Abstract
Protein fatty-acylation is the covalent addition of a lipid chain at specific amino acids. This modification changes the inherent hydrophobicity of a protein, often targeting it to cellular membrane compartments. Acylation may also regulate protein activity, stability, and protein-protein interactions. Its study is therefore critical to understanding the biology of the hundreds of proteins described to be lipid-modified, as well as those that are continually being discovered. Fatty-acylation can be analyzed using chemical reporters that mimic natural lipids and contain bioorthogonal chemical handles allowing them to be reacted with detection tags such as fluorophores or affinity tags. Our laboratory has successfully utilized alkynyl-chemical reporters of protein myristoylation, S-palmitoylation, prenylation and acetylation. Protocol 1 describes metabolic incorporation of these chemical reporters onto proteins in living cells. Protocol 2 describes the global visualization of reporter-labeled proteins by selectively reacting alkyne-containing chemical reporter-labeled proteins in cell lysates with azido-rhodamine via the click chemistry and fluorescence gel scanning. Protocol 3 describes analysis of protein acylation on individual candidate proteins using immunoprecipitation, click chemistry and fluorescence gel scanning. Finally, Protocol 4 allows identification of novel fatty acylated proteins by reacting chemical reporter-labeled proteins with azido-biotin via click chemistry and selective retrieval using streptavidin beads. This may be particularly valuable for the examination of S-palmitoylomes in different cell types or activation states, as these modifications do not occur on readily predicted consensus amino acid motifs. Overall, these techniques provide robust, non-radioactive methods for examining the acylation states of full cellular proteomes and individual proteins of interest.
Keywords: fatty-acylation, S-palmitoylation, click chemistry
Introduction
Protein fatty acylation is the covalent addition of a lipid chain at specific amino acids. This modification changes the inherent hydrophobicity of a protein, often targeting it to cellular membrane compartments(Linder and Deschenes, 2007). Acylation may also regulate protein activity, stability, and protein-protein interactions(Linder and Deschenes, 2007). Its study is therefore critical to understanding the biology of the hundreds of proteins already described to be lipid-modified, as well as those that are continually being discovered. Fatty acylation can be analyzed using chemical reporters that mimic natural lipids and contain bioorthogonal chemical handles allowing them to be reacted with secondary detection tags such as fluorophores or affinity tags(Charron et al., 2009b). For example, our laboratory has successfully utilized alkynyl-chemical reporters of protein myristoylation(Charron et al., 2009b), S-palmitoylation(Charron et al., 2009b; Yount et al., 2010; Zhang et al., 2010) (Fig 1), prenylation(Charron et al., 2010) (Fig 1) and acetylation(Yang et al., 2010a). Basic Protocol 1 describes metabolic incorporation of these chemical reporters onto proteins in living cells. Basic Protocol 2 describes the global visualization of reporter-labeled proteins by selectively reacting alkyne-containing chemical reporter-labeled proteins in cell lysates with azido-rhodamine via the click chemistry and fluorescence gel scanning. Basic Protocol 3 describes analysis of protein acylation on individual candidate proteins using immunoprecipitation, click chemistry and fluorescence gel scanning. Finally, Basic Protocol 4 allows identification of novel fatty acylated proteins by reacting chemical reporter-labeled proteins with azido-biotin via click chemistry and selective retrieval using streptavidin beads. This may be particularly valuable for the examination of S-palmitoylomes in different cell types or activation states, as these modifications do not occur on readily predicted consensus amino acid motifs. Overall, these techniques provide robust, non-radioactive methods for examining the acylation states of full cellular proteomes and individual proteins of interest (Fig 2).
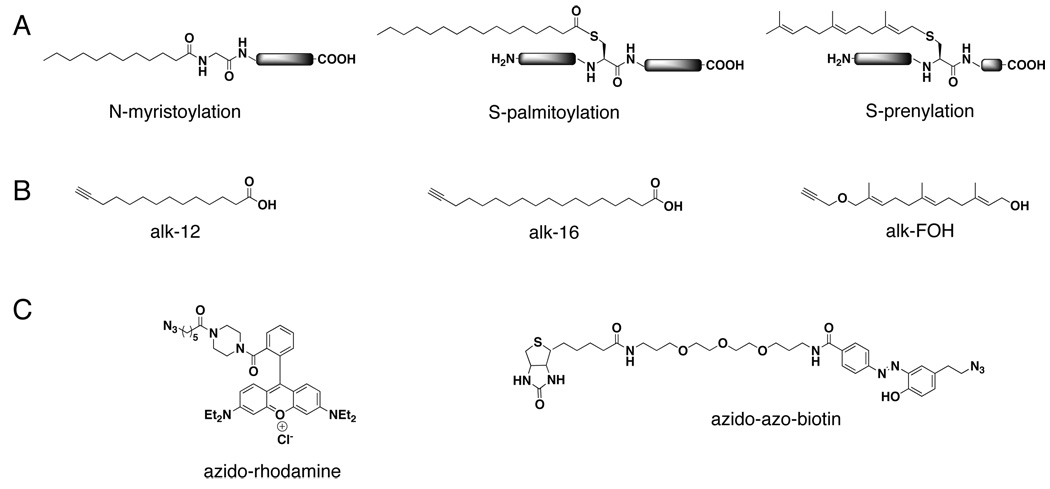
Figure 1. Chemical tools for studying protein fatty acylation.
