Abstract
The success of belatacept in late-stage clinical trials inaugurates the arrival of a new class of immunosuppressants based on costimulatory blockade, an immunosuppression strategy that disrupts essential signals required for alloreactive T cell activation. Despite having improved renal function, kidney transplant recipients treated with belatacept experienced increased rates of acute rejection. This finding has renewed focus on costimulatory blockade-resistant rejection and specifically the role of alloreactive memory T cells in mediating this resistance. To study mechanisms of costimulatory blockade-resistant rejection and enhance the clinical efficacy of costimulatory blockade, we developed an experimental transplant system that models a donor-specific memory CD8+ T cell response. After confirming that graft-specific memory T cells mediate costimulatory blockade-resistant rejection, we characterized the role of integrins in this rejection. The resistance of memory T cells to costimulatory blockade was abrogated when costimulatory blockade was coupled with either anti-VLA-4 or anti-LFA-1. Mechanistic studies revealed that in the presence of costimulatory blockade, anti-VLA-4 impaired T cell trafficking to the graft but not memory T cell recall effector function, whereas anti-LFA-1 attenuated both trafficking and memory recall effector function. As antagonists against these integrins are clinically approved, these findings may have significant translational potential for future clinical transplant trials.
Keywords: Costimulatory blockade, memory T cells, integrins, LFA-1, VLA-4
Introduction
Costimulatory blockade (CoB) is a novel immunosuppression strategy that disrupts essential costimulatory signals (such as CD40-CD154 or CD28-B7 interactions) required for full activation of alloreactive T cells (1-3). In experimental systems employing immunologically naïve recipients, costimulatory blockade dramatically prolongs transplant survival (4, 5). More recently, the significant clinical potential of costimulatory blockade was demonstrated in the phase III BENEFIT trial of belatacept (a second-generation CD28 antagonist), which revealed that renal transplant patients treated with belatacept had superior long-term graft function compared to those treated with cyclosporine (6, 7).
Paradoxically, treatment with belatacept was associated with a higher incidence and severity of acute rejection. Thus, costimulatory blockade must fail to tolerize a subset of alloreactive T cells. The mechanisms responsible for CoB-resistant rejection are not fully elucidated, but alloreactive memory T cells potentially play a central role in mediating this resistance, as memory T cells possess diminished requirements for costimulation and are therefore resistant to suppression by CoB (8-12). The resistance of memory T cells to the effects of costimulatory blockade may therefore pose a substantial barrier to the clinical success of belatacept and other CoB regimens.
Indeed, donor-specific memory T cells pose an unaddressed challenge to transplantation even with conventional immunosuppression, as higher pre-transplant frequencies of donor-specific memory T cells correlate with worse long-term graft function and acute rejection rates (8, 13-15). These donor-specific memory T cells may be induced by either prior exposure to donor MHC (via failed prior transplant, blood transfusion or pregnancy) or by heterologous immunity, in which memory T cells generated in response to pathogens prove cross-reactive to donor antigens (16). A recent study highlighted the importance of heterologous immunity in generating alloreactive memory T cells in human patients, revealing that 45% of memory T cells generated after exposure to common viruses possessed alloreactive potential (17).
In order to dissect the immunologic pathways employed by these memory T cells during costimulation-independent activation, we sought to identify critical molecules utilized by memory T cells to mediate transplant rejection in the presence of costimulatory blockade. We initially targeted integrins such as LFA-1 (leukocyte functional antigen-1) and VLA-4 (very late antigen-4), as they play a central role in T cell activation, effector functions and trafficking to inflamed tissues (18-23). The immunomodulatory properties of integrins spurred the clinical development of integrin antagonists against both LFA-1 (efalizumab) and VLA-4 (natalizumab) to treat autoimmune diseases such as psoriasis, multiple sclerosis and Crohn's disease (24, 25).
Given their translational potential, several groups have executed limited studies of integrin antagonists in a transplant setting. For example, LFA-1 or VLA-4 blockade proved efficacious in prolonging the survival of murine skin (26), cardiac (27-30) and islet transplants (27, 31-35). Recently, two clinical studies also utilized anti-LFA-1 to promote engraftment and insulin-independence in recipients of single-donor islet transplants (36, 37). A smaller number of animal studies have also examined LFA-1 blockade in combination with costimulatory blockade and found that this regimen also suppresses graft rejection (38-43). Importantly, virtually all of these studies utilized immunologically naïve recipients, and the role of integrins in mediating transplant rejection via CoB-resistant alloreactive memory T cells remains poorly characterized.
In the following study, we demonstrate that in a murine model of costimulatory blockade-resistant transplant rejection, combined integrin and costimulatory blockade specifically inhibits graft rejection mediated by CD8+ memory T cells.
Materials and Methods
Mice
Adult male 6–8-week-old C57BL/6 mice (NCI-Frederick), TCR transgenic OT-I mice (Taconic Farms), μMT mice (Jackson Laboratories) and Act-mOVA mice (gifted by Dr. Marc Jenkins, University of Minnesota, Minneapolis, MN) (44) were obtained. Animals received humane care and treatment in accordance with Emory University Institutional Animal Care and Use Committee guidelines.
B6.OT-IMemory mouse generation
After quantification of OT-I cells from whole blood of OT-I mice by TruCount bead analysis (BD Pharmingen, San Diego, CA), 104 OT-I cells (along with syngeneic carrier splenocytes) were adoptively transferred into each naïve C57BL/6 mouse. Two days later, the mice were infected with 104 CFU of LM-OVA (45) by i.p. injection.
Skin grafting
Full thickness tail skin grafts (~1cm2) were transplanted onto the dorsal thorax of recipient mice. Where indicated, recipients of skin grafts received treatment with costimulatory blockade [500 μg each of hamster anti-mouse-CD154 mAb (MR-1, BioXcell, West Lebanon, NH) and human CTLA-4 Ig (Bristol-Meyers Squibb, New York, NY)], 250 μg of rat anti-mouse-VLA-4 mAb (PS/2, BioXcell), and/or 250 μg of rat anti-mouse-LFA-1 mAb (M17/4, BioXcell). All monoclonal antibodies were administered i.p. on post-transplant day 0, 2, 4 and 6.
Flow cytometric analyses for frequency and absolute number
Splenocytes, blood, and/or cells obtained from axillary draining lymph nodes (dLNs) were stained with Thy1.1-PerCP, CD8a-APC, CD11a-FITC and/or CD49d-PE (Pharmingen) for analysis on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA). Absolute numbers of OT-I T cells were determined by TruCount Bead analysis according to manufacturer's instructions. Data were analyzed using FlowJo Software (Tree Star, San Carlos, CA).
Intracellular cytokine staining
Splenocyte suspensions were incubated with 10 nM OVA257-264 (SIINFEKL) (Emory University Core Facility) and 10 μg/ml Brefeldin A (Pharmingen). Replicates without peptide were also performed. After 5 hr in culture, cells were processed using an intracellular staining kit (Pharmingen) according to manufacturer's instructions and stained with anti-TNF-PE and anti-IFN-γ-FITC (Pharmingen). The adjusted % dual-producers of TNF and IFN-γ for each sample was calculated by subtracting the % dual-producers from the non-stimulated samples from the matched SIINFEKL-stimulated sample. Outliers (values greater than or less than median ± 3*SEM) for each group were excluded.
