Abstract
Interferon-activated monocytes are known to exert cytocidal activity against tumor cells in vitro. Here, we have examined whether a combination of IFN-α2a and IFN-γ and human monocytes mediate significant antitumor effects against human ovarian and melanoma tumor xenografts in mouse models. OVCAR-3 tumors were treated i.t. with monocytes alone, IFN-α2a and IFN-γ alone or combination of all three on day 0, 15 or 30 post-tumor implantation. Mice receiving combination therapy beginning day 15 showed significantly reduced tumor growth and prolonged survival including complete regression in 40% mice. Tumor volumes measured on day 80 in mice receiving combination therapy (206 mm3) were significantly smaller than those of mice receiving the IFNs alone (1,041 mm3), monocytes alone (1,111 mm3) or untreated controls (1,728 mm3). Similarly, combination therapy with monocytes and IFNs of much larger tumor also inhibited OVCAR-3 tumor growth. Immunohistochemistry studies showed a large number of activated macrophages (CD31+/CD68+) infiltrating into OVCAR-3 tumors and higher densities of IL-12, IP10 and NOS2, markers of M1 (classical) macrophages in tumors treated with combination therapy compared to the controls. Interestingly, IFNs-activated macrophages induced apoptosis of OVCAR-3 tumor cells as monocytes alone or IFNs alone did not mediate significant apoptosis. Similar antitumor activity was observed in the LOX melanoma mouse model, but not as profound as seen with the OVCAR-3 tumors. Administration of either mixture of monocytes and IFN-α2a or monocytes and IFN-γ did not inhibit Lox melanoma growth; however, a significant inhibition was observed when tumors were treated with a mixture of monocytes, IFN-α2a and IFN-γ. These results indicate that monocytes and both IFN-α2a and IFN-γ may be required to mediate profound antitumor effect against human ovarian and melanoma tumors in mouse models.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1152-x) contains supplementary material, which is available to authorized users.
Keywords: IFNs, Monocytes, Combination therapy, M1 macrophages, Mouse model
Introduction
Macrophages are a part of the mononuclear phagocyte system and are professional antigen-presenting cells for adaptive immunity. Mononuclear phagocytes migrate out from bone marrow, circulate briefly in the blood as monocytes and then enter into the tissues and inflammatory foci where they differentiate into macrophages [1]. Compelling evidence has emerged over the past decades for macrophages playing an important role in the defense against harmful microorganisms and tumor cells [2, 3]. Monocyte/macrophage infiltration is an important aspect of host response to tumor growth, but the exact function of macrophages in tumor growth remains controversial [4]. Two major types of macrophages have been identified: the “classical” phenotype (M1) and the alternative (M2) phenotype (Gordon 2003). The classical (M1) macrophages are activated in response to microbial products or interferon-γ and are characterized by high interleukin-12 (IL-12) and IL-23 production as well as increased major histocompatibility complex (MHC) class II and inducible nitric oxide synthesis (NOS2) expression [5–7]. M2 macrophages are characterized by decreased expression of NOS2, MHC class II and IL-12, but elevated expression of anti-inflammatory cytokines such as IL-10. The M2 phenotype is promoted by Th2 cytokines such as IL-4 and IL-13. M1 macrophages can serve as potent effector cells that kill microorganisms and tumor cells, present antigens and produce large amounts of proinflammatory cytokines [8], while M2 macrophages have potent immunosuppressive properties and act by different mechanisms to inhibit the development of an efficient antitumor response [9].
The modulation of macrophages has been postulated as an effective antitumor therapy [10–14], because activated macrophages can selectively kill tumor cells without damaging normal cells [15]. It has been reported that interferon-activated monocytes can exert near eradicative cytocidal activity against some human tumor cells (HOS, A549 and LOX) in vitro, under defined conditions [16]. Transfer of tumor cytotoxic macrophages activated in vitro exerts a therapeutic effect against lymphomas in animal models [17]. Chen et al. showed that, Kupffer cells, a liver organ-specific macrophage, inhibit the growth of tumor by inducing apoptosis in murine tumor model systems [18]. Indeed, evidence from many studies suggests that activated macrophages are capable of selectively lysing tumor cells in vitro and play an important role in the immune-mediated destruction of tumor cells in vivo [18–20].
Interferons (IFNs) have been shown to exhibit antiviral, antiproliferative and immunomodulatory effects [21–23]. As early as 1969, it was shown that IFNs also have antitumor activity. Indeed, mice inoculated with syngeneic tumor cells exhibited significantly increased survival when treated with IFN preparations [24]. This seminal study triggered a wealth of research eventually leading to clinical testing of IFNs in cancer therapy [25–27]. IFNs also have important roles in regulating the innate and adaptive arms of the immune system. For example, type I IFNs can activate dendritic cells (DCs), upregulate the expression of MHC class I molecules, promote the priming and survival of T cells, enhance humoral immunity and increase the cytotoxic activity of natural killer (NK) cells, macrophages and CD8+ T cells. Additionally, it is reported that IFNs responsiveness in the host hematopoietic compartment is necessary and sufficient to evoke antitumor activity, suggesting that the crucial targets of IFNs responses are the host cells rather than the tumor cells [28]. Thus, by exerting antiproliferative, angiostatic and immune cell-activating functions, IFNs likely play a vital role in tumor growth control. In addition, human IFN-α2a is approved for clinical use by the FDA for several types of cancer including hairy cell leukemia, chronic myelogenous leukemia, malignant melanoma, follicular lymphoma and AIDS-related Kaposi’s sarcoma.
Recently, it has been demonstrated that IFN-α2a plus IFN-γ-activated monocytes strongly increase human monocyte cytocidal activity and eradicate high concentrations of human tumor cells in vitro [29]. Based on this study, we hypothesized that a combination of type I and type II IFNs and monocytes may mediate significant antitumor effects against human ovarian and melanoma tumor xenografts in mouse models. We also examined whether IFNs and monocytes can cause eradication of established human ovarian and melanoma tumors and what is the mechanism of tumor eradication. Our results indicate that a combination local therapy with monocytes and IFN-α2a plus IFN-γ mediate significant antitumor effect against ovarian and melanoma tumor growth by infiltration of activated monocytes and induction of tumor cell apoptosis that results in near eradication of the tumors especially in the case of the ovarian tumors (OVCAR-3). Mice receiving a combination therapy survived significantly longer than mice treated with either monocytes alone or IFNs alone, and this combination was effective in mice not only with smaller tumors but also with larger tumors.
Materials and methods
Monocytes, IFNs and cell lines
Elutriated monocytes were obtained from healthy adult donors, after signing informed consent forms, from the Department of Transfusion Medicine at the National Institutes of Health using the Gambro Elutra method [30]. The preparation, in general, contained more than 85% monocytes, <10% neutrophils and <5% lymphocytes. As reported earlier, this level of contaminating lymphocytes and neutrophils in the monocyte preparation do not contribute to the antitumor activity observed here [16]. Interferon-α2a was obtained from Hoffmann LaRoche (Nutley, NJ, USA). Interferon-γ was obtained from Intermune Pharmaceutical Inc (Brisbane, CA). The human OVCAR-3 and LOX melanoma tumor cell lines were obtained from ATCC.