A. Chemical structures of protein myristoylation on N-terminal glycines, palmitoylation on cysteine residues, and prenylation on C-terminal CaaX motifs. B. Alkyne-chemical reporters used for studying protein myristoylation (alk-12), palmitoylation (alk-16), and prenylation (alk-FOH). C. Secondary detection reagents for visualization (azido-rhodamine) or affinity enrichment (azido-azo-biotin) of alkyne-chemical reporter-labeled proteins.
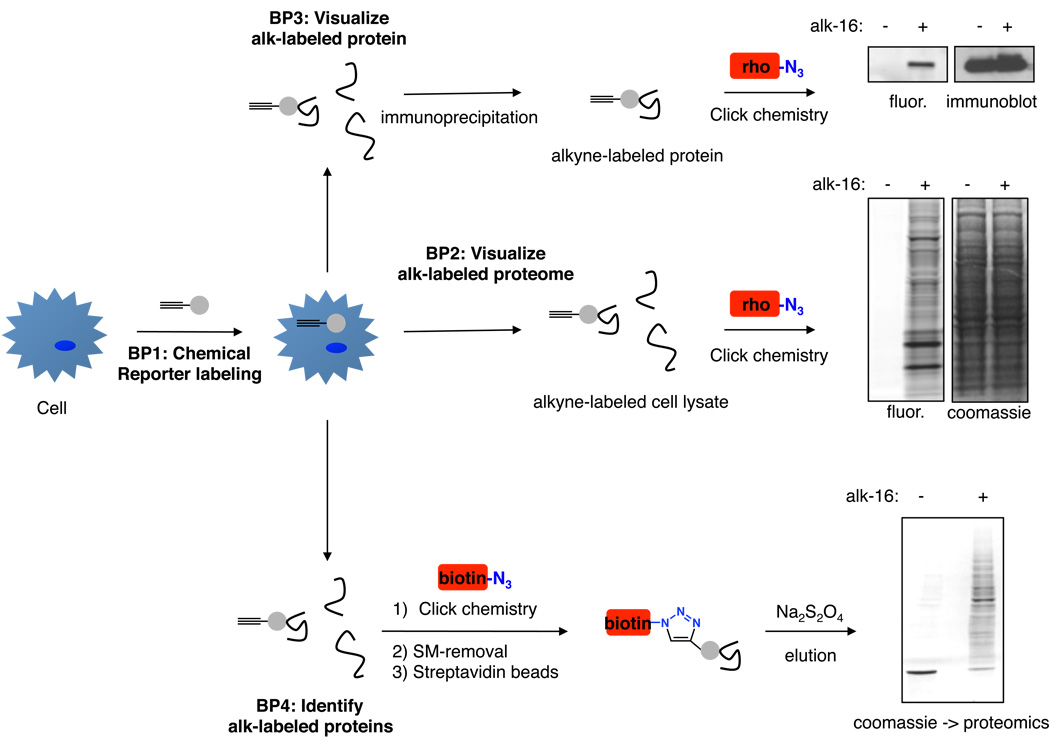
Figure 2. Workflow of protocols used for studying protein fatty acylation.
Basic protocol 1 describes labeling of proteins in cells with alkyne-chemical reporters of fatty acylation. Basic protocols 2–4 utilize the labeled cells for visualization of labeled proteomes by bioorthogonal ligation with azido-rhodamine (rho, Basic protocol 2), visualization of individual candidate proteins using immunoprecipitation and bioorthogonal ligation with azido-rhodamine (Basic protocol 3), and/or affinity enrichment of labeled proteins by bioorthogonal ligation with azido-azo-biotin (biotin) and selective elution from streptavidin beads (Basic protocol 4). Data shown for Basic protocols 2 and 4 is from DC2.4 cells labeled with 50 uM alk-16 for 1 h. Data shown for Basic protocol 3 is from HeLa cells transfected with HA-tagged IFITM3 protein and labeled with 50 uM alk-16 for 1 h followed by immunoprecipitation and immunoblotting with anti-HA antibodies.
Basic Protocol 1: Metabolic incorporation of chemical reporters of protein fatty acylation in living cells
Cellular growth medium is supplemented with alkyne-bearing chemical reporters of fatty acylation allowing cellular uptake and metabolic incorporation of the reporters onto proteins at sites of acylation.
Materials
Cultured cells for metabolic labeling
Complete tissue culture media for growing the cell type of interest
Appropriate media for growing the cell type of interest supplemented with 2% charcoal/dextran filtered fetal bovine serum, 37 °C (Note: charcoal/dextran filtering removes lipids present in serum allowing cellular uptake of lipid chemical reporters without competition from serum lipids.)
DMSO (USB grade)
50 mM stock of chemical reporter in DMSO (Note: Alk-12 is useful for studying myristoylation, alk-16 is useful for studying palmitoylation, and alk-FOH is useful for studying prenylation. Alk-16 is commercially available (Sigma-Aldrich O8382) and chemical synthesis has been published for alk-12(Charron et al., 2009b), alk-16(Charron et al., 2009b), and alk-FOH(Charron et al., 2010).
PBS (144 mg/L KH2PO4, 9 g/L NaCl, 795 mg/L Na2HPO4-7H2O pH 7.4), ice-cold
Cell scrapers
Refrigerated Centrifuge
Dry ice/ethanol bath or liquid nitrogen
-
1.