CD107a/b degranulation assay
As previously described (46), splenocyte suspensions were incubated in R10 media at 37°C in a 96-well plate (4 × 106 cells/well) for five hours with monensin and anti-CD107a/b-FITC in the presence or absence of 10 nM OVA257-264 peptide. After incubation, surface staining with anti-Thy1.1-PerCP and CD8a-Pacific Blue was performed. Degranulation was measured as the adjusted MFI of CD107a/b (peptide-stimulated – unstimulated).
In vivo CTL assay
As previously published (47), CD45.1-congenic splenocyte target cells were labeled with high (1 μM) or low (100 nM) concentrations of CFSE. The CFSELo target cells were pulsed at 10 nM OVA257-264 peptide; CFSEHi target cells were incubated without peptide. 106 target cells in a 50:50 mixture of unloaded and peptide-loaded target cells were adoptively transferred i.v. into each mOVA skin graft recipient. Twelve hours after adoptive transfer, splenocytes were harvested and assessed for CD45.1 expression and CFSE labeling. Outliers (%specific lysis greater than or less than median ± 2*SEM) were excluded.
Immunohistochemistry
Explanted skin grafts were fixed in OTC and frozen. Sections were stained with anti-Thy1.1 mAb and developed with horseradish peroxidase to visualize infiltrating OT-I cells. Representative images are shown magnified 40x.
Quantitative real-time PCR for OT-I TCR
Explanted skin grafts were homogenized on a TissueLyser II bead mill (Qiagen). mRNA was extracted using a RNeasy Fibrous Tissue kit (Qiagen), and cDNA was generated using a TaqMan RNA-to-Ct 2-step kit (Applied Biosystems). Quantitative real-time PCR for OT-I TCR expression was performed in triplicate using TaqMan Gene Expression Master Mix (Applied Biosystems) and custom primer/probe pairs (Applied Biosystems) specific for the CDR3 region of the OT-I TCR, as previously published (48). Relative quantification was employed using the 2-ΔΔCt method, normalizing OT-I TCR expression against beta-actin expression and comparing to the average normalized OT-I TCR expression in untreated mOVA skin graft recipients (which is set at a normalized value of 1.0).
T cell trafficking experiment
Naïve C57BL/6 recipients were transplanted with mOVA skin grafts. Other C57BL/6 mice previously transferred with OT-I cells were infected with LM-OVA, and after harvesting splenocytes eight days later, effector OT-I cells were counted using TruCount tubes. The equivalent of 106 activated effector OT-I T cells were adoptively transferred into each mOVA skin graft recipient following in vitro treatment for 30 minutes with an immunosuppressive regimen (100μg/ml for each agent) matching the regimen used to treat the transplant recipient. Twenty-four hours after transfer, skin grafts were explanted and OT-I trafficking was assessed by quantitative rt-PCR for OT-I TCR expression.
Statistical analyses
Skin graft experiments are presented on Kaplan-Meier survival curves and were compared with log-rank test. All other assays were compared with the Mann-Whitney nonparametric test. Statistical analyses were conducted using GraphPad Prism (La Jolla, CA).
Results
A novel murine transplant system models rejection by donor-specific memory T cells
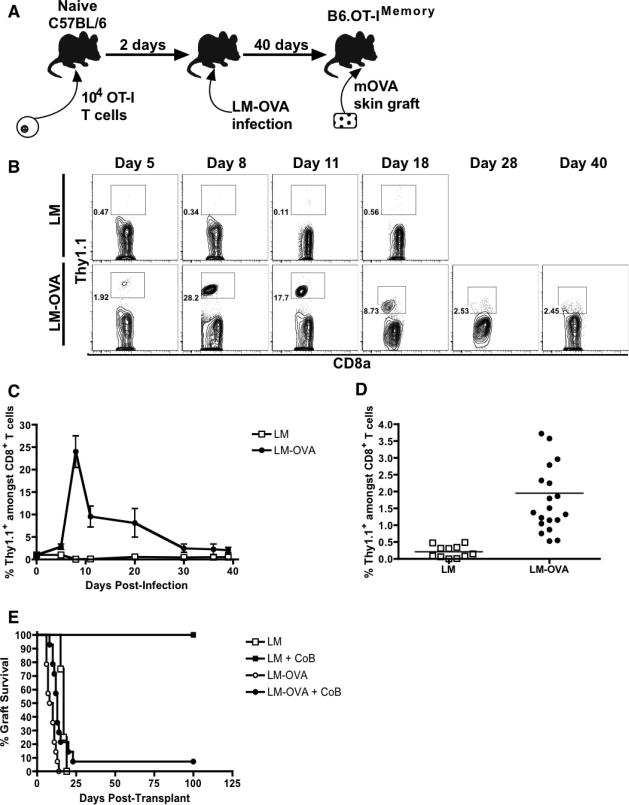
To study CoB-resistant rejection, we developed a novel transplant system that models a donor-specific memory T cell response (Figure 1A). We adoptively transferred OT-I CD8+ TCR transgenic T cells (49) specific for an ovalbumin (OVA) peptide into naïve C57BL/6 mice. These transferred cells expressed the Thy1.1 marker, enabling us to specifically track them. Mice were subsequently infected with genetically-modified Listeria monocytogenes expressing the OVA257-264 epitope (LM-OVA) (45), generating recipients containing memory OT-I cells (B6.OT-IMemory mice). Following LM-OVA infection, naïve OT-I T cells rapidly proliferated until post-infection day 8-9, after which they contracted into a stable memory T cell population constituting 0.5-4% of all CD8+ T cells in the blood by post-infection day 28 (Figures 1B-D). After this development of memory OT-I cells, these B6.OT-IMemory mice were re-challenged with skin grafts from mOVA transgenic mice that ubiquitously express OVA in their tissues, thus precipitating rejection by memory OT-I cells (44). We demonstrated that B6.OT-IMemory recipients rejected mOVA skin grafts with second-set kinetics despite costimulatory blockade with CTLA-4Ig and anti-CD154 (Figure 1E), thereby recapitulating costimulatory blockade-resistant rejection mediated by donor-reactive memory T cells. Adoptive transfer of MACS-sorted memory OT-I cells was sufficient to mediate this CoB-resistant rejection (Figure 2A), and transplants in B cell-deficient recipients were also promptly rejected, further demonstrated that B cells and/or antibodies are not required for CoB-resistant rejection in this system (Figure 2B). The inability of LM-OVA infection to induce an anti-OVA antibody response was also confirmed via ELISA assay (Figure 2C).
Figure 1. The mOVA transplant system models a donor-specific memory response.
(A) Schematic of mOVA transplant system to model graft-specific memory responses. 104 CD8+ TCR transgenic T cells specific for ovalbumin (OVA) were adoptively transferred into naïve C57BL/6 mice that were then infected with genetically-modified Listeria that expresses the OVA257-264 epitope (LM-OVA). Forty days after infection, the recipients were re-challenged with a skin graft from a mOVA transgenic mouse that ubiquitously expresses OVA in all tissues. (B) Representative flow cytometry demonstrating time course of OT-I CD8+ T cell expansion as measured by frequency of CD8+ Thy1.1+ T cells (in box) after wild-type Listeria infection (n=2) or LM-OVA infection (n=5). (C) Summary of kinetics of OT-I CD8+ T cell expansion after wild-type Listeria (n=2) or LM-OVA infection (n=5). (D) OT-I frequency thirty days after infection with wild-type Listeria (n=11) or LM-OVA (n=20). Combined results from four independent experiments for each group are shown. (E) Survival curves of mOVA skin grafts in C57BL/6 recipients adoptively transferred with naïve OT-I T cells and then infected with either wild-type Listeria or LM-OVA. The recipients were either treated with costimulatory blockade or left untreated. Combined results from two independent experiments (4-14 mice/group) are shown. All error bars represent the mean ± SEM.
