; interquartile range [IQR], 2.83–8.89), compared with subjects without GBV-C viremia (median, 2.77%; IQR, 2.10–4.24; P = .038; Figure 1A). The GBV-C load correlated with double-negative T-cell levels (Figure 1B and C).
Figure 1.
GB virus C (GBV-C) viremia is associated with an increase in double-negative T cells. The percentage of CD3+ T cells that lacked surface expression of both CD4 and CD8 was significantly higher among human immunodeficiency virus (HIV)–infected subjects with GBV-C viremia, compared with those without GBV-C viremia (A). Among GBV-C–viremic subjects, the double-negative T-cell percentage (B) and the absolute T cell count (C) were correlated with the GBV-C load. The Spearman test was used to calculate r and P values, and results of best-fit linear regression analysis are shown. Gated cells were normalized to 105 total events.
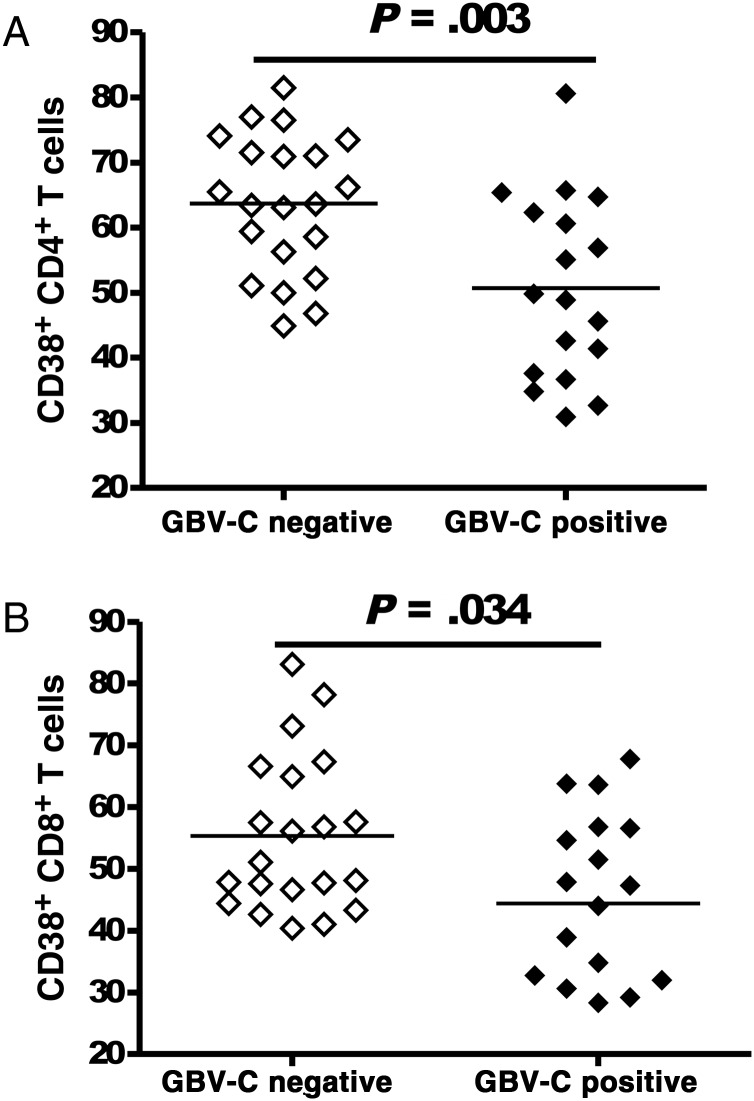
Expression of CD38 on the surface of CD4+ and CD8+ T cells has proven useful in quantifying the level of T-cell activation in subjects with HIV infection [9, 12, 14]. We found that CD38 surface expression on CD4+ and CD8+ T cells was significantly lower among subjects with GBV-C viremia, compared with subjects without GBV-C viremia: for CD4+ T cells, median values were 49.35% (IQR, 30.9–80.6) and 63.6% (IQR, 44.9–81.5; P = .003; Figure 2A); for CD8+ T cells, median values were 45.65% (IQR, 18.50–67.80) and 51.10% (IQR, 40.40–83.10; P = .034; Figure 2B).