Replace serum-containing cellular media with 37 °C media supplemented with 2% charcoal/dextran filtered fetal bovine serum and either DMSO as a solvent control or alkyne-fatty acid chemical reporter (20 – 100 uM).
Media at 37 °C allows solubilization of fatty acid chemical reporters.
Fatty acid chemical reporters are typically effective and show minimal toxicity when used at concentrations of 20–100 uM supplied from 50 mM stocks in DMSO. A concentration of 20 uM can be used as a starting point for most experiments and can be increased if enhanced signal strength is desired..
-
2.
Incubate cells with chemical reporters for 1h at 37 °C.
Longer incubation times may be necessary to label protein sites with minimal turnover and to label sites that are modified upon de novo protein synthesis. This should be titrated for each protein of interest, though a 1 h labeling time is sufficient for most proteins.
-
3.
Harvest cells and wash twice with ice cold PBS to remove serum proteins. Pellet cells by centrifugation at 1000 × g for 3 min at 4 °C.
Use a cell scraper to remove adherent cells. Use of trypsin may degrade cell surface proteins.
-
4.
Freeze cell pellets in liquid nitrogen or a dry ice/ethanol bath and store at −80 °C, or continue to Basic Protocol 2, 3, or 4.
Cell pellets have been stored for up to 6 months without apparent loss of signal.
Basic Protocol 2: Global fluorescent profiling of fatty acylated proteins in whole cell lysates
Chemical reporters mimic natural lipids but contain bioorthogonal groups allowing a selective chemical reaction appending detection tags to the labeled proteins. Proteins labeled with alkyne-bearing chemical reporters of fatty acylation are reacted with the azide bearing detection tag azido-rhodamine via click chemistry allowing fluorescent visualization of protein bands after SDS-PAGE.
Materials
Chemical reporter-labeled cell pellets from Basic Protocol 1
4% SDS buffer (See Reagents and Solutions)
EDTA-free protease inhibitor cocktail tablets (Roche)
Benzonase (Sigma-Aldrich, ultrapure)
BCA assay reagents (Pierce Protein Research)
5 mM azido-rhodamine in DMSO (Synthesis has been described(Charron et al., 2009b), tetramethylrhodamine-5-carbonyl azide has also been used in click chemistry reactions(Martin and Cravatt, 2009) (V and is available from Invitrogen)
50 mM TCEP, freshly prepared in H2O
2 mM TBTA in 1:4 DMSO:Butanol (v/v)
50 mM CuSO4, freshly prepared in H2O
Methanol, ice-cold
Chloroform, ice-cold
H2O, ice-cold
4× protein loading buffer (See Reagents and Solutions)
2-mercaptoethanol
Protein Molecular Weight Standards
Destaining solution (40% methanol, 50% acetic acid, 10% H2O)
Coomassie blue staining reagents (Pierce Protein Research)
1.5 mL tubes
18-well 4–20% Tris-HCl protein gels
Fluorescence gel scanner (For azido-rhodamine(Charron et al., 2009b), a 532 nm excitation and a 580 nm detection filter with 30 nm band-pass can be used)
Lyse cells with SDS-containing buffer in order to maximally solubilize proteins
-
1.
Lyse cells labeled with chemical reporters in Basic Protocol 1 by vortexing in 50 uL of 4% SDS buffer supplemented with EDTA-free protease inhibitor cocktail and 250 units Benzonase / 1 × 106 cells.
Protease inhibitors containing EDTA are not compatible with Benzonase or click chemistry reactions.
-
2.
Quantify protein concentrations using a standard BCA assay.
Protein concentrations obtained for cell lines are generally 1–10 mg/mL depending on the cell type.
-
3.
Aliquot equal amounts of protein for each sample into 1.5 mL tubes and bring the volume up to 44.5 uL with 4% SDS lysis buffer used in step 1.
50 ug total protein is generally adequate for visualization of fatty acylation with chemical reporters. Using less protein makes precipitation more difficult in later steps.
Ligate chemical reporter-labeled proteins with azido-rhodamine for visualization using the click chemistry reaction
-
4.
Prepare a master mix of click chemistry reagents adding the following volumes per sample: 1 uL 5 mM azido-rhodamine, 1 uL 50 mM TCEP, 2.5 uL 2 mM TBTA, and 1 uL 50 mM CuSO4.
Solutions of TCEP and CuSO4 should be prepared fresh for each experiment.
A master mix ensures equal amounts of each reagent are added to each sample.
-
5.
Add 5.5 uL of the click chemistry master mix to each protein sample for a final volume of 50 uL and vortex to mix.
-
6.
Incubate the click chemistry reaction for 1 h at room temperature.
Remove unreacted molecules by chloroform/methanol extraction
-
7.
Perform a chloroform/methanol precipitation of protein to remove unreacted azido-rhodamine by adding 200 uL methanol, 75 uL chloroform, and 150 uL H2O. Precipitation reagents should be ice-cold. Vortex and centrifuge for 15 min at 20,000 × g at 4 °C.
-
8.
Remove and discard the upper aqueous phase (should be clear) leaving the lower organic phase (contains the pink colored rhodamine) and the white layer of protein that should have formed between the two layers.
-
9.