Figure 2. CD8+ memory T cells are sufficient to mediate costimulatory blockade-resistant transplant rejection.
(A) Survival curves of mOVA skin grafts in naïve C57BL/6 mice after adoptive transfer of 106 memory OT-I that were MACS-sorted from B6.OT-IMemory mice. Graft recipients were either treated with costimulatory blockade or left untreated. Combined results from two independents (6-13 mice/group) are shown. (B) Naïve μMT mice lacking B-cells or serum immunoglobulin were adoptively transferred with OT-I T cells and infected with either wild-type Listeria (LM) or ovalbumin-expressing Listeria (LM-OVA). After waiting 30 days, the mice were transplanted with mOVA skin grafts and then left untreated or else treated with CoB. Combined results from 2 independent experiments (4-5 mice/group) are shown. (C) ELISA for anti-OVA IgG in naïve uninfected C57BL/6 mice, LM-OVA infected mice, naïve mice receiving an mOVA skin graft one month previously, and naïve mice receiving an mOVA skin graft that also received peri-operative CoB (n= 3 mice/group). Error bars represent the mean ± SEM.
Combined integrin and costimulatory blockade prolongs graft survival against a donor-specific memory response
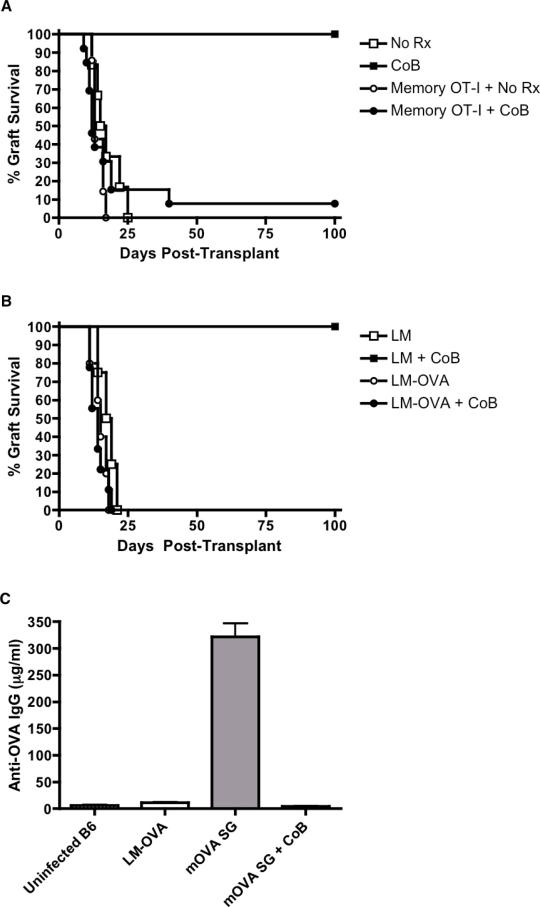
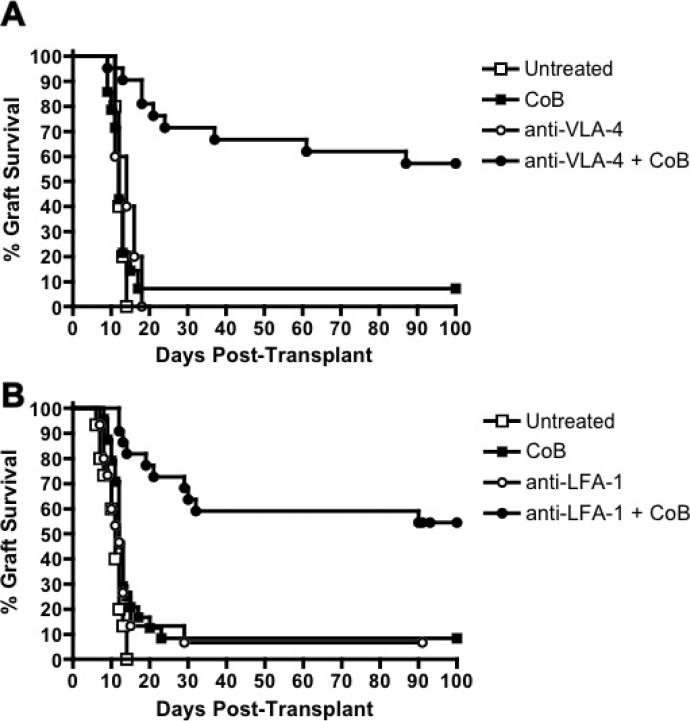
Surface expression of both LFA-1 and VLA-4 was upregulated in memory compared to naïve OT-I cells, suggesting that integrin antagonists might target donor-specific memory T cells (Figure S1). To evaluate whether combined integrin/costimulatory blockade could prolong graft survival, we transplanted mOVA skin grafts onto B6.OT-IMemory mice. These graft recipients were treated with costimulatory blockade (CTLA-4Ig + anti-CD154) alone, integrin antagonist alone, or a dual blockade regimen (i.e. anti-LFA-1 + CoB or anti-VLA-4 + CoB). Whereas recipients treated with CoB or integrin antagonists alone all displayed accelerated graft rejection similar to untreated controls, the recipients treated with dual blockade regimens displayed significantly prolonged graft survival, with a median graft survival time >100 days (Figures 3A and B, and Table S1). Thus, combined costimulatory and integrin blockade prolongs graft survival, even against a donor-specific memory T cell response. However, donor-specific memory precursor frequencies may impact susceptibility to these combined blockade regimens, as mOVA graft survival was not prolonged when performed on recipients whose frequency of memory OT-I cells was increased dramatically (to ~15% of total CD8+ T cells) by repetitive LM-OVA infection prior to grafting (Figures S2A and B).
Figure 3. Dual costimulatory/integrin blockade prolongs graft survival.
(A) Survival curves of mOVA skin grafts in C57BL/6 recipients in which memory OT-I T cells were induced by LM-OVA infection. Recipients were either left untreated (n=15) or treated on POD#0,2,4 and 6 with costimulatory blockade alone (CTLA-4Ig + anti-CD154, n=24), anti-VLA-4 alone (n=5) or combined CoB + anti-VLA-4 (n=22). (B) Survival curves of mOVA skin grafts in C57BL/6 recipients in which memory OT-I T cells were induced by LM-OVA infection. Recipients were either left untreated or treated on POD#0,2,4 and 6 with costimulatory blockade alone (CTLA-4Ig + anti-CD154), anti-LFA-1 alone (n=15) or combined CoB + anti-LFA-1 (n=22). Combined results from four independent experiments are shown. All error bars represent the mean ± SEM.