Figure 2.
GB virus C (GBV-C) viremia is associated with reduced T-cell activation in subjects with a nondetectable human immunodeficiency virus load who were receiving antiretroviral therapy (ART). T-cell activation, as measured by the percentage of CD38-expressing CD4+ (A) and CD8+ (B) T cells, was significantly lower in GBV-C–viremic subjects receiving combination ART, compared with subjects without GBV-C viremia.
To assess the relationship of T-cell activation with double-negative T-cell level and with GBV-C load, we examined the correlation between CD38 expression and either double-negative T-cell level or GBV-C load. Although there were significant differences in levels of double-negative T cells and levels of activation between GBV-C–viremic subjects and nonviremic controls, we did not observe a statistically significant correlation between levels of CD38 expression and either the double-negative T-cell level (Spearman r = −0.02) or the GBV-C load (for CD4+CD38+ T cells, Spearman r = −0.22; for CD8+CD38+ T cells, Spearman r = 0.18).
DISCUSSION
Chronic immune activation during HIV infection is characterized by increased HIV load, lower CD4+ T-cell gain after ART, loss of CD4+ T cells by activation-induced cell death, and immune dysfunction [7, 9]. Several, although not all, studies observed a beneficial association between GBV-C infection and survival in HIV-infected individuals [4]. Although the mechanisms for this protective effect are not fully understood, recent studies indicate that GBV-C is associated with reduced T-cell activation in vivo and in vitro [10, 12]. In this study, we found that GBV-C viremia is associated with a reduction of T-cell activation among HIV-infected subjects with a suppressed HIV load, compared with HIV-infected subjects without GBV-C viremia (Figure 2). Because combination ART also reduces T-cell activation, the effect of GBV-C viremia on T-cell activation in these subjects appears to have been modest, compared with the effect previously reported for individuals with GBV-C and HIV viremia [12]. Nevertheless GBV-C viremia was associated with reduced T-cell activation in subjects with suppressed HIV RNA load who were receiving combination ART.
A role for double-negative T cells in the regulation of immune responses to viral infections is increasingly being recognized [13], and human double-negative T cells are important modulators of cytokine production and T-cell proliferation [15]. Higher levels of double-negative T cells are associated with reduced T-cell activation and proliferation during acute HIV-1 infection [14]. The effect on T-cell activation appears to be related to the secretion of the immunosuppressive cytokines interleukin 10 and transforming growth factor β. Among HIV-positive individuals successfully treated with ART for >6 months, we found that GBV-C–viremic subjects had a significantly higher percentage of double-negative T cells, compared with subjects without GBV-C viremia (Figure 1A). The percentage of double-negative T cells in the peripheral blood correlated significantly with GBV-C load, suggesting a causal relationship between increased GBV-C viremia and an increased percentage of double-negative T cells (Figure 1B). Although the mechanism for higher levels of double-negative T cells during GBV-C infection has not been studied, the immunosuppressive functions of double-negative T cells may contribute to lower immune activation in these individuals. Although the levels of double-negative T cells were higher and CD38 expression lower in GBV-C–viremic subjects, we did not observe a significant correlation between levels of double-negative T cells and activation, as measured by the expression of CD38 on CD4+ or CD8+ T cells. Thus, although the higher levels of double-negative T cells may contribute to the reduction in immune activation in GBV-C–viremic subjects, other mechanisms of inhibition of activation by GBV-C may be involved. Current studies are underway to better characterize the mechanisms by which GBV-C reduces immune activation.