Add 1 mL of ice cold methanol to each sample and mix gently. This should allow the protein pellet to sink to the bottom of the tube. Centrifuge at 20,000 × g for 10 minutes and remove all liquid by pipeting. The protein pellet should remain in the tube and should be a light pink color.
-
10.
Wash the protein pellet by adding 1 mL of ice-cold methanol and inverting the tube. Centrifuge at 20,000 × g for 10 min at 4 °C.
-
11.
Carefully remove the methanol leaving behind the protein pellet. Allow remaining methanol to evaporate by leaving the sample tubes open on the bench for 20 min at room temperature. The dried pellet will be a grey/white color.
Resolubilize proteins for SDS-PAGE
-
12.
Add 50 uL of 4% SDS buffer to dissolve the protein pellets. A bath sonicator may be used at this step to speed the solubilization process. Pellets are generally dissolved within 5 s using a sonicator.
-
13.
Add 17.5 uL 4× loading buffer and 3.5 uL 2-mercapto ethanol to each sample giving a final concentration of 5% 2-mercaptoethanol and vortex samples. Note: DTT has been used successfully as an alternative to 2-mercaptoethanol.
-
14.
Denature proteins at 95 °C for 5 min using a heating block, vortex, and centrifuge for 1 min at 5000 × g at room temperature.
-
15.
Load 20 uL of samples on an 18-well 4–20% Tris-HCl gel and run for 1 h at 200 V.
A fluorescent protein ladder should be used as a molecular weight standard. Commercially available protein ladders may require as much as a 1:10,000 dilution with 4% SDS-buffer and appropriate addition of 4× loading buffer for the fluorescence gel to avoid saturation and bleed-through of the standard into other lanes of the gel.
Destain the gel prior to fluorescent gel scanning
-
16.
Destain the gel by rocking for 1 h in 40% methanol/10% acetic acid/50% dH2O at room temperature to remove remaining traces of loading buffer and unreacted rhodamine.
-
17.
Scan the gel using a fluorescence gel scanner.
For the azido-rhodamine derivative pictured in Fig 1, an Amersham Biosciences Typhoon 9400 scanner has been successfully used with 532 nm excitation and a 580 nm detection filter with 30 nm band-pass).
-
18.
Stain the gel with coomassie blue in order to demonstrate equal protein loading.
Basic Protocol 3: Fluorescent visualization of fatty acylation on candidate proteins
Proteins labeled with chemical reporters of fatty acylation are immunoprecipitated prior to click chemistry ligation with azido-rhodamine and SDS-PAGE.
Materials
Chemical reporter-labeled cell pellets from Basic Protocol 1
1% Brij97 buffer (See Reagents and Solutions)
EDTA-free protease inhibitor cocktail tablets (Roche)
BCA assay reagents (Pierce Protein Research)
Immunoprecipitation reagents for the protein of interest
4% SDS buffer (See Reagents and Solutions)
5 mM azido-rhodamine in DMSO (Synthesis has been described(Charron et al., 2009b), tetramethylrhodamine-5-carbonyl azide has also been used in click chemistry reactions(Martin and Cravatt, 2009) (V and is available from Invitrogen)
50 mM TCEP, freshly prepared in H2O
2 mM TBTA in 1:4 DMSO:Butanol (v/v)
50 mM CuSO4, freshly prepared in H2O
Optional: 25% NH2OH (w/v) in H2O, pH 7.0
Protein Molecular Weight Standards
4× protein loading buffer (See Reagents and Solutions)
2-mercaptoethanol
Destaining solution (50% methanol/10% acetic acid/40% H2O)
Western blotting equipment and reagents for the protein of interest
2.0 mL dolphin microcentrifuge tubes
1.5 mL tubes
18-well 4–20% Tris-HCl protein gels
Fluorescent gel scanner (For azido-rhodamine(Charron et al., 2009b), a 532 nm excitation and a 580 nm detection filter with 30 nm band-pass can be used)
-
1.
Lyse chemical reporter-labeled cells prepared in Basic Protocol 1 using 50 uL 1% Brij97 buffer supplemented with EDTA-free protease inhibitor cocktail per 1× 106 cells. Centrifuge at 1,000 × g for 5 min at 4 °C to remove cellular debris.
Other lysis buffers compatible with immunoprecipitations may be substituted for Brij97 buffer.
Protease inhibitors containing EDTA are not compatible with click chemistry.
-
2.
Quantify protein concentrations using a standard BCA assay and aliquot 500 ug protein into eppendorf tubes. Normalize the volumes to 100 uL with 1% Brij97 buffer used in step 1.
Increased quantities of proteins may be needed, particularly when examining proteins of low cellular abundance. 500 ug is generally adequate for study of overexpressed proteins and most native proteins.
-
3.
Perform standard immunoprecipitation techniques for the protein of interest and wash the agarose beads at least 3 times with lysis buffer.
-
4.
Add 22.25 uL of 4% SDS buffer to the pelleted agarose beads used for immunoprecipitations.
4% SDS buffer is not compatible with most immunoprecipitations and its addition will likely dissociate antibody-protein complexes. Thus, proteins will no longer be associated with beads and additional washes subsequent to this step should not be performed.
-
5.
Add 2.75 uL of click chemistry reagent master mix containing the following volumes per sample: 0.5 uL 5 mM azido-rhodamine, 0.5 uL 50 mM TCEP, 1.25 uL 2 mM TBTA, and 0.5 uL 50 mM CuSO4. Vortex to mix.