Combined integrin and costimulatory blockade does not curtail recall accumulation of donor-specific memory T cells
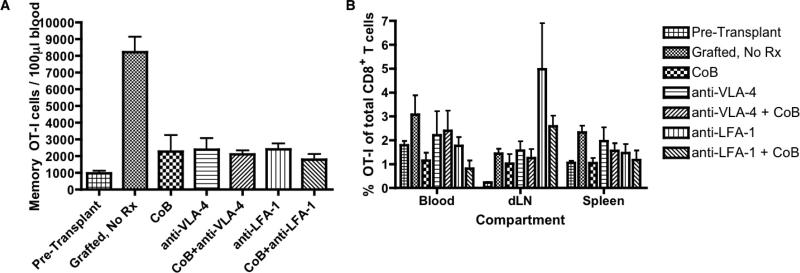
By what mechanisms do integrin antagonists prolong graft survival in recipients treated with CoB? We first assessed whether integrin antagonists function by curtailing the accumulation of OT-I cells during the memory recall response. We transplanted mOVA skin grafts onto B6.OT-IMemory recipients that were treated with an immunosuppression regimen prior to sacrifice on POD#7. Absolute quantification of OT-I T cells from the memory recall response in the blood, draining lymph nodes and spleen was performed using flow cytometry (50). Despite a decrease in the absolute number of OT-I T cells in the blood in treated versus untreated recipients, there were no significant differences between the absolute number of OT-I T cells in the dual costimulatory/integrin blockade recipient groups compared to treatment with CoB alone (Figure 4A). Similar results were noted in the spleens and draining lymph nodes of mOVA skin graft recipients (Figures S3A and B). Comparing recipients treated with CoB alone and those treated with one of the dual costimulatory/integrin blockade regimens, there was no difference in the frequency of OT-I cells within the total population of CD8+ T cells (Figure 4B). However, an enrichment of antigen-specific OT-I cells was evident only in the draining lymph nodes of recipients re-challenged with an mOVA skin graft, consistent with some of our previous observations.
Figure 4. Dual blockade does not inhibit donor-reactive memory CD8+ T cell expansion and accumulation compared to CoB alone.
Skin grafts from mOVA donors were transplanted onto C57BL/6 recipients in which memory OT-I cells were generated by prior LM-OVA infection. The transplant recipients were left untreated or were treated on POD#0, 2, 4 and 6 with costimulatory blockade alone, integrin antagonist alone, or a dual blockade regimen. (A) On POD#7, the absolute number of memory OT-I T cells in the peripheral blood was quantified by flow cytometry. (B) Frequency of memory OT-I T cells as a percentage of total CD8+ T cells on POD#7 is shown for the blood, draining lymph node and spleen compartments amongst the recipients treated with different immunosuppression regimens. Cumulative data from two independent experiments (3-6 mice/group) are shown. Error bars represent the mean ± SEM.
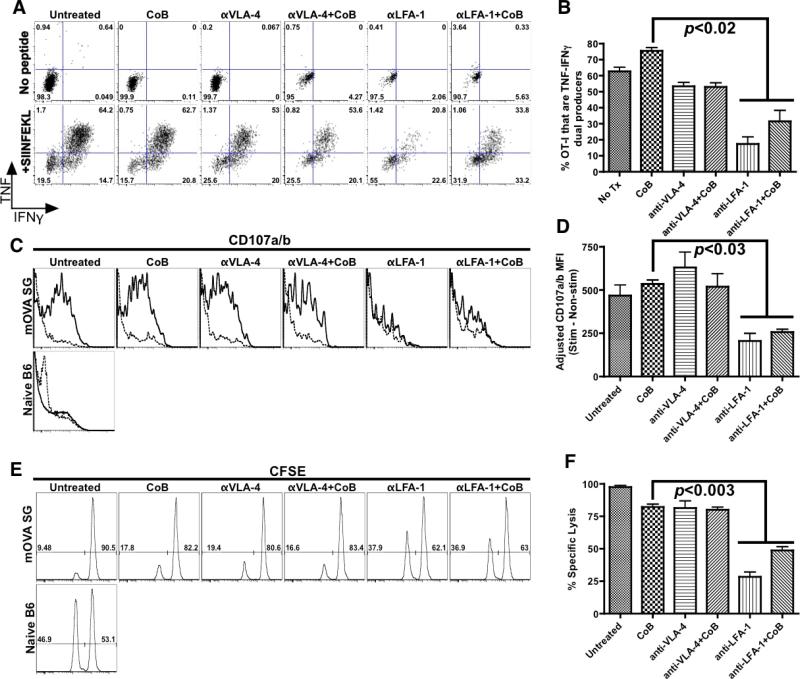
LFA-1 blockade attenuates donor-specific memory T cell effector responses
As the dual blockade regimens did not impact recall accumulation, we next evaluated whether integrin antagonists might inhibit T cell effector functions during the memory recall response. We again transplanted mOVA skin grafts onto B6.OT-IMemory recipients, which were treated with an immunosuppressive regimen prior to sacrifice on POD#7. Intracellular cytokine staining revealed that immunosuppressive regimens containing anti-LFA-1, unlike those containing anti-VLA-4, substantially decreased the ability of donor-reactive T cells to become highly active dual-producers of TNF and IFN-γ following stimulation with the OVA257-264 epitope (Figures 5A and B). Similarly, a flow cytometric degranulation assay based on surface localization of CD107a/b (46) revealed that LFA-1 blockade (but not regimens including anti-VLA-4) potently hindered cytotoxic granule release by OT-I cells during memory recall (Figures 5C and D). Finally, an in vivo CTL assay (47) validated these ex vivo findings, demonstrating that anti-LFA-1 significantly suppressed the generation of graft-specific cytotoxicity, whereas regimens containing anti-VLA-4 had only a minimal impact on OT-I effector functions during a memory recall response (Figures 5E and F).
Figure 5. LFA-1 blockade attenuates CD8+ memory T cell effector responses.
(A) Intracellular cytokine staining of memory OT-I T cells harvested from C57BL/6 recipients on POD#7 after mOVA skin graft placement. Splenocytes were either left unstimulated or stimulated for four hours with OVA257-264 peptide (SIINFEKL). (B) LFA-1 blockade inhibits the ability of memory OT-I T cells to become dual-producers of TNF and IFN-gamma upon ex vivo restimulation. Results show (A) representative data or (B) summary of combined data from three independent experiments (5-6 mice/group). (C) CD107-based degranulation assay of memory OT-I T cells harvested from C57BL/6 recipients on POD#7 after mOVA skin graft placement. Splenocytes from these recipients were either left unstimulated (dashed line) or stimulated for four hours with SIINFEKL peptide (solid line). (D) Adjusted MFI of CD107a/b surface localization of memory OT-I T cells. Results show (C) representative data or (D) summary of combined data from two independent experiments (3-6 mice/group). (E and F) In vivo CTL of memory OT-I T cells in recipients of mOVA skin grafts to assess impact of different immunosuppression regimens on memory T cell cytotoxicity. Results show (E) representative data or (F) summary of combined data from five independent experiments (4-10 mice/group). All error bars represent the mean ± SEM.