In summary, we found that GBV-C viremia is associated with reduced T-cell activation and an increased percentage of double-negative T cells in HIV-positive subjects receiving combination ART. Because chronic immune activation contributes to HIV disease progression, GBV-C infection–mediated diminution of immune activation and proliferation may protect against HIV disease, and the observed increase in double-negative T cells among those with GBV-C infection may contribute to this reduction in immune activation during HIV infection. Further understanding of the mechanisms by which GBV-C alters immune activation and proliferation and regulates double-negative T cells may lead to novel approaches to HIV therapy.
Notes
Acknowledgments. We thank our patients in the University of Iowa HIV Clinic, for participating in this study; Wendy Wallace, for specimen collection; and Donna Klinzman, for technical assistance.
Financial support. This work was supported in parts by the Department of Veterans Affairs (Merit Review Grant to J. T. S.), the National Institute of Allergy and Infectious Diseases (RO1 AI-58740 to J. T. S.), the Doris Duke Scholars Program (to R. T. R.), and the Fulbright Scholars Program (to E. T. C.).
Potential conflicts of interest. J. T. S. holds patents related to the use of GBV-C as potential HIV therapy. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mohr EL, Stapleton JT. GB virus type C interactions with HIV: the role of envelope glycoproteins. J Viral Hepat. 2009;16:757–68. doi: 10.1111/j.1365-2893.2009.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Chaloner K, Tillmann HL, Williams CF, Stapleton JT. Effect of early and late GB virus C viraemia on survival of HIV-infected individuals: a meta-analysis. HIV Med. 2006;7:173–80. doi: 10.1111/j.1468-1293.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 3.Tillmann HL, Heiken H, Knapik-Botor A, et al. Infection with GB virus C and reduced mortality among HIV-infected patients. N Engl J Med. 2001;345:715–24. doi: 10.1056/NEJMoa010398. [DOI] [PubMed] [Google Scholar]
- 4.Bhattarai N, Stapleton JT. GB virus C: the good boy virus? Trends Microbiol. 2012;20:124–30. doi: 10.1016/j.tim.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George SL, Varmaz D, Stapleton JT. GB virus C replicates in primary T and B lymphocytes. J Infect Dis. 2006;193:451–4. doi: 10.1086/499435. [DOI] [PubMed] [Google Scholar]
- 6.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008;22:439–46. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 7.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–95. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 8.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–33. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rydze RT, Bhattarai N, Stapleton JT. GB virus C infection is associated with a reduced rate of reactivation of latent HIV and protection against activation-induced T cell death. Antivir Ther. 2012 doi: 10.3851/IMP2309. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stapleton JT, Chaloner K, Zhang J, et al. GBV-C viremia is associated with reduced CD4 expansion in HIV-infected people receiving HAART and interleukin-2 therapy. AIDS. 2009;23:605–10. doi: 10.1097/QAD.0b013e32831f1b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maidana-Giret MT, Silva TM, Sauer MM, et al. GB virus type C infection modulates T-cell activation independently of HIV-1 viral load. AIDS. 2009;23:2277–87. doi: 10.1097/QAD.0b013e32832d7a11. [DOI] [PubMed] [Google Scholar]
- 13.D'Acquisto F, Crompton T. CD37 + CD4-CD8- (double negative) T cells: saviours or villains of the immune response? Biochem Pharmacol. 2011;82:333–40. doi: 10.1016/j.bcp.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Petitjean G, Chevalier MF, Tibaoui F, et al. Level of double negative T cells, which produce TGF-beta and IL-10, predicts CD8 T-cell activation in primary HIV-1 infection. AIDS. 2012;26:139–48. [Google Scholar]
- 15.Voelkl S, Gary R, Mackensen A. Characterization of the immunoregulatory function of human TCR-alphabeta+ CD4- CD8- double-negative T cells. Eur J Immunol. 2011;41:739–48. doi: 10.1002/eji.201040982. [DOI] [PubMed] [Google Scholar]