Murine tumor models
All murine experiments were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and the studies were approved by the Center for Biologics Evaluation and Research (CBER) Animal Care and Use Committee. OVCAR-3 (10 × 106) or LOX (0.5 × 106) were suspended in 200 μl RPMI-1640 and inoculated subcutaneously (SC) into the right dorsal flank of four- to six-week-old (~20 g in body weight) female BALB/c nude mice. Tumor growth was monitored using Vernier calipers every third day. Tumor size (mm3) was calculated by (tumor length) × (tumor width)2 × 0.4 on a given day. Treatments were started on day 0, day 15 or day 30 post-tumor implantation in OVCAR-3, and day 0 or day 9 in LOX melanoma model, when palpable tumors reached a mean size of ~20 mm3 (4–5 mm diameters). Mice were divided randomly into different therapeutic groups and one control group. Mice were injected with RPMI-1640, monocytes (3 × 107) or IFNs (20 ng/mouse of IFN-α2a plus 20 ng/mouse of IFN-γ), or mixture of monocytes and both IFNs (20 ng IFN-α2a and 20 ng of IFN-γ) by intratumoral (IT; 200 μL using a 27-gauge needle) routes. Five to six mice were used for each group.
Immunohistochemistry
Frozen sections were co-stained with rat anti-human CD31 (1 μg/mL; MCA1767, Serotec, Oxford, United Kingdom), rat anti-human CD68 (1 μg/mL; MCA1108G, Serotec), goat anti-human IL-12 or goat anti-human IL-10 (1 μg/mL; R&D Systems), rabbit anti-human CXCL10 (IP10) (1 μg/mL; Peprotec, Inc., Rocky Hill, NJ, USA), rabbit anti-NOS2 (1 μg/mL Santa Cruz Biotechnology, Inc.) antibody or respective isotype control for 18 h at 4°C. All antibodies were resuspended in PBS. All tissue sections were visualized with Alexa 555 or FITC-conjugated streptavidin as the secondary reporting reagent, and the slides were analyzed with laser scanning by fluorescence microscopy.
Apoptosis detection by TUNEL staining in vitro
Tumor samples were harvested and fixed with 10% formalin. Paraffin-embedded sections were deparaffinized by xylene treatment and washed successively with alcohol (100–50%) and PBS. Sections were stained using the TUNEL (terminal deoxynucleotidyl transferase mediated dUTP nick end labeling)-based apoptosis detection kit (Millipore, Billerica) as per manufacturer’s instructions. Apoptotic cells were assessed and measured by fluorescent microscopy. Automated image processing and analysis software, Metamorph (Universal Imaging Corporation, Downingtown, PA), was used to identify and quantitate the number of apoptotic cells.
Statistical analysis
The tumor volumes in the treatment and control groups were analyzed by ANOVA. The statistical significance of data was calculated using two-tailed Student’s unpaired t test. Survival curves were generated by Kaplan–Meier method and compared using the log-rank test.
Results
Intratumoral administration of IFNs and monocytes reduced tumor burden and prolonged survival of mice with established human ovarian tumors
To determine the efficacy of a combination of monocytes and IFNs, a number of experiments were performed to evaluate the therapeutic antitumor effect in the established human ovarian tumor model (OVCAR-3). First, we optimized the number of monocytes with or without IFN-α2a plus IFN-γ needed to inhibit OVCAR-3 tumor growth in a murine model. As shown in Supplementary Fig. S1, IFNs alone and monocytes alone, when co-injected at the time of tumor cell injection, had a marginal effect on tumor growth. However, when monocytes and IFNs were mixed together and administered with tumor cells, a significant delay in tumor growth was observed in a monocyte dose-dependent manner. Co-administration of 3 × 107 monocytes and IFNs inhibited tumor growth by 32% compared to the mice which received lower dose of monocytes (1 × 107) and IFNs (305 mm3 vs. 445 mm3) when tumors were measured on day 30 (Fig S1A). Next, we optimized the concentration of IFNs needed for optimal antitumor effect in vivo (Fig S1B and Fig S1C). When mice were injected with dosages of IFN-γ and IFN-α2a (2 ng/mouse or 20 ng/mouse each IFN, respectively), an optimal delay in tumor growth was observed compared to low dose of IFNs (0.2 ng/mouse). On day 96 post-tumor implant, the higher dose of IFNs (20 ng/mouse each) completely eliminated tumor growth. Interestingly, a lower dose of IFNs (2.0 ng/mouse each) also inhibited tumor growth completely until day 72. After that, tumors began to grow. These tumors were much smaller (105 mm3) compared to the 0.2 ng/mouse dose of each IFN (792 mm3), a decrease of 87% tumor burden measured on day 96, the last day of the experiment (Fig. S1B). The lowest dose of IFN (0.02 ng/mouse each) was also effective in slowing the tumor growth but not as much as higher doses of IFNs (Fig. S1C). Cumulatively, these results demonstrate that a combination therapy with IFNs and monocytes can cause significant inhibition of ovarian tumor growth in animal models. The highest dose of IFNs (200 ng/mouse each) in LOX melanoma model produced optimum antitumor effects (not shown).