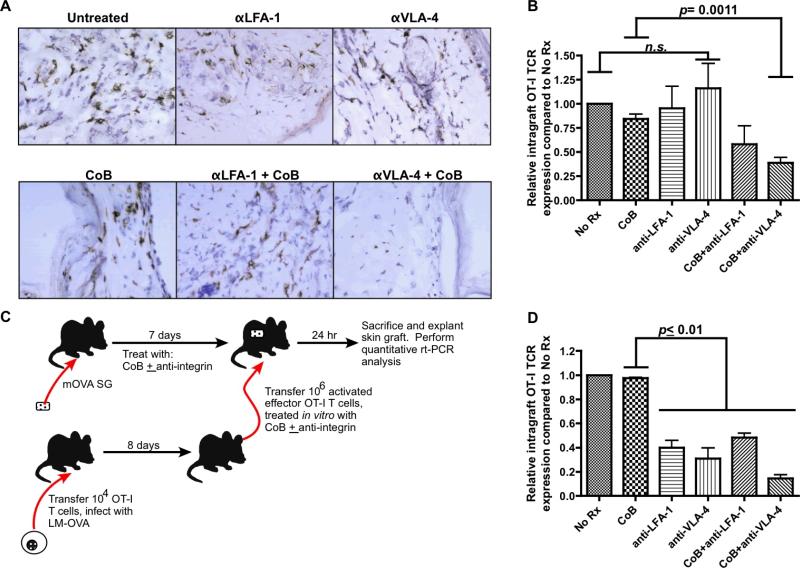
Both VLA-4 and LFA-1 blockade inhibit donor-specific memory T cell trafficking to graft
These results did not explain why combined costimulatory and VLA-4 blockade prolonged graft survival, so we next determined whether combined blockade regimens impact trafficking of T cells from the memory recall response to the graft. We explanted mOVA skin grafts on POD#7 after transplant onto B6.OT-IMemory recipients that were treated with different immunosuppression regimens, and performed immunohistochemistry staining for Thy1.1 to assess the relative infiltration of grafts by memory/effector OT-I cells. Whereas grafts from recipients treated with CoB alone had an abundant OT-I T cell infiltrate, those treated with CoB + anti-VLA-4 had almost no infiltration (Figure 6A). To better quantify the degree of OT-I T cell infiltrate in these explanted mOVA grafts, we performed quantitative real-time PCR on graft tissue explanted on POD#7 utilizing a primer-probe pair specific for the OT-I T cell receptor (48). Using this more sensitive assay, a small decrease in OT-I cell infiltration was detected in recipients treated with regimens containing anti-LFA-1. However, there was a significant decline in memory/effector OT-I infiltration in recipients treated with anti-VLA-4+CoB (Figure 6B).
Figure 6. VLA-4 and LFA-1 blockade inhibit donor-specific CD8+ memory T cell trafficking to the graft.
(A) Immunohistochemistry analysis of POD#7 explanted mOVA skin grafts from recipients with memory OT-I T cells, staining for Thy1.1 marker found only on memory OT-I T cells (shown 40x magnification). Representative images over two independent experiments (3 mice/group) are shown. (B) Quantitative real-time PCR assay for OT-I TCR expression using cDNA generated from mOVA skin grafts explanted on POD#7. Combined results from two independent experiments (3-6 mice/group) are shown. (C) Experimental design for assay to determine the effect of integrin blockade on donor-specific effector T cell trafficking to transplanted skin grafts. (D) VLA-4 blockade inhibits trafficking of adoptively transferred OT-I to mOVA skin grafts. Combined results from three independent experiments (8-10 mice/group) are shown. All error bars represent the mean ± SEM.
To specifically assess how integrin blockade impacts primed graft-specific effector T cell trafficking, effector OT-I T cells were harvested from LM-OVA-infected mice, blocked in vitro with an integrin antagonist, and then adoptively transferred into a naïve C57BL/6 mouse that had received an mOVA skin graft seven days previously (i.e. cells were transferred after graft neovascularization). The grafts were explanted 24 hours after adoptive transfer, homogenized and then subjected to rt-PCR analysis to detect OT-I TCR (Figure 6C). Both VLA-4 and LFA-1 blockade were associated with a dramatic decrease in T cell trafficking to the graft (Figure 6D). Interestingly, treatment with anti-VLA-4 in the absence of CoB dramatically impaired trafficking of recently primed effectors (Figure 6D), but not the trafficking of memory T cells and secondary effectors during the recall response (Figures 6A and B), perhaps reflecting greater dependence of primary effectors on VLA-4 for trafficking into the graft.
Discussion
Costimulatory blockade is a novel immunosuppression strategy that offers many benefits compared to conventional calcineurin inhibitor-based regimens. Chief amongst these advantages is a general lack of off-target toxicities such as nephrotoxicity, enabling better long-term graft function. While promising, theoretical concerns have been raised about costimulatory blockade due to the fact that memory T cells have diminished requirements for costimulation, and therefore alloreactive memory T cells may resist suppression by costimulatory blockade. These concerns were supported by the BENEFIT trial of belatacept, a second-generation CD28 antagonist. In this trial, the belatacept-treated renal transplant recipients experienced a higher incidence and severity of acute rejection compared to the patients treated with cyclosporine. The ultimate clinical success of costimulatory blockade may require a characterization of immunological pathways critical for costimulation-independent activation of donor-specific memory T cells, enabling the identification of adjunct immunosuppressive therapies that could augment the clinical efficacy of costimulatory blockade.
In this report, we developed a unique transplant system that specifically models CoB-resistant rejection by a donor-specific CD8+ memory T cell recall response. Using this experimental system, we identified the integrins LFA-1 and VLA-4 as playing important roles in the costimulation-independent donor-specific memory T cell recall response. We demonstrated that coupling costimulatory blockade to antagonists against either of these integrins enables long-term graft survival, even against a donor-specific CD8+ memory T cell response. It remains an area of ongoing investigation whether eventual graft rejection in this model is due to a lack of maintenance blockade or potentially the activity of recent thymic emigrants that never received treatment with combined blockade. Finally, we characterized the immunological pathways by which LFA-1 and VLA-4 prolong graft survival. These findings are of obvious translational significance, as humanized monoclonal antibodies against both LFA-1 and VLA-4 have been developed and FDA-approved for clinical use against autoimmune diseases. In contrast, several groups have explored other regimens to improve the efficacy of CoB against memory alloresponses, but these have employed reagents that are not clinically relevant (42, 51, 52).
The transplant system utilized in this current study possesses several notable advantages over older models of memory T cell-mediated rejection, most of which relied upon anti-donor memory induced by the rejection of a primary graft in an untreated transplant recipient. Because the OT-I cells employed in the mOVA transplant system express the Thy1.1 marker, our system enables the graft-specific memory T cells to be specifically identified and tracked, permitting mechanistic studies previously limited with other transplant systems. Additionally, as LM-OVA infection does not induce an appreciable anti-OVA antibody response, our system allowed us to specifically focus on rejection mediated by a memory T cell recall response. In other transplant systems used to study memory alloresponses, the contribution of memory T cells to graft rejection is often confounded by the contribution of anti-donor antibody. Finally, the initial priming stimulus in this system is of limited duration, as LM-OVA infection is completely cleared after 6 days (53). Thus, this system ensures that donor-specific T cells at the time of transplant are true memory T cells, as they persist for over 30 days by the time of re-challenge despite an absence of antigen.