-
6.
Incubate for 1 h at room temperature on the benchtop.
-
7.
Add 9 uL 4× loading buffer and 1.8 uL 2-mercaptoethanol to each sample giving a final concentration of 5% 2-mercapto ethanol and vortex samples.
Optional: At this step, samples can be split into 2 tubes where 2 uL of 25% neutral NH2OH is added to one tube and 2 uL H2O is added to the second tube and mixed by vortexing. NH2OH is known to cleave thioester bonds characteristic of protein palmitoylation and thus fluorescence signal should be lost or greatly reduced for proteins that are labeled with alkyne-palmitate reporter on cysteine residues. The final concentration of NH2OH should be 2.5%. Proceed to step 8.
-
8.
Denature proteins at 95 °C for 5 min using a heating block, vortex, and centrifuge for 1 min at 9000 × g at room temperature to pellet agarose beads used for immunoprecipitations.
-
9.
Load 15 uL of samples onto duplicate 18-well 4–20% Tris-HCl gels and run for 1 h at 200 V.
Gel 1 will be used for fluorescent gel scanning and gel 2 will be used in western blotting for the protein of interest as a loading control.
If samples were split for NH2OH treatment, additional lysis buffer and 4× loading buffer can be added before loading onto gels. Alternatively, lower volumes can be loaded.
A fluorescent protein ladder should be used as a molecular weight standard. Commercially available protein ladders may require as much as a 1:10,000 dilution with 4% SDS-buffer and appropriate addition of 4× loading buffer for the fluorescent gel to avoid saturation and bleed-through of the standard into other lanes of the gel.
-
10a.
Destain gel 1 and perform fluorescence gel scanning as described in Basic Protocol 2.
-
10b.
Transfer proteins from gel 2 onto nitrocellulose membrane and probe for the protein of interest using standard western blotting techniques.
Basic Protocol 4: Proteomic identification of proteins labeled with chemical reporters of fatty acylation
Proteins labeled with alkyne-bearing chemical reporters of fatty acylation are reacted with the azide bearing detection tag azido-azo-biotin via “click chemistry” allowing selective retrieval of labeled proteins using streptavidin beads. Na2SO4 cleavage of the azo group allows specific elution of labeled proteins from beads without boiling.
Materials
Chemical reporter-labeled cell pellets from Basic Protocol 1
4% SDS buffer (See Reagents and Solutions)
1% Brij97 buffer (See Reagents and Solutions)
EDTA-free protease inhibitor cocktail tablets (Roche)
BCA assay reagents (Pierce Protein Research)
streptavidin agarose
5 mM azido-azo-biotin (Synthesis has been described(Yang et al., 2010b) and azide-biotin is available from Invitrogen and has been successfully used in click chemistry reactions and proteomic studies(Martin and Cravatt, 2009))
50 mM TCEP, freshly prepared in H2O
2 mM TBTA in 1:4 DMSO:Butanol (v/v)
50 mM CuSO4, freshly prepared in H2O
Protein Molecular Weight Standards
0.5M EDTA
PBS, ice cold, pH 7.4
PBS/0.2% SDS, ice cold, pH 7.4
250 mM ammonium bicarbonate in H2O (ABC buffer), ice cold
8 M Urea
200 mM TCEP, freshly prepared in H2O
400 mM iodoacetamide, freshly prepared in H2O
Na2S2O4 Elution buffer (See Reagents and Solutions)
Green Centricons (Millipore, YM-10 membranes with 10 kDa molecular weight cutoff)
1% SDS in H2O
2-mercaptoethanol
NuPAGE 4× LDS buffer (Invitrogen)
Coomassie blue staining reagents (Pierce Protein Research)
15 mL tubes
Nutating mixer
-
1.
Lyse cells labeled with chemical reporters in Basic Protocol 1 by vortexing in 50 uL of 4% SDS buffer supplemented with EDTA-free protease inhibitor cocktail and 250 units of Benzonase / 1 × 106 cells.
Protease inhibitors containing EDTA are not compatible with Benzonase or click chemistry reactions.
-
2.
Quantify protein concentrations using a standard BCA assay.
-
3.
Aliquot equal amounts of protein for each sample and bring the volume up to 4.45 mL with 4% SDS lysis buffer.
A minimum of 10 mg total protein is recommended.
Ligate chemical reporter-labeled proteins with azido-azo-biotin for visualization using the click chemistry reaction
-
4.
Prepare a master mix of click chemistry reagents adding the following volumes per sample: 100 uL 5 mM azido-azo-biotin, 100 uL 50 mM TCEP, 250 uL 2 mM TBTA, and 100 uL 50 mM CuSO4.
Solutions of TCEP and CuSO4 should be prepared fresh for each experiment.
-
5.
Add 550 uL of the click chemistry master mix to each protein sample for a final volume of 5 mL and vortex to mix.
-
6.
Incubate the click chemistry reaction for 1.5 h at room temperature on the benchtop.
-
7.
Perform a methanol/chloroform precipitation of protein as in Basic Protocol 1 scaling up volumes proportionately. Wash the protein pellet twice with 50 mL ice cold methanol dissociating the protein pellet by pipeting. Dry the protein pellet for 20 minutes at room temperature to remove methanol.