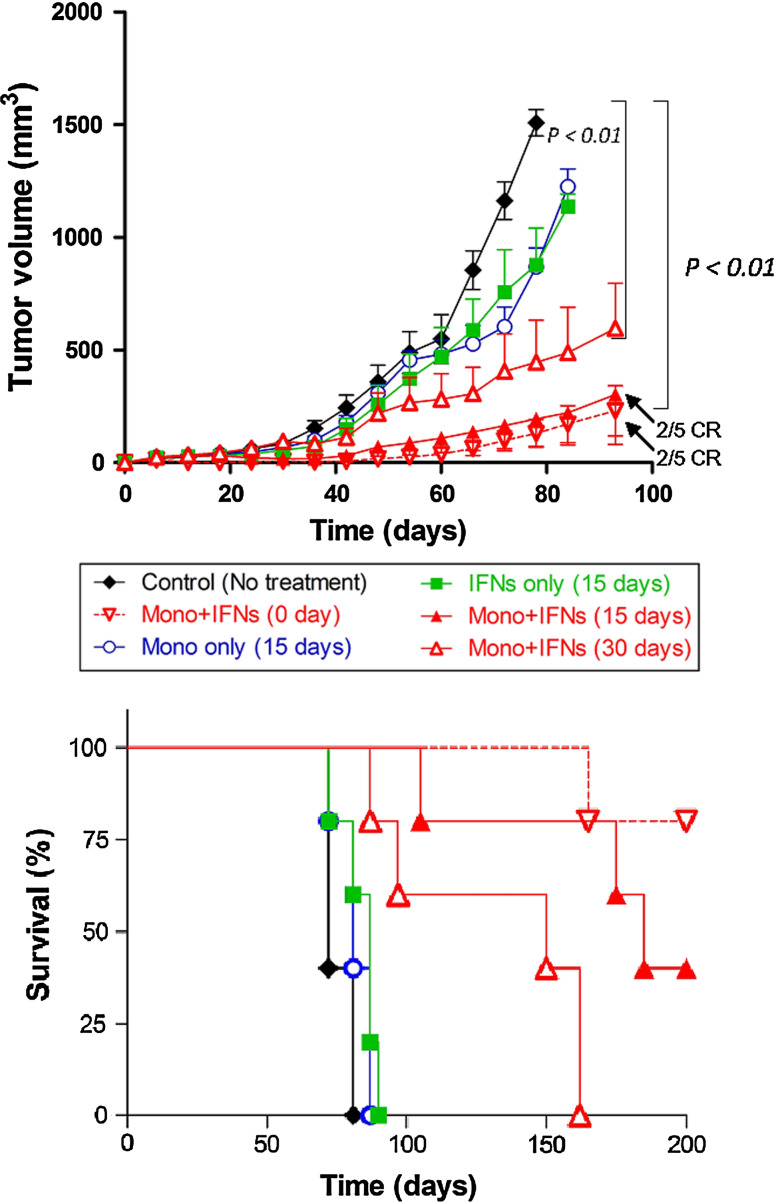
Based on above data, we determined the antitumor effect of a combination of IFNs and monocytes in established murine tumor models. Nude mice with palpable subcutaneous human OVCAR-3 tumors of 50–70 mm3 volume were injected i.t. with monocytes (3 × 107) alone, IFN-α2a plus IFN-γ (20 ng/each) alone or both on day 0, 5 or 30 post-tumor implantation. As shown in Fig. 1a, the treatment of mice with IFNs or monocytes alone on day 15 slightly delayed the OVCAR-3 tumor growth compared to the excipient-treated control. On the other hand, the mice receiving combination therapy with IFNs and monocytes on day 15 significantly reduced the tumor burdens. On day 80, the tumor volume in mice receiving combination therapy (206 mm3) was significantly smaller than that of mice receiving the IFNs alone (1,041 mm3, P < 0.001), monocytes alone (1,111 mm3, P < 0.001) or untreated control (1,728 mm3, P < 0.0001). Furthermore, we found that the administration of monocytes, IFNs or combination of both at day 30 in larger tumors could also inhibit the OVCAR-3 tumor growth. The tumor volume of mice receiving combination therapy at day 30 was 73% smaller (475 mm3) than the untreated control mice on day 80 (P < 0.001), IFNs-treated mice or monocytes-treated mice on day 87 (P < 0.01). We also tested whether retreatment with IFNs and monocytes further reduced tumor burden compared to the one time treatment. The tumor growth of mice treated on day 15 and 25 or day 30 and 40 was smaller than that of the mice treated one time on day 15 or 30. Repeat treatment in early or late treatment models made no significant difference in tumor regression (data not shown).
Fig. 1.
Intratumoral administration of IFNs-activated monocytes inhibited tumor growth and prolonged survival of animals in established OVCAR-3 ovarian tumors. a Mice were subcutaneously injected with 10 × 106 of OVCAR-3 tumors and treated with monocytes (3 × 107) or IFN-α2a and IFN-γ (20 ng each), or combination of both IFNs plus monocytes by i.t. injection on day 0, 15 or 30 post-tumor implantation. The mice receiving combination therapy showed significant inhibition of tumor growth compared to IFNs alone, monocytes alone and PBS-treated mice in the OVCAR-3 tumor model. b Kaplan–Meier survival curves of mice with OVCAR-3 tumors are shown. Each group contained 5 mice. CR represents complete regression. Tumor volumes were measured by Vernier caliper, and overall survival time was calculated based on the killing of mice when tumors size reached >2 cm in diameter. Experiment was repeated twice; bars, SD
The overall survival time (OST) of animals (tumor-bearing mice were killed when tumor volume reached 2 cm in diameter according to NIH animal guidelines) was 72 days in the untreated OVCAR-3 control group, whereas OST of animals was slightly increased to 87 and 81 days in the IFNs alone and monocytes alone groups, respectively (Fig. 1b). However, when IFNs and monocytes were combined, a significantly prolonged OST was observed in the combination therapy group compared to controls in both early and later tumor models. Prolonged survival time in the mice with the combination therapy group of early tumor model (170 days, P < 0.001) was more than double the untreated control group. Furthermore, complete responses (tumor-free survival) were observed in 40% mice treated with combination therapy on day 15 and 70% complete responses when monocytes and IFNs were injected on day 0. These results indicate that intratumoral combination therapy with monocytes and IFNs could be effective in significantly inhibiting tumor growth and prolonging survival of OVCAR-3 tumor-bearing mice.
Combination therapy with monocytes and IFNs reduced LOX melanoma tumor size and prolonged survival
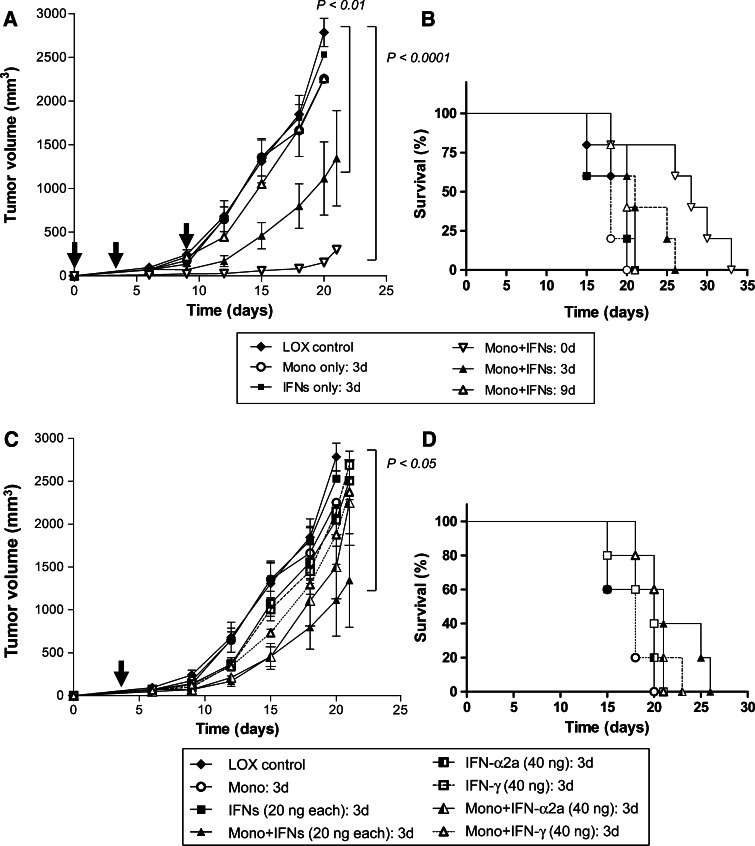
We also evaluated the efficacy of combination therapy in LOX melanoma tumor models. Immunodeficient mice with established LOX melanoma tumors received i.t. injection of monocytes (3 × 107) or IFNs (20 ng/each of IFN-α2a plus IFN-γ) on day 0 or both IFNs and monocytes on day 0, 3 or 9 post-tumor implantation. As shown in Fig. 2a, treatment of mice with IFNs alone or monocytes alone has little or no impact on tumor growth; however, when IFN-α2a, IFN-γ and monocytes were combined and injected either on day 0, or 3, LOX melanoma tumor growth was inhibited compared to the PBS-treated control. On day 20, the LOX tumor volume in mice receiving combination therapy on day 0 (153 mm3, P < 0.0001) or day 3 (1,114 mm3, P < 0.01) was significantly smaller than that of untreated control mice (2,784 mm3), IFNs alone (2,532 mm3) or monocytes alone (2,254 mm3). Later treatment on day 9 showed no inhibition of tumor growth (2,258 mm3). The OST of the mice was 20 days in control groups, whereas it was significantly increased to 33 days in the combination therapy group (treated on day 0, P < 0.05) and 26 days in the combination therapy group (treated on day 3) (Fig. 2b). The mice that received combination treatment with monocytes and IFNs on day 9 did not show any prolongation in survival.