The mOVA transplant system shares some aspects of alloreactive memory generated by heterologous immunity, as well as some elements of memory generated by prior exposure to donor MHC (such as occurs with a failed prior graft). In this system, pathogen infection results in the generation of memory T cells that react against shared pathogen and graft antigens, very similar to heterologous immunity. Unlike typical heterologous immunity, however, the donor-specific memory T cells are recognizing an identical (rather than merely cross-reactive) epitope in the graft and pathogen. The extent to which the degree of cross-reactivity (i.e. TCR affinity for cross-reactive antigen) impacts the T cells’ susceptibility to costimulation/integrin therapy is an important area of future research.
Utilizing this mOVA transplant system, we defined several mechanisms of action of anti-LFA-1 and anti-VLA-4 in prolonging graft survival against a CoB-resistant memory T cell response. As demonstrated by T cell functional assays, VLA-4 antagonism in the setting of concurrent costimulatory blockade dramatically suppresses trafficking of alloreactive memory/effector T cells to the graft, whereas LFA-1 blockade both impairs T cell trafficking to the graft and additionally attenuates T cell effector functions during the memory recall response such as cytotoxicity and cytokine production. These functional assays therefore highlight the distinct immunological pathways employed by integrins during the costimulation-independent activation and function of memory T cells. One recent report also evaluated LFA-1 blockade on early CD8+ memory T cell infiltration of allografts and found that it dramatically reduced trafficking to allografts (54). Notably, we also demonstrate that anti-LFA-1 blockade diminishes trafficking to the graft (Figure 6), and differences in the degree of impaired trafficking noted in these two studies may potentially be attributed to the different time-points and different target organs (heart vs. skin) evaluated.
Despite its advantages, our experimental approach also has some limitations. First, given that our in vitro assays are measured 7 days after re-challenge with an mOVA graft, it is difficult to assess whether the activity of the combined blockade regimen is impacting memory CD8+ T cells themselves or CD8+ effector cells that have differentiated during the memory recall response. Second, the mOVA system best models a single minor antigen mismatch transplant system that may not be relevant to fully allogeneic memory recall responses. Finally, we employ a non-vascularized skin graft model, which may differ from the memory recall responses encountered during vascularized clinical transplants. Evaluation of combined costimulatory and integrin blockade immunosuppression regimens in vascularized animal transplant models and in a fully allogeneic model of heterologous immunity (16) is ongoing.
Interestingly, although prolonged graft survival was only observed in recipients treated with dual costimulatory/integrin blockade regimens, treatment with anti-LFA-1 alone appeared to have similar effects on memory T cell effector functions. Importantly, all of these functional assays only evaluated the effect of immunosuppression regimens on the Thy1.1+ graft-specific memory T cells, leaving open the possibility that the addition of CoB prolongs enhances graft survival through mechanisms independent of graft-specific T cells. For example, several investigators have found that costimulatory blockade can induce an accumulation of CD4+FoxP3+ Tregs in grafts (55, 56). An alternative hypothesis is that costimulatory blockade may prolong graft survival through independent tolerance-promoting effects on innate immune cells such as dendritic cells, which were not assessed in this report (57, 58). Finally, the synergistic survival benefit conferred by CoB and anti-LFA-1 may be due to augmented inhibition of memory/effector T cell trafficking to the graft, as less donor-specific T cell infiltration is observed in the graft with either combined integrin/costimulatory blockade regimen compared to either integrin antagonist alone (Figure 6B).
How does costimulatory blockade synergize with anti-LFA-1 and anti-VLA-4 to inhibit the trafficking of memory/effector T cells to the graft? Several potential mechanisms can be invoked. First, graft infiltration by T cells in CoB-treated recipients is dramatically reduced when neutrophil infiltration of the graft is impaired (59). Given that both LFA-1 and VLA-4 are involved in the transmigration of neutrophils into tissues (60), the synergistic reduction in graft infiltration observed with combined blockade regimens compared to CoB treatment alone may be due to an effect on these innate immune cells. An alternative explanation is based on the recent finding that although memory T cell effector responses are costimulation-independent, the ability of memory T cells to activate innate immune cells and thereby enhance inflammation and tissue injury remains critically dependent on CD154 signaling (61). As local tissue inflammation augments the expression of integrin ligands such as ICAM-1 and to a greater extent VCAM-1 (19), CoB may synergize with integrin antagonists by inhibiting graft-expression of integrin ligands, further inhibiting integrin-mediated transmigration of memory/effector T cells into the graft. This synergy between costimulatory and integrin blockade may be particularly critical for memory and secondary effector T cell trafficking (Figures 6A and B), as integrin antagonists alone were sufficient to prevent trafficking of primary effectors (Figure 6D). We are currently studying the differential role of integrins in trafficking by memory and primed effector T cells, as both have been shown to participate in the rejection response (62).
Although our study reveals the potential of integrin antagonists as adjunct immunosuppressive agents, enthusiasm for these drugs must be tempered by important safety concerns raised by the early clinical experience with efalizumab and natalizumab, as a small handful of patients receiving chronic therapy with these integrin antagonists were reported to develop progressive multifocal leukoencephalopathy (PML), leading to the voluntary withdrawal of efalizumab from the market in June 2009 (63-66). Importantly, the risk of PML is directly correlated with the duration of anti-integrin therapy. For example, whereas the overall risk of developing PML in patients treated with efalizumab was 1 in 10,000, the incidence dropped to 1 in 400 after receiving 3 years of therapy (63).
The remote risk of developing a fatal neurodegenerative condition such as PML is unacceptable for patients with conditions that are not life-threatening such as psoriasis, but the same risk may be entirely tolerable in the transplant arena. Additionally, integrin antagonists employed in the transplant setting would likely be used as short-term perioperative induction immunosuppression (as they were utilized in this study), and this short duration of therapy should substantially mitigate the risks of PML development, as this risk increases dramatically with duration of anti-integrin therapy (66).
Thus, while the risks of integrin blockade must not be neglected, they certainly do not exclude integrins as a viable target for transplant immunosuppression. The enormous potential of integrin antagonists is especially clear in the setting of calcineurin inhibitor-sparing transplant immunosuppression regimens based on costimulatory blockade. Given the early clinical experience with belatacept, the significant barrier posed by the resistance of alloreactive memory T cells threatens to derail this promising new era of targeted immunosuppression. However, our study offers the potential that combined integrin and costimulatory blockade might be an effective immunosuppression regimen, especially for patients with high frequencies of donor-specific memory T cells who would otherwise be vulnerable to transplant rejection if treated with costimulatory blockade alone. Further primate and clinical trials with combined integrin and costimulatory blockade are certainly merited in the future to evaluate the efficacy and risks of this novel immunosuppression strategy.
Supplementary Material
Acknowledgements
We thank K. Newell, T. Pearson, S. Knechtle, A. Mehta, and A. Adams for their experimental and technical advice. This work was supported by grants from the US National Institutes of Health (R01 AI073707 and R56 AI081789 to M.L.F.). W.H.K. is supported by a Roche Laboratories Scientist scholarship from the American Society of Transplant Surgeons.