-
8.
Resuspend the protein pellet in 1 mL 4% SDS buffer supplemented with 20 uL 0.5 M EDTA solution.
EDTA will chelate remaining copper and prevent any further click chemistry reaction.
-
9.
Quantify resuspended protein by BCA assay.
50–80% of proteins are generally recovered after precipitation.
Bind fatty acylation chemical reporter-labeled and biotin-ligated proteins to streptavidin beads
-
10.
Add 5mg protein in 1 mL 4% SDS buffer to individual 15 mL falcon tubes.
-
11.
Add 2 mL of 1% Brij97 buffer to dilute the SDS concentration to a level compatible with biotin/streptavidin binding, i.e., less than 1.5% SDS.
-
12.
Wash 100 uL streptavidin agarose per 5 mg protein with 50 mL PBS three times. Centrifuge beads for 2 min at 4000×g for each wash.
-
13.
Resuspend the streptavidin agarose in 100 uL 1% Brij97 buffer per sample and add 100 uL agarose to each protein sample.
-
14.
Rock the protein/bead mixture on a nutating mixture for 1 h at room temperature.
Wash streptavidin beads to minimize non-specific pulldown of proteins
-
15.
Wash beads by inverting several times in 10 mL PBS containing 0.2% SDS and centrifuge at 4000 ×g for 2 min.
-
16.
Wash beads three times with 10 mL PBS centrifuging at 4000 × g for 2 min between washes.
-
17.
Wash beads twice with 10 mL 250 mM ABC buffer centrifuging at 4000 × g for 2 min between washes.
Cap cysteines for mass spectrometry analysis
-
18.
Add 500 uL 8 M Urea, 25 uL 200 mM TCEP, and 25 uL 400 mM iodoacetamide. Mix by pipeting and incubate for 30 min at room temperature.
-
19.
Add 10 mL ABC buffer and centrifuge at 4000 × g for 2 min.
-
20.
Resuspend beads in 1 mL ABC buffer and transfer to dolphin tubes.
-
21.
Centrifuge at 4000 × g for 2 min and remove the supernatant.
Elute labeled proteins from streptavidin beads
-
22.
Add 250 uL Na2S2O4 elution buffer, resuspend beads by pipeting, and incubate for 30 min at room temperature.
-
23.
Centrifuge at 4000 × g for 2 min and save the supernatant containing eluted proteins.
-
24.
Add an additional 250 uL Na2S2O4 elution buffer to the beads and incubate for 30 min at room temperature.
-
25.
Centrifuge at 4000 × g for 2 min, collect the eluent, combine with the previously eluted proteins.
Concentrate eluted proteins for SDS-PAGE
-
26.
Wash a green centricon (YM-10 membrane with 10 kDa molecular weight cutoff) with 500 uL 1% SDS in H2O and centrifuge at 9000 × g for 30 min.
-
27.
Wash centricon again with 500 uL H2O and centrifuge at 9000 × g for 30 min.
-
28.
Add 500 uL of eluted proteins to the centricon and centrifuge at 9000 × g for 30 min.
-
29.
Turn the centricon upside down and spin into a collection tube at 1000 × g for 3 min.
-
30.
Add 50 uL 1% SDS containing 75 mM 2-mercaptoethanol to the centricon reservoir, pipet up and down several times, and centrifuge the upside down centricon into the same collection tube for 3 min at 1000 × g.
-
31.
Repeat step 30.
The final volume will be approximately 125 uL.
-
32.
Speedvac the samples to a solid white powder.
Perform SDS-PAGE of eluted proteins
-
33.
Resuspend the white powder in 25 uL 1× LDS buffer containing 5% 2-mercaptoethanol by vorexing vigorously.
-
34.
Incubate samples for 5 min on a 95 °C heating block.
-
35.
Centrifuge at 1000 × g for 1 min at room temperature.
-
36.
Load 20 uL protein mixture onto a 4–20% Tris-HCl gel.
If gel slices will be cut for proteomic analysis, blank lanes should be placed between samples. This will aid in preventing contamination during gel slicing for gel-based proteomics. Blanks should contain LDS buffer with ~20% SDS and 5% 2-mercaptoethanol. If SDS is not added to the blank lanes, the sample proteins may expand horizontally in the gel during electrophoresis.
A fraction of the retrieved proteins can be saved for western blotting to confirm mass spectrometry results.
-
37.
Run the gel for 1 h at 200 V.
-
38.
Cut gel slices for proteomic analysis according to standard procedures.