Fig. 2.
Combination therapy with monocytes and IFNs reduced LOX melanoma tumor size and prolonged survival. a Mice were injected s.c. with 0.5 × 106 of LOX melanoma tumors and treated with monocytes (3 × 107) or IFN-α2a (20 ng) plus IFN-γ (20 ng) or combination by i.t. injection on day 0, 3 or 9 post-tumor implantation. Tumor volume in mice receiving combination therapy on day 0 and on day 3 was significantly smaller than that of untreated control mice, IFNs alone or monocytes alone. b Kaplan–Meier survival curves of LOX tumor models were plotted. Each group contained 5 mice. Time (OST) was calculated based on the killing of mice when tumors reached >2 cm. The OST of the mice was significantly increased in the combination therapy group (treated on either day 0 or day 3). The mice that received combination treatment on day 9 did not show any significant prolongation in survival. Experiment was repeated twice; bars, SD. c Individual IFN-α2a (40 ng/mouse) or IFN-γ (40 ng/mouse) was administrated with monocytes on day 3 after tumor injection in LOX melanoma tumor model. The treatment of mice with IFN-α2a or IFN-γ with monocytes slightly delayed the tumor growth compared to the excipient treated control on day 18 after tumor injection. On the other hand, the mice receiving combination therapy with IFNs (both IFN-α2a and IFN-γ) with monocytes significantly reduced the tumor burdens on day 20 compared to individual treatments of IFN-α2a or IFN-γ combined with monocytes. d Kaplan–Meier survival curve of mice treated under (c)
We also investigated whether monocytes combined with either IFN-α2a or IFN-γ mediated antitumor effects compared to when both IFNs were mixed with monocytes. As shown in Fig. 2c, monocytes when combined with either IFN-α2a or IFN-γ mediated very little inhibition of LOX melanoma tumor growth. In contrast, when both IFNs were combined with monocytes, they mediated a statistically significant inhibition of tumor growth (P < 0.05) compared to control tumors. The Kaplan–Meier survival curve showed that mice receiving both IFNs and monocytes lived for 26 days compared to mice receiving monocytes and either IFN-α2a (23 days) or IFN-γ (21 days) although differences between groups were not statistically significant (Fig. 2d).
These results indicate that intratumoral combination therapy with monocytes and IFNs could be effective in significantly inhibiting tumor growth and prolonging survival of LOX melanoma tumor-bearing mice. It appears that combination of both IFNs is more effective in reducing tumor burden than either IFN alone.
Activated macrophages are infiltrated into OVCAR-3 tumors
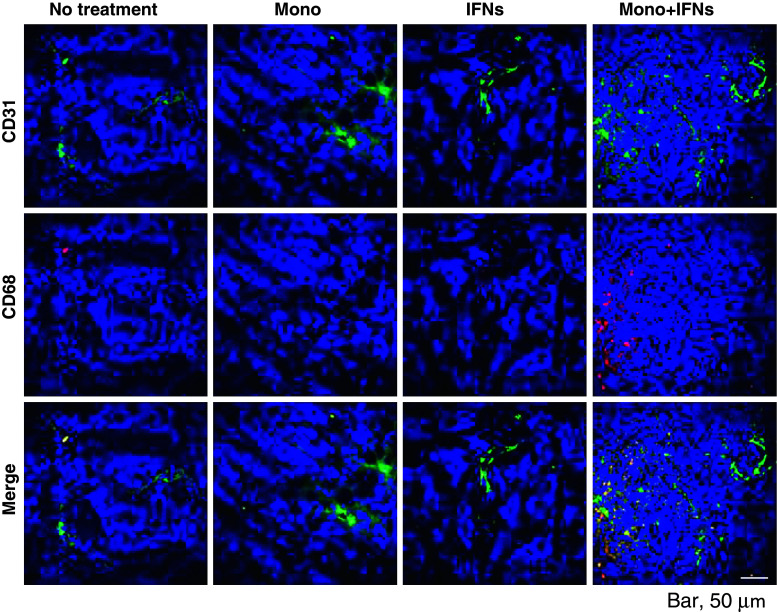
The next question we addressed whether IFN-activated human monocytes when injected into tumor bed were differentiated into activated macrophages in vivo. It is reported that one of the functionally important receptors in macrophage biology is PECAM-1 (CD31) [31]. CD31, through homophilic adhesion, determines which cells will be phagocytosed. In addition, CD68 is predominantly expressed on the intracellular lysosomes of monocytes and macrophages [32]. By co-staining with both CD31 and CD68, one can determine whether monocytes are activated. As shown in Fig. 3, a large number of activated macrophages (CD31+/CD68+) were present in OVCAR-3 tumors harvested on day 2 after combination therapy. In contrast, few activated macrophages were observed when treated with IFNs alone or monocytes alone. On day 10 after treatment, although tumors had shrunk significantly, there was still evidence of CD31+CD68+ macrophages infiltrating tumor stroma although their numbers were significantly lower compared to tumors harvested on day 2 after treatment (Supplementary Fig. 2A and Fig. 3).
Fig. 3.