Abbreviations
- CoB
costimulatory blockade
- CTL
cytotoxic T lymphocyte
- ELISA
enzyme-linked immunosorbent assay
- IFN-γ
interferon-gamma
- LFA-1
leukocyte functional antigen-1
- MACS
magnet-assisted cell sorting
- MHC
major histocompatibility complex
- OVA
ovalbumin
- PBMC
peripheral blood mononuclear cells
- PML
progressive multifocal leukoencephalopathy
- SEM
standard error of mean
- TCR
T cell receptor
- TNF
tumor necrosis factor
- VLA-4
very late antigen-4
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Weaver T, Charafeddine A, Kirk A. Costimulation blockade: towards clinical application. Front Biosci. 2008;13:2120–2139. doi: 10.2741/2829. [DOI] [PubMed] [Google Scholar]
- 2.Larsen CP, Knechtle SJ, Adams A, Pearson T, Kirk AD. A new look at blockade of T-cell costimulation: a therapeutic strategy for long-term maintenance immunosuppression. Am J Transplant. 2006;6(5 Pt 1):876–883. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 3.Vadivel N, Trikudanathan S, Chandraker A. Transplant tolerance through costimulation blockade--are we there yet? Front Biosci. 2007;12:2935–2946. doi: 10.2741/2283. [DOI] [PubMed] [Google Scholar]
- 4.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381(6581):434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 5.Lin H, Bolling SF, Linsley PS, Wei RQ, Gordon D, Thompson CB, et al. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178(5):1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10(3):535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 7.Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant. 2010;10(3):547–557. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 8.Brook MO, Wood KJ, Jones ND. The impact of memory T cells on rejection and the induction of tolerance. Transplantation. 2006;82(1):1–9. doi: 10.1097/01.tp.0000226082.17507.da. [DOI] [PubMed] [Google Scholar]
- 9.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169(8):4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 11.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152(6):2675–2685. [PubMed] [Google Scholar]
- 12.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104(12):1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. Am J Transplant. 2005;5(8):1971–1975. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 14.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163(4):2267–2275. [PubMed] [Google Scholar]
- 15.Poggio ED, Augustine JJ, Clemente M, Danzig JM, Volokh N, Zand MS, et al. Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation. 2007;83(7):847–852. doi: 10.1097/01.tp.0000258730.75137.39. [DOI] [PubMed] [Google Scholar]
- 16.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amir AL, D'Orsogna LJA, Roelen DL, van Loenen MM, Hagedoorn RS, de Boer R, et al. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115(15):3146–3157. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- 18.Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu Rev Immunol. 2004;22:157–180. doi: 10.1146/annurev.immunol.22.012703.104649. [DOI] [PubMed] [Google Scholar]
- 19.Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol. 2009;29(2):87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, et al. Integrins in immunity. J Cell Sci. 2009;122(Pt 2):215–225. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 21.Sims TN, Dustin ML. The immunological synapse: integrins take the stage. Immunol Rev. 2002;186:100–117. doi: 10.1034/j.1600-065x.2002.18610.x. [DOI] [PubMed] [Google Scholar]
- 22.Langer HF, Chavakis T. Leukocyte-endothelial interactions in inflammation. J Cell Mol Med. 2009;13(7):1211–1220. doi: 10.1111/j.1582-4934.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 24.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9(10):804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 25.González-Amaro R, Mittelbrunn M, Sánchez-Madrid F. Therapeutic anti-integrin (alpha4 and alphaL) monoclonal antibodies: two-edged swords? Immunology. 2005;116(3):289–296. doi: 10.1111/j.1365-2567.2005.02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isobe M, Suzuki J, Yamazaki S, Sekiguchi M. Acceptance of primary skin graft after treatment with anti-intercellular adhesion molecule-1 and anti-leukocyte function-associated antigen-1 monoclonal antibodies in mice. Transplantation. 1996;62(3):411–413. doi: 10.1097/00007890-199608150-00019. [DOI] [PubMed] [Google Scholar]
- 27.Grazia TJ, Gill RG, Gelhaus HC, Doan AN, Sleater ML, Pietra BA. Perturbation of leukocyte function-associated antigen-1/intercellular adhesion molecule-1 results in differential outcomes in cardiac vs islet alograft survival. J Heart Lung Transplant. 2005;24(9):1410–1414. doi: 10.1016/j.healun.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Isobe M, Suzuki J, Yagita H, Okumura K, Yamazaki S, Nagai R, et al. Immunosuppression to cardiac allografts and soluble antigens by anti-vascular cellular adhesion molecule-1 and anti-very late antigen-4 monoclonal antibodies. J Immunol. 1994;153(12):5810–5818. [PubMed] [Google Scholar]
- 29.Paul LC, Davidoff A, Benediktsson H, Issekutz TB. The efficacy of LFA-1 and VLA-4 antibody treatment in rat vascularized cardiac allograft rejection. Transplantation. 1993;55(5):1196–1199. [PubMed] [Google Scholar]
- 30.Miwa S, Isobe M, Suzuki J, Makuuchi M, Miyasaka M, Yamazaki S, et al. Effect of anti-intercellular adhesion molecule-1 and anti-leukocyte function associated antigen-1 monoclonal antibodies on rat-to-mouse cardiac xenograft rejection. Surgery. 1997;121(6):681–689. doi: 10.1016/s0039-6060(97)90057-x. [DOI] [PubMed] [Google Scholar]
- 31.Arai K, Sunamura M, Wada Y, Takahashi M, Kobari M, Kato K, et al. Preventing effect of anti-ICAM-1 and anti-LFA-1 monoclonal antibodies on murine islet alograft rejection. Int J Pancreatol. 1999;26(1):23–31. doi: 10.1385/IJGC:26:1:23. [DOI] [PubMed] [Google Scholar]
- 32.Nicolls MR, Coulombe M, Yang H, Bolwerk A, Gill RG. Anti-LFA-1 therapy induces long-term islet alograft acceptance in the absence of IFN-gamma or IL-4. J Immunol. 2000;164(7):3627–3634. doi: 10.4049/jimmunol.164.7.3627. [DOI] [PubMed] [Google Scholar]
- 33.Nishihara M, Gotoh M, Ohzato H, Ohta Y, Luo Z, Dono K, et al. Awareness of donor alloantigens in antiadhesion therapy induces antigen-specific unresponsiveness to islet alografts. Transplantation. 1997;64(7):965–970. doi: 10.1097/00007890-199710150-00005. [DOI] [PubMed] [Google Scholar]
- 34.Stegall MD, Dean PG, Ninova D, Cohen AJ, Shepard GM, Gup C, et al. alpha4 integrin in islet alograft rejection. Transplantation. 2001;71(11):1549–1555. doi: 10.1097/00007890-200106150-00011. [DOI] [PubMed] [Google Scholar]
- 35.Stegall MD, Ostrowska A, Haynes J, Karrer F, Kam I, Gill RG. Prolongation of islet alograft survival with an antibody to vascular cell adhesion molecule 1. Surgery. 1995;118(2):366–369. doi: 10.1016/s0039-6060(05)80346-0. discussion 369-370. [DOI] [PubMed] [Google Scholar]