Reagents and Solutions
4% SDS buffer, pH 7.4, storage at room temperature for >1 yr if sterile filtered
4% (w/v) SDS
150 mM NaCl
50 mM triethanol amine
1% Brij97 buffer, pH 7.4, storage at room temperature for >1 yr if sterile filtered
1% (w/v) Brij97
150 mM NaCl
50 mM triethanol amine
4× loading buffer, storage at room temperature for 1 yr
40% Glycerol
240 mM Tris/HCl, pH 6.8
8% SDS
0.04% (w/v) bromophenol blue
Na2S2O4 Elution buffer, prepared fresh for each experiment
250 mM ammonium bicarbonate
0.1% (w/v) SDS
25 uM Na2S2O4
COMMENTARY
Background Information
The use of chemical reporters and bioorthogonal ligation chemistries has a rich history in the field of protein glycosylation(Prescher and Bertozzi, 2006), and has recently been extended to the study of protein myristoylation(Charron et al., 2009b), S-palmitoylation(Charron et al., 2009b; Yount et al., 2010; Zhang et al., 2010), prenylation(Charron et al., 2010) (Fig 1a,b) and acetylation(Yang et al., 2010a). Protein acylation has classically been studied using radiolabeled lipids. However, this method is hazardous and provides low sensitivity often requiring film exposure times of weeks or months. For example, the bacterial effector protein SifA was demonstrated to be prenylated using radiolabeled lipids in an in vitro translation system, but radiolabeled lipids did not provide the sensitivity needed to detect SifA prenylation in living cells(Reinicke et al., 2005). In contrast, study of protein acylation with alkyne-bearing chemical reporters and bioorthogonal ligation with azido-rhodamine provides a robust and sensitive way to visualize these modifications(Charron et al., 2009a). Accordingly, we recently confirmed SifA prenylation in living cells using a prenylation reporter and were able to identify classes of cellular transferases responsible for SifA prenylation(Charron et al., 2010). The increased sensitivity of chemical reporters is also accompanied by an increase in efficiency of experimental workflow as a typical cellular labeling and visualization procedure can be completed in a single workday without long exposure times.
Chemical reporters coupled with biotin and selective elution of labeled proteins allows the identification of novel acylated proteins(Martin and Cravatt, 2009; Yount et al., 2010). This method has proved fruitful in the identification of new palmitoylated proteins, which has traditionally been difficult as this modification does not occur on a predictable motif. The Interferon-induced transmembrane protein 3 (IFITM3) was discovered in this way to be palmitoylated and using subsequent labeling and visualization of IFITM3 mutants, the sites of palmitoylation were mapped.(Yount et al., 2010) Palmitoylation was found to be essential for full antiviral activity of IFITM3 against influenza virus demonstrating the unique and interesting biological findings that can be elucidated with a chemical reporter approach(Yount et al., 2010). Other methods for identifying novel palmitoylated proteins have been described such as Acyl-biotin exchange (ABE) chemistry which was important in expanding our knowledge of the multitude of specific proteins on which palmitoylation can occur(Roth et al., 2006). ABE chemistry and chemical reporters afford complementary tools for analyzing S-fatty acylation.
Chemical reporters of acylation are likely to be especially useful for identifying novel acylated proteins during different cellular states such as differentiation, apoptosis, or cellular infection. For example, chemical reporters of myristoylation, which occurs predictably on N-terminal glycines, could allow identification of new myristoylation sites that are revealed upon caspase cleavage of proteins during apoptosis. Likewise, C-terminal CaaX motifs may also be identified after protein cleavage events or on uncharacterized protein isoforms with the use of chemical reporters and proteomic methods.
Critical Parameters and Troubleshooting
Cellular labeling of proteins with reporters of fatty acylation requires complete solubilization of the reporter, as precipitates are toxic to most cell types. To this end, chemical reporter should always be added to media at 37 °C and mixed thoroughly. Washes of labeled cells should be done quickly and with ice-cold PBS to avoid turnover of some types of acylation thus maximizing the detected lipidation signal. The choice of detergents for lysing cells is critically important to the success of the experiment, particularly when performing immunoprecipitations. While SDS-containing buffer maximally solubilizes proteins and is especially useful for acylated proteome profiling, Brij97-containing buffer also solubilizes many lipidated membrane proteins and is compatible with immunoprecipitations.
When performing the click chemistry reaction, it is imperative that fresh solutions of TCEP and CuSO4 are used. TCEP may become oxidized over time and Cu(I) is required for correct coordination of the reaction. Upon completion of SDS-PAGE, destaining the gel destined for fluorescence gel scanning greatly improves signal to noise as residual azido-rhodamine in the lanes may cause background signal. High quality reagents should be used to prepare the destain solution to avoid any contaminants that may provide fluorescent signal. Likewise, the gel should be handled only on the edges as physical manipulations of the gel can often appear as smudges or fingerprints in fluorescence gel scans even when gloves are worn. The fluorescence gel molecular weight standard should also be titrated so that bleedthrough into other lanes does not occur. Wiping the pipet tip before loading and starting the current immediately upon addition may also help if bleedthrough of the standard is observed.
For selective retrieval of acylated proteins, we have found that protein input of 5–10 mg is a good starting point. Smaller amounts of protein may limit detection to only the most abundant proteins. In the precipitation step following click chemistry with azido-azo-biotin, it is important that the protein pellet is washed twice with ice-cold methanol so that unreacted biotin does not block streptavidin binding sites on the beads used for pulldown of labeled proteins. Following the methanol washes, using 4% SDS buffer and sonication is the most effective way that we have found to completely resolubilize the protein pellet. 4% SDS is not compatible with the biotin-streptavidin binding so the SDS concentration must be diluted for this step. It has also been reported that two elutions using Na2S2O4 are more effective than one longer elution(Verhelst et al., 2007), and we have also observed increases in protein recovery from the streptavidin beads when performing two elutions. Concentration of the eluted protein with a centricon is necessary for buffer exchange prior to SDS-PAGE.