Activated macrophages are accumulated into OVCAR-3 tumors. The tumor samples were collected on day 17 (2 days after 15-day post-inoculation treatment) from the mice in Fig. 1a. The immunofluorescence microscopy of tumors from mice was done using antibodies specific for CD31 and CD68 counter-stained with 4′,6-diamidino-2-phenylindole (DAPI). The representative images of a tumor from each group are shown (magnification ×400)
Combination therapy induced classical (M1) macrophages in OVCAR-3 tumors
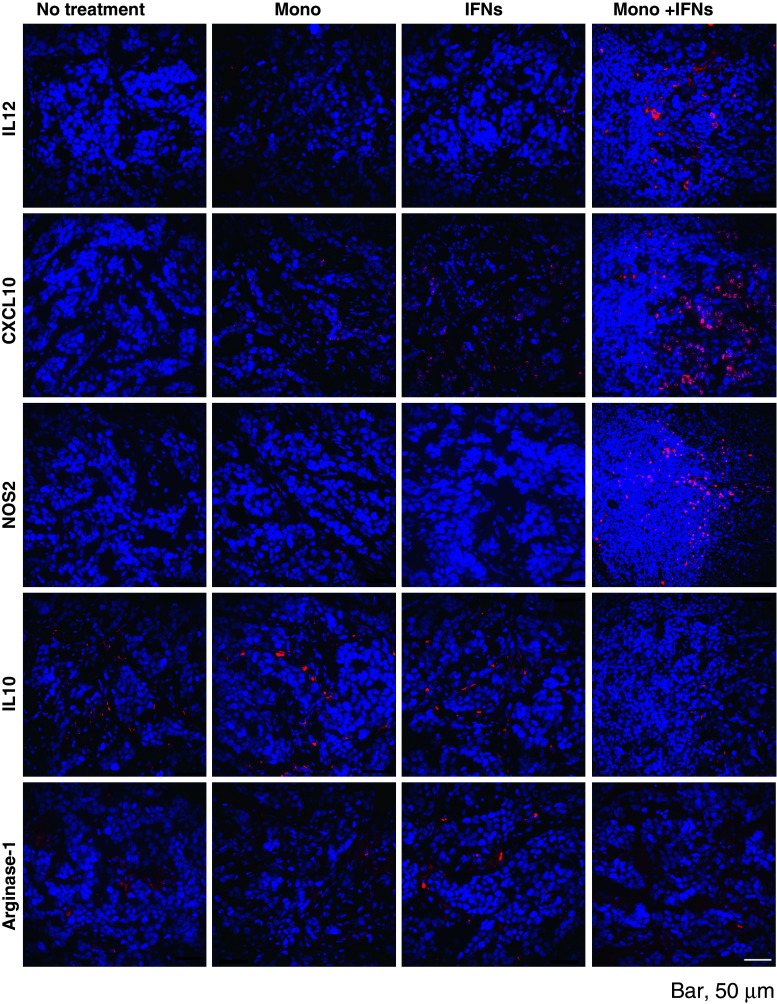
Because we found that activated macrophages are accumulated in tumors after combination therapy with monocytes and IFNs in the murine OVCAR-3 tumor model, we hypothesized that those macrophages were classically activated macrophages (M1). To examine this hypothesis, the tumor samples were collected on day 17 or 25 (2 days or 10 days after treatment on day 15, respectively) from the mice of Fig. 1a, and then, immunohistochemistry was performed using specific antibodies. Tumor samples were stained with anti-IL-12 or anti-IL-10 antibodies. These cytokines were selected because the M1 macrophages are characterized by an IL-12 high and IL-10 low phenotype, whereas regulatory macrophages (M2) are characterized by an IL-10 high and IL-12 low phenotype [33]. As shown in Fig. 4, the higher density of IL-12 was identified in tumor samples of combination therapy compared to the PBS-treated control, monocytes alone or IFNs alone tumors. In contrast, lower density of IL-10 was observed in the sample from combination therapy compared to the other groups. Additionally, we examined the arginase-1 expression in tumor samples, as it is reported that arginase-1 is one of the markers of M2 macrophage in addition to IL-10 [34]. But the density of arginase-1 expression was not different between each group.
Fig. 4.
IFNs activate differentiation of monocytes to classical (M1) macrophages in OVCAR-3 tumors. The tumor samples were collected on day 17 (2 days after 15-day post-inoculation treatment) from the mice in Fig. 1a, and the immunofluorescence microscopy was done using antibodies specific for IL-12, CXCL10 (IP-10) and NOS2, IL-10, and Arginase-1 (magnification ×400)
Tumor samples were also stained with anti-IP10 (CXCL10) or anti-NOS2 antibodies, because these chemokines are also reported as characteristic and potential biomarkers of M1 macrophages [35, 36]. Similar to IL-12 staining, density of both CXCL10 and NOS2-positive cells was higher in the combination therapy group compared to the other groups (Fig. 4). On day 10, although the density of IL-12 and NOS2-positive cells was lower than day 2 tumors, IL-12 and NOS2-positive cells were still present in the tumors (Supplementary Fig. 2B). These results suggest that combination therapy with IFNs and monocytes induces the M1 macrophages activated by IFNs, and they can survive surrounding tumor. These cells may serve as a potent effector cells that kill OVCAR-3 tumors, inducing apoptosis and producing certain chemokines in tumors.
IFNs-activated monocytes induce apoptosis in OVCAR-3 tumors
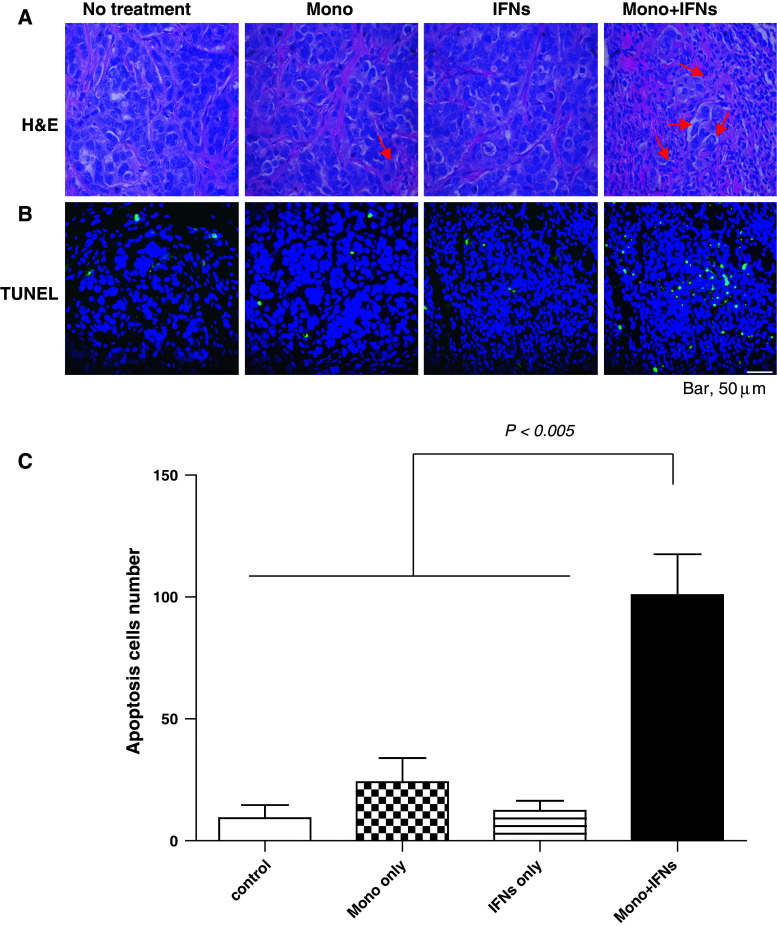
The primary mechanisms responsible for tumor cytotoxicity by activated macrophages are phagocytosis and apoptosis induced by molecules produced by activated macrophages [37]. To examine the role of apoptosis, TUNEL assays were performed in the tumor sections obtained from various treatments including combination therapy. The tumor samples were collected on day 17 or 25 (2 days or 10 days after treatment, respectively) from the mice of Fig. 1a and stained with hematoxylin and eosin (H&E) and TUNEL as described in “Materials and methods”. The H&E-stained samples showed accumulation of large number of macrophages into the tumor receiving combination therapy compared to the PBS-treated control, monocytes alone and IFNs alone groups (Fig. 5a). Similar to the number of accumulated macrophages, the number of apoptotic cells in the tumors of mice receiving combination therapy (n = 101/field) was also higher than that of the PBS-treated control group mice (n = 9/field), monocytes alone (n = 24/field) and IFNs alone (n = 12/field) (P < 0.01) (Fig. 5b, c). These results suggest that macrophages activated by IFNs induced apoptosis of OVCAR-3 tumor cells in murine tumor model.