- 36.Posselt AM, Bellin MD, Tavakol M, Szot GL, Frassetto LA, Masharani U, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant. 2010;10(8):1870–1880. doi: 10.1111/j.1600-6143.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turgeon NA, Avila JG, Cano JA, Hutchinson JJ, Badell IR, Page AJ, et al. Experience with a novel efalizumab-based immunosuppressive regimen to facilitate single donor islet cell transplantation. Am J Transplant. 2010;10(9):2082–2091. doi: 10.1111/j.1600-6143.2010.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. J Clin Invest. 2010;120(12):4520–4531. doi: 10.1172/JCI43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berney T, Pileggi A, Molano RD, Poggioli R, Zahr E, Ricordi C, et al. The effect of simultaneous CD154 and LFA-1 blockade on the survival of allogeneic islet grafts in nonobese diabetic mice. Transplantation. 2003;76(12):1669–1674. doi: 10.1097/01.TP.0000092525.17025.D0. [DOI] [PubMed] [Google Scholar]
- 40.Corbascio M, Ekstrand H, Osterholm C, Qi Z, Simanaitis M, Larsen CP, et al. CTLA4Ig combined with anti-LFA-1 prolongs cardiac allograft survival indefinitely. Transpl Immunol. 2002;10(1):55–61. doi: 10.1016/s0966-3274(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 41.Malm H, Corbascio M, Osterholm C, Cowan S, Larsen CP, Pearson TC, et al. CTLA4ig induces long-term graft survival of allogeneic skin grafts and totally inhibits T-cell proliferation in LFA-1-deficient mice. Transplantation. 2002;73(2):293–297. doi: 10.1097/00007890-200201270-00024. [DOI] [PubMed] [Google Scholar]
- 42.Nicolls MR, Coulombe M, Beilke J, Gelhaus HC, Gill RG. CD4-dependent generation of dominant transplantation tolerance induced by simultaneous perturbation of CD154 and LFA-1 pathways. J Immunol. 2002;169(9):4831–4839. doi: 10.4049/jimmunol.169.9.4831. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Gao D, Lunsford KE, Frankel WL, Bumgardner GL. Targeting LFA-1 synergizes with CD40/CD40L blockade for suppression of both CD4-dependent and CD8-dependent rejection. Am J Transplant. 2003;3(10):1251–1258. doi: 10.1046/j.1600-6143.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 44.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3(11):1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 45.Dudani R, Chapdelaine Y, Faassen Hv Hv, Smith DK, Shen H, Krishnan L, et al. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J Immunol. 2002;168(11):5737–5745. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- 46.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281(1-2):65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 47.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171(1):27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 48.Wright KO, Murray DA, Crispe NI, Pierce RH. Quantitative PCR for detection of the OT-1 transgene. BMC Immunol. 2005;6:20. doi: 10.1186/1471-2172-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 50.Nicholson JK, Stein D, Mui T, Mack R, Hubbard M, Denny T. Evaluation of a method for counting absolute numbers of cells with a flow cytometer. Clin Diagn Lab Immunol. 1997;4(3):309–313. doi: 10.1128/cdli.4.3.309-313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie B, Chen J, Wang F, Lan T, Wang Y, Xia J, et al. Monoclonal antibody treatment to prolong the secondary cardiac allograft survival in alloantigen-primed mice. Scand J Immunol. 2010;71(5):345–352. doi: 10.1111/j.1365-3083.2010.02387.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Q-W, Rabant M, Schenk A, Valujskikh A. ICOS-Dependent and -independent functions of memory CD4 T cells in allograft rejection. Am J Transplant. 2008;8(3):497–506. doi: 10.1111/j.1600-6143.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- 53.Floyd TL, Koehn BH, Kitchens WH, Robertson JM, Cheeseman JA, Stempora L, et al. Limiting the amount and duration of antigen exposure during priming increases memory T cell requirement for costimulation during recall. J Immunol. 2011;186(4):2033–2041. doi: 10.4049/jimmunol.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Setoguchi K, Schenk AD, Ishii D, Hattori Y, Baldwin WM, 3rd, Tanabe K, et al. LFA-1 Antagonism Inhibits Early Infiltration of Endogenous Memory CD8 T Cells into Cardiac Allografts and Donor-Reactive T Cell Priming. Am J Transplant. 2011;11(5):923–935. doi: 10.1111/j.1600-6143.2011.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oderup C, Malm H, Ekberg H, Qi Z, Veress B, Ivars F, et al. Costimulation blockade-induced cardiac allograft tolerance: inhibition of T cell expansion and accumulation of intragraft cD4(+)Foxp3(+) T cells. Transplantation. 2006;82(11):1493–1500. doi: 10.1097/01.tp.0000244064.66136.04. [DOI] [PubMed] [Google Scholar]
- 56.Verbinnen B, Billiau AD, Vermeiren J, Galicia G, Bullens DMA, Boon L, et al. Contribution of regulatory T cells and effector T cell deletion in tolerance induction by costimulation blockade. J Immunol. 2008;181(2):1034–1042. doi: 10.4049/jimmunol.181.2.1034. [DOI] [PubMed] [Google Scholar]
- 57.Jiang XF, Cui ZM, Zhu L, Guo DW, Sun WY, Lin L, et al. CD40-CD40L costimulation blockade induced the tolerogenic dendritic cells in mouse cardiac transplant. Int Surg. 2010;95(2):135–141. [PubMed] [Google Scholar]
- 58.Guillot C, Ménoret S, Guillonneau C, Braudeau C, Castro MG, Lowenstein P, et al. Active suppression of allogeneic proliferative responses by dendritic cells after induction of long-term allograft survival by CTLA4Ig. Blood. 2003;101(8):3325–3333. doi: 10.1182/blood-2002-07-2076. [DOI] [PubMed] [Google Scholar]
- 59.El-Sawy T, Belperio JA, Strieter RM, Remick DG, Fairchild RL. Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation. 2005;112(3):320–331. doi: 10.1161/CIRCULATIONAHA.104.516708. [DOI] [PubMed] [Google Scholar]
- 60.Henderson RB, Lim LH, Tessier PA, Gavins FN, Mathies M, Perretti M, et al. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils. J Exp Med. 2001;194(2):219–226. doi: 10.1084/jem.194.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen X, Reng F, Gao F, Uchida Y, Busuttil RW, Kupiec-Weglinski JW, et al. Alloimmune activation enhances innate tissue inflammation/injury in a mouse model of liver ischemia/reperfusion injury. Am J Transplant. 2010;10(8):1729–1737. doi: 10.1111/j.1600-6143.2010.03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. Am J Transplant. 2008;8(8):1652–1661. doi: 10.1111/j.1600-6143.2008.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carson KR, Focosi D, Major EO, Petrini M, Richey EA, West DP, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009;10(8):816–824. doi: 10.1016/S1470-2045(09)70161-5. [DOI] [PubMed] [Google Scholar]
- 64.Clifford DB, De Luca A, Deluca A, Simpson DM, Arendt G, Giovannoni G, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9(4):438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- 65.Piccinni C, Sacripanti C, Poluzzi E, Motola D, Magro L, Moretti U, et al. Stronger association of drug-induced progressive multifocal leukoencephalopathy (PML) with biological immunomodulating agents. Eur J Clin Pharmacol. 2010;66(2):199–206. doi: 10.1007/s00228-009-0739-z. [DOI] [PubMed] [Google Scholar]
- 66.Tyler KL. Progressive multifocal leukoencephalopathy: can we reduce risk in patients receiving biological immunomodulatory therapies? Ann Neurol. 2010;68(3):271–274. doi: 10.1002/ana.22185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.