Anticipated Results
Using alkyne-bearing chemical reporters and azido-modified detection reagents, negligible signal should be seen in the DMSO-treated control lanes. An experiment is dubbed successful if signal is detected above these control lanes for both rhodamine visualization or coomassie staining. The magnitude of the signal can be increased or decreased as necessary by changing the concentration of chemical reporter used or by modifying the amount of protein that is added to each lane of the gel. If signal is undetectable, the investigator should label Jurkat T cells with the alk-16 reporter as a positive control as these cells are efficiently labeled with this reporter at 20 uM for 1 h. Likewise, an immunoprecipitation for endogenous palmitoylated Lck from alk-16 labeled Jurkat cell lysate can also serve as a positive control using a mouse anti-Lck monoclonal antibody (Clone 3A5 from Invitrogen)(Zhang et al., 2010). For proteomic experiments, we have routinely identified greater than 100 selectively retrieved lipidated proteins when starting with 5 mg or more of total protein in cell lysate. When using lipidation reporters of different chain lengths, alk-12 is preferentially labels myristoylated proteins while alk-16 preferentially labels palmitoylated proteins. Minimal, but observable, crosstalk has been observed for both reporters(Charron et al., 2009b; Martin and Cravatt, 2009; Wilson et al., 2010; Yount et al., 2010), likely due to substrate promiscuity of the respective acyltransferases.
Time Considerations
Basic Protocol 1 can typically be completed in 2 h and cell pellets can be frozen or cells can be utilized in Basic Protocols 2 or 3 in the same day. Both protocols typically require ~ 6 h before fluorescence gel scans are obtained. Loading controls for these experiments, i.e., coomassie gel staining and western blotting, respectively, can also be completed the same day. Alternatively, membranes for blotting can be blocked overnight and probed the following day. Likewise, the coomassie stain can be applied overnight or the destain procedure can be performed overnight at 4 °C. Throughout protocols 2 and 3 there are at least three 1 h incubation periods during which the experimenter can focus on other tasks. Basic Protocol 4 is a more lengthy procedure and requires approximately 15 h to prepare samples for proteomic analysis, though this can easily be spread over 2 days as there are several stopping points within the protocol. There are also several 1 h or 30 min incubation periods. If gel based proteomics is desired, then another 2 days of sample preparation time should be planned.
Literature Cited
- Charron G, Tsou LK, Maguire W, Yount JS, Hang HC. Alkynyl-farnesol reporters for detection of protein S-prenylation in cells. Mol Biosyst. 2010;7:67–73. doi: 10.1039/c0mb00183j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron G, Wilson J, Hang HC. Chemical tools for understanding protein lipidation in eukaryotes. Curr Opin Chem Biol. 2009a;13:382–391. doi: 10.1016/j.cbpa.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Charron G, Zhang MM, Yount JS, Wilson J, Raghavan AS, Shamir E, Hang HC. 2009b [Google Scholar]
- Robust fluorescent detection of protein fatty-acylation with chemical reporters. J Am Chem Soc. 131:4967–4975. doi: 10.1021/ja810122f. [DOI] [PubMed] [Google Scholar]
- Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- Martin BR, Cravatt BF. Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods. 2009;6:135–138. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescher JA, Bertozzi CR. Chemical technologies for probing glycans. Cell. 2006;126:851–854. doi: 10.1016/j.cell.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Reinicke AT, Hutchinson JL, Magee AI, Mastroeni P, Trowsdale J, Kelly AP. A Salmonella typhimurium effector protein SifA is modified by host cell prenylation and S-acylation machinery. J Biol Chem. 2005;280:14620–14627. doi: 10.1074/jbc.M500076200. [DOI] [PubMed] [Google Scholar]
- Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR, 3rd, Davis NG. Global analysis of protein palmitoylation in yeast. Cell. 2006;125:1003–1013. doi: 10.1016/j.cell.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhelst SH, Fonovic M, Bogyo M. A mild chemically cleavable linker system for functional proteomic applications. Angew Chem Int Ed Engl. 2007;46:1284–1286. doi: 10.1002/anie.200603811. [DOI] [PubMed] [Google Scholar]
- Wilson JP, Raghavan AS, Yang YY, Charron G, Hang HC. Proteomic analysis of fatty-acylated proteins in mammalian cells with chemical reporters reveals S-acylation of histone H3 variants. Mol Cell Proteomics. 2010 doi: 10.1074/mcp.M110.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YY, Ascano JM, Hang HC. Bioorthogonal chemical reporters for monitoring protein acetylation. J Am Chem Soc. 2010a;132:3640–3641. doi: 10.1021/ja908871t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YY, Grammel M, Raghavan AS, Charron G, Hang HC. Comparative analysis of cleavable azobenzene-based affinity tags for bioorthogonal chemical proteomics. Chem Biol. 2010b;17:1212–1222. doi: 10.1016/j.chembiol.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount JS, Moltedo B, Yang YY, Charron G, Moran TM, Lopez CB, Hang HC. Palmitoylome profiling reveals S-palmitoylation-dependent antiviral activity of IFITM3. Nat Chem Biol. 2010;6:610–614. doi: 10.1038/nchembio.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, Tsou LK, Charron G, Raghavan AS, Hang HC. Tandem fluorescence imaging of dynamic S-acylation and protein turnover. Proc Natl Acad Sci U S A. 2010;107:8627–8632. doi: 10.1073/pnas.0912306107. [DOI] [PMC free article] [PubMed] [Google Scholar]