Fig. 5.
Monocytes activated by IFNs induced apoptosis in OVCAR-3 tumors. a The tumor samples were collected on day 17 (2 days after 15-day post-inoculation treatment) from the mice in Fig. 1a and stained with H&E. Large number of macrophages (arrow heads) were accumulated around tumors (a). Tumor samples were also stained using the TUNEL. Apoptotic cells were assessed and measured by fluorescent microscopy (b). The numbers of apoptotic cells were counted in each group. The numbers of apoptotic cells in tumors harvested from combination therapy–treated mice were significantly higher (P < 0.005) compared to control, monocytes or IFNs-only-treated tumors (c) (per 400× field view)
Combination therapy causes no toxicity to vital organs
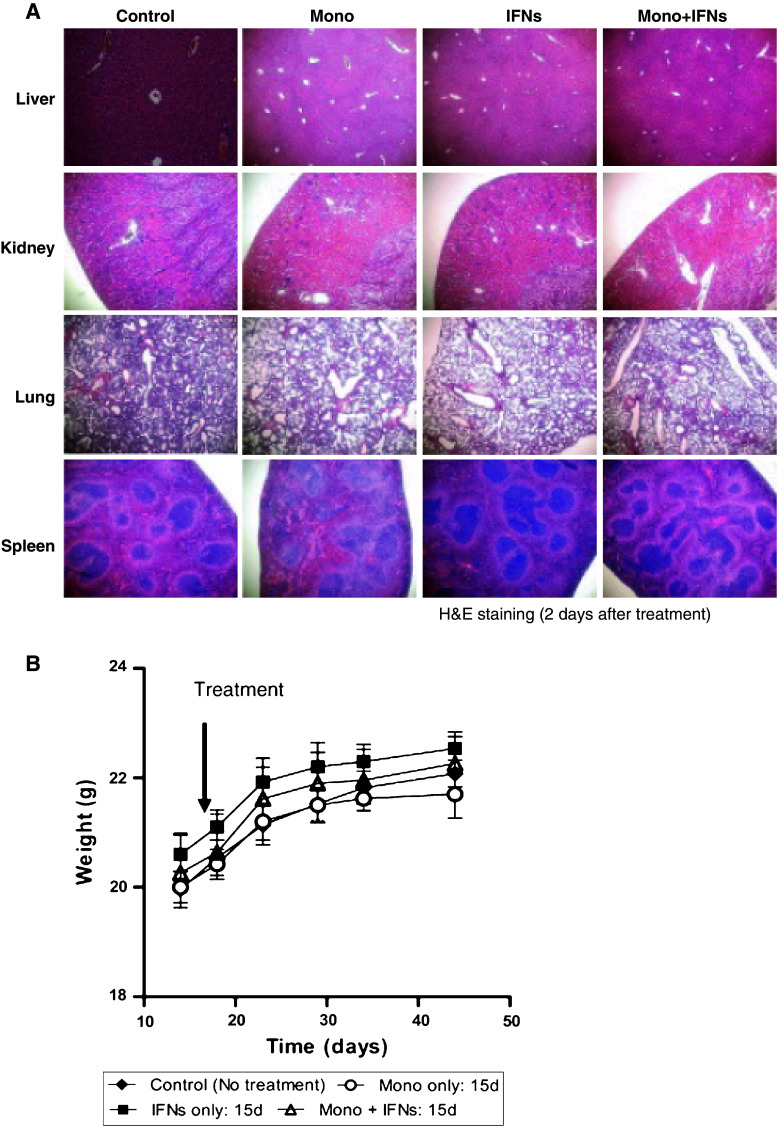
The administration of high dose of IFNs is reported to show serious side effects in the clinic. These adverse events include flu-like symptoms, leukopenia, thrombocytopenia, pancreatitis, hyperthyroidism, diabetes mellitus and jaundice. To assess the toxicity profile of IFNs and monocytes, we examined the histological changes in various vital organs. No histological changes were observed by H&E in any organ including liver, kidney, lung and spleen in either the control or treated groups (Fig. 6a). In addition, no adverse effect on body weight and physical well-being were observed (Fig. 6b). Thus, combination therapy with monocytes and IFNs has significant antitumor efficacy but without any observed adverse side effects in mice.
Fig. 6.
Combination therapy caused no toxicity to vital organs. a Vital organs such as liver, kidney, lung and spleen of treated mice were examined for hepatocellular, tubular, pulmonary and splenic necrosis. No histological changes were observed in these organs. b Body weight was observed before and after treatment with combination therapy group compared to the controls. No body weight changes were observed with combination therapy. Each group contained 5 mice
Discussion
We demonstrate that IFNs, when co-administered with human monocytes, can mediate synergistic antitumor effects against human ovarian and melanoma tumors in vivo. The impressive antitumor effects were demonstrated by (a) regression of small and large tumor nodules, (b) enhanced survival of animals, (c) infiltration of M1-activated macrophages but not regulatory M2 macrophages in OVCAR-3 tumors and (d) apoptosis of macrophage-induced OVCAR-3 tumor cells. These remarkable antitumor effects were mediated without any evidence of local or systemic toxicity to the mouse; however, we must acknowledge the species specificity of IFNs in that human IFNs are at least 4-logs less active on mouse cells in comparison with human cells. In contrast, macrophages can act across species. Although previous studies with interferon showed its broad ability to cause antiviral, antiproliferative, immunomodulatory and antitumor effects in vitro [21–23, 38–41], ours is the first report to demonstrate that a direct intratumoral administration of IFN-α2a-activated monocytes can regress or eradicate tumors in a mouse model. Current studies were based on our in vitro observations that direct cell-to-cell contact of IFN-activated monocytes and optimum concentration of IFN is necessary for tumor cell cytotoxicity in vitro [16, 29]. Thus, our in vitro results predicted the in vivo outcome.
The role of macrophages in cancer has been controversial, and many issues remain unresolved. Early evidence indicate that macrophage surveillance mechanisms are essential for preventing the growth of transformed or pre-transformed cells into tumors, and there is strong evidence showing that activated macrophages can kill transformed cells in vitro [16, 29, 42]. However, there is also evidence that macrophage depletion has little effect on the host’s susceptibility to cancer and in some cases may even be beneficial to the host [43, 44]. It is proposed that macrophages can have contrasting roles in cancer depending on their phenotype [45]. Two major types of macrophages have been identified: the “classical” phenotype (M1) and the alternative regulatory (M2) phenotype [9]. Classically activated macrophages are generally cytotoxic to tumor cells but not to normal cells, and therefore, classical macrophages contribute to the early eradication of transformed cells [46, 47]. It is required that they contact tumor cells in order to kill them [16, 29]. Our immunohistological findings suggest that monocytes/macrophages not only accumulated into the tumor treated with combination therapy with IFNs and monocytes but also expressed CD31+/CD68+ phenotype, indicating that they were activated and involved in phagocytosis of tumors [31, 32]. These macrophages seem to express high levels of IL-12, CXCL10 and NOS2 as determined by immunofluorescence and low levels of IL-10 and arginase-1 in the tumor. These results indicate that intratumoral injection of IFNs and monocytes induces M1 macrophages rather than regulatory M2 (suppressor) macrophages and M1 macrophages contribute to tumor cell killing. Three mechanisms of tumor cell killing by macrophages may include phagocytosis, necrosis and apoptosis [37, 45]. In the present study, TUNEL assays showed that combination therapy induced apoptosis in ovarian tumor model. Although, in vivo results do not provide direct evidence that macrophages are involved in direct killing of tumor cells, our previous in vitro results showed that IFNs-activated monocytes directly kill the tumor cells [16]. Since transplanted monocytes by themselves do not mediate killing nor show TUNEL-positive cells in tumors, these TUNEL-positive cells were observed only when monocytes were mixed with IFNs. These results indicate that TUNEL-positive cells were tumor cells but not monocytes. It is also possible that other factor(s) produced from tumor stroma facilitate macrophage-mediated killing of tumor cells in vivo. Future studies will determine whether the tumor cell killing may be caused by nitric oxide-induced necrosis and production of other cytokines (e.g., TNF and chemokines) by macrophage [45].
Previous in vitro findings showed that low concentrations of tumor cells are more sensitive to killing by IFN-activated monocytes than are high concentrations [16, 29]. The present finding that the growth of small OVCAR-3 tumors is inhibited more profoundly than larger tumors is consistent with the in vitro finding. Thus, the small target size of early tumors may increase their susceptibility to the IFN-activated monocytes [29]. This may have clinical implications such as that some clinically detectable early human tumors may be treatable. Also delayed treatment for the OVCAR-3 tumors with the monocytes and IFNs appeared to be effective in mice up to 30 days post-tumor cell injection. Another finding is the absence of toxicity of the injected IFN and monocytes for the mice and its organs. Our earlier report also showed that in vitro growth of normal diploid cells was not inhibited by IFN-activated monocytes [16]. These findings are also consistent with clinical trials in which low toxicity was seen in patients treated with autologous monocytes [48–50]. These observations imply that IFN-activated monocyte may be relatively selective for tumor cells. It is likely that such an antitumor strategy could be widely applicable to and relevant for possible clinical applications.
Innate and acquired immune systems defenses can eradicate large amounts of invading viruses and bacteria in vivo [51]. A similarly potent immune defense against tumors in vivo has not yet been found [51, 52]. Cancer therapy with IFN, other cytokines, leukocytes or vaccines is only partially effective [16]. The eradication of tumors by cytokines and leukocytes remains an important goal and has been reported in vitro [16, 29]. The present study provides the first in vivo evidence for existence of such a potent innate immune defense against a number of tumors.
In summary, we demonstrated that intratumoral administration of monocytes, and IFN-α2a plus IFN-γ inhibited tumor growth and prolonged survival of animals in established tumor models. The near eradication of the tumors especially OVCAR-3 was caused by accumulation of M1 macrophages and induction of tumor cell apoptosis. These results indicate that monocytes combined with IFN-α2a plus IFN-γ may provide effective therapy for the treatment for localized cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Contributor Information
Raj K. Puri, Phone: +301-827-0471, FAX: +301-827-0449, Email: raj.puri@fda.hhs.gov
Kathryn C. Zoon, Phone: +301-496-3006, FAX: +301-402-0166, Email: kzoon@niaid.nih.gov
References
- 1.Adams D. Macrophages. Methods Enzymol. 1979;LVIII:494–506. doi: 10.1016/S0076-6879(79)58164-6. [DOI] [PubMed] [Google Scholar]
- 2.Evans R. Macrophage accumulation in primary and transplanted tumors growing in C5-deficient B10.D2/oSn mice. Int J Cancer. 1980;26:227–229. doi: 10.1002/ijc.2910260215. [DOI] [PubMed] [Google Scholar]
- 3.Gauci CL, Alexander P. The macrophage content of some human tumours. Cancer Lett. 1975;1:29–32. doi: 10.1016/S0304-3835(75)94826-0. [DOI] [PubMed] [Google Scholar]
- 4.Conti I, Rollins BJ, CCL2 Monocyte chemoattractant protein-1 and cancer. Semin Cancer Biol. 2004;14:149–154. doi: 10.1016/j.semcancer.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Taylor PR, Gordon S. Monocyte heterogeneity and innate immunity. Immunity. 2003;19:2–4. doi: 10.1016/S1074-7613(03)00178-X. [DOI] [PubMed] [Google Scholar]
- 6.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 7.Verreck FA, de Boer T, Langenberg DM, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sher A, Pearce E, Kaye P. Shaping the immune response to parasites: role of dendritic cells. Curr Opin Immunol. 2003;15(4):421–429. doi: 10.1016/S0952-7915(03)00072-4. [DOI] [PubMed] [Google Scholar]
- 9.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 10.Xie K, Fidler IJ. Therapy of cancer metastasis by activation of the inducible nitric oxide synthase. Cancer Metastasis Rev. 1998;17(1):55–75. doi: 10.1023/A:1005956721457. [DOI] [PubMed] [Google Scholar]
- 11.Whitworth PW, Pak CC, Esgro J, Kleinerman ES, Fidler IJ. Macrophages and cancer. Cancer Metastasis Rev. 1990;8(4):319–351. doi: 10.1007/BF00052607. [DOI] [PubMed] [Google Scholar]
- 12.Dima VF, Balotescu C, Dima SV. Potentiation of the activity of mucosal vaccines by immunological adjuvants. Roum Arch Microbiol Immunol. 2000;59(3):157–210. [PubMed] [Google Scholar]
- 13.Thiounn N, Pages F, Mejean A, Descotes JL, Fridman WH, Romet-Lemonne JL. Adoptive immunotherapy for superficial bladder cancer with autologous macrophage activated killer cells. J Urol. 2002;168(6):2373–2376. doi: 10.1016/S0022-5347(05)64148-1. [DOI] [PubMed] [Google Scholar]
- 14.North RJ. The concept of the activated macrophage. J Immunol. 1978;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- 15.Andreesen R. Macrophage-directed tumour immunotherapy revisited—past and future of an old doctor’s dilemma. Res Immunol. 1993;144(4):291–298. doi: 10.1016/0923-2494(93)80111-B. [DOI] [PubMed] [Google Scholar]
- 16.Baron S, Hernandez J, Bekisz J, et al. Clinical model: interferons activate human monocytes to an eradicative tumor cell level in vitro. J Interferon Cytokine Res. 2007;27(2):157–163. doi: 10.1089/jir.2006.0083. [DOI] [PubMed] [Google Scholar]
- 17.Wang BS, Lumanglas AL, Durr FE. Immunotherapy of a murine lymphoma by adoptive transfer of syngeneic macrophages activated with bisantrene. Cancer Res. 1986;46(2):503–506. [PubMed] [Google Scholar]
- 18.Chen GG, Lau WY, Lai PB, et al. Activation of Kupffer cells inhibits tumor growth in a murine model system. Int J Cancer. 2002;99(5):713–720. doi: 10.1002/ijc.10412. [DOI] [PubMed] [Google Scholar]
- 19.Chen GG, Chak EC, Chun YS, et al. Glioma apoptosis induced by macrophages involves both death receptor-dependent and independent pathways. J Lab Clin Med. 2003;141(3):190–199. doi: 10.1067/mlc.2003.22. [DOI] [PubMed] [Google Scholar]
- 20.Fan D, Liaw A, Denkins YM, et al. Type-1 transforming growth factor-beta differentially modulates tumoricidal activity of murine peritoneal macrophages against metastatic variants of the B16 murine melanoma. J Exp Ther Oncol. 2002;2(5):286–297. doi: 10.1046/j.1359-4117.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 21.Bekisz J, Schmeisser H, Hernandez J, Goldman ND, Zoon KC. Human interferons alpha, beta and omega. Growth Factors. 2004;22(4):243–251. doi: 10.1080/08977190400000833. [DOI] [PubMed] [Google Scholar]
- 22.Gresser I. How does interferon inhibit tumour growth? Philos Trans R Soc Lond B Biol Sci. 1982;299(1094):69–76. doi: 10.1098/rstb.1982.0107. [DOI] [PubMed] [Google Scholar]
- 23.Isaacs A. Foreign nucleic acids. Sci Am. 1963;209:46–50. doi: 10.1038/scientificamerican1063-46. [DOI] [PubMed] [Google Scholar]
- 24.Gresser I, Bourali C, Levy JP, Fontaine-Brouty-Boye D, Thomas MT. Increased survival in mice inoculated with tumor cells and treated with interferon preparations. Proc Natl Acad Sci USA. 1969;63(1):51–57. doi: 10.1073/pnas.63.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brassard DL, Grace MJ, Bordens RW. Interferon-alpha as an immunotherapeutic protein. J Leukoc Biol. 2002;71(4):565–581. [PubMed] [Google Scholar]
- 26.Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):119–134. doi: 10.1016/S1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 27.Bekisz J, Baron S, Balinsky C, Morrow A, Zoon KC. Antiproliferative properties of type I and type II interferon. Pharmaceuticals (Basel) 2010;3(4):994–1015. doi: 10.3390/ph3040994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6(11):836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 29.Baron S, Finbloom J, Horowitz J, et al. Near eradication of clinically relevant concentrations of human tumor cells by interferon-activated monocytes in vitro. J Interferon Cytokine Res. 2011;31(7):569–573. doi: 10.1089/jir.2010.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Hoecker P, Zeng J, Dettke M. Combination of Cobe AutoPBSC and Gambro Elutra as a platform for monocyte enrichment in dendritic cell (DC) therapy: clinical study. J Clin Apher. 2008;23(5):157–162. doi: 10.1002/jca.20173. [DOI] [PubMed] [Google Scholar]
- 31.McKenney JK, Weiss SW, Folpe AL. CD31 expression in intratumoral macrophages: a potential diagnostic pitfall. Am J Surg Pathol. 2001;25(9):1167–1173. doi: 10.1097/00000478-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Mordue DG, David SL. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. J Leukoc Biol. 2003;74:1015–1025. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- 33.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42(6):717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien J, Lyons T, Monks J, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176(3):1241–1255. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 36.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 37.Meyn RE, Milas L, Stephens LC. Apoptosis in tumor biology and therapy. Adv Exp Med Biol. 1997;400B:657–667. [PubMed] [Google Scholar]
- 38.Chen AR, Koren HS. Impaired oxidative burst does not affect human monocyte tumoricidal activity. J Immunol Mar. 1985;134(3):1909–1913. [PubMed] [Google Scholar]
- 39.Martinet N, Beck G, Bernard V, et al. Mechanism for the recruitment of macrophages to cancer site. In vivo concentration gradient of monocyte chemotactic activity. Cancer. 1992;70(4):854–860. doi: 10.1002/1097-0142(19920815)70:4<854::AID-CNCR2820700422>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 40.Galligioni E, Quaia M, Spada A, et al. Activation of cytolytic activity in peripheral blood monocytes of renal cancer patients against non-cultured autologous tumor cells. Int J Cancer. 1993;55(3):380–385. doi: 10.1002/ijc.2910550307. [DOI] [PubMed] [Google Scholar]
- 41.Khammari A, Nguyen JM, Pandolfino MC, et al. Long-term follow-up of patients treated by adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2007;56(11):1853–1860. doi: 10.1007/s00262-007-0340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klimp AH, de Vries EG, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol. 2002;44(2):143–161. doi: 10.1016/S1040-8428(01)00203-7. [DOI] [PubMed] [Google Scholar]
- 43.Teng MW, Swann JB, Koebel CM, Schreiber RD, Smyth MJ. Immune-mediated dormancy: an equilibrium with cancer. J Leukoc Biol. 2008;84(4):988–993. doi: 10.1189/jlb.1107774. [DOI] [PubMed] [Google Scholar]
- 44.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67(11):5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 45.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romieu-Mourez R, Solis M, Nardin A, et al. Distinct roles for IFN regulatory factor (IRF)-3 and IRF-7 in the activation of antitumor properties of human macrophages. Cancer Res. 2006;66(21):10576–10585. doi: 10.1158/0008-5472.CAN-06-1279. [DOI] [PubMed] [Google Scholar]
- 47.Swann JB, Vesely MD, Silva A, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci USA. 2008;105(2):652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faradji A, Bohbot A, Frost H, et al. Phase I study of liposomal MTP-PE-activated autologous monocytes administered intraperitoneally to patients with peritoneal carcinomatosis. J Clin Oncol. 1991;9(7):1251–1260. doi: 10.1200/JCO.1991.9.7.1251. [DOI] [PubMed] [Google Scholar]
- 49.Faradji A, Bohbot A, Schmitt-Goguel M, et al. Apheresis-elutriation program for adoptive immunotherapy with autologous activated monocytes in cancer patients. Int J Artif Organs. 1991;14(5):304–312. [PubMed] [Google Scholar]
- 50.Hossne NA, Jr, Invitti AL, Buffolo E, et al. Refractory angina cell therapy (ReACT) involving autologous bone marrow cells in patients without left ventricular dysfunction: a possible role for monocytes. Cell Transpl. 2009;18(12):1299–1310. doi: 10.3727/096368909X484671. [DOI] [PubMed] [Google Scholar]
- 51.Baron S, Fons M, Albrecht T (1996) Viral pathogenesis. In: Baron S (ed) Medical microbiology, 4th edn. University of Texas Medical Branch at Galveston, Galveston Exas Chapter 45 [PubMed]
- 52.Baron S, Pan J, Poast J. Frequency of revaccination against smallpox. Emerg Infect Dis. 2003;9(11):1489–1490. doi: 10.3201/eid0911.020820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.