Abstract
Biolistic vaccination using gene gun is developed as a safer tool for delivery of DNA vaccines, a technique that combines high vaccine efficiency with lower antigen dosage and lower cost per vaccine dose. In this study, we compared the protective responses in mice after delivering the BmALT-2 DNA vaccine using the conventional intradermal approach or with the needleless gene gun delivery approach. BmALT-2 is a leading vaccine candidate against Brugia malayi, a lymphatic filarial parasite of human. After optimizing the DNA dose and gene gun parameters for delivery into mouse skin, groups of mice were biolistically vaccinated with 5µg of BmALT-2pVAX. Groups of mice vaccinated intradermally with 5µg or 100µg of BmALT-2pVAX was used for comparison of vaccine efficacy. Results demonstrated that gene gun vaccination with 5µg of BmALT-2pVAX conferred significant protection against challenge infection that was comparable to the degree of protection conferred by intradermal vaccination with 100µg of BmALT-2pVAX. This observation was further supported by an in vitro antibody dependent cellular cytotoxicity (ADCC) assay. Analysis of the immune response showed that the gene gun vaccination predominantly induced an IgG1 antibody response and significantly high Th2 cytokine response (IL-4) from spleen cells compared to intradermal BmALT-2 DNA delivery that induced predominantly an IgG2a and Th1 cytokine response (IFN-γ, IL-12 and TNF-α). These findings show that host protective responses could be achieved with 20 fold decrease in DNA dose using a gene gun and could prove to be an efficient delivery method in BmALT-2 DNA vaccination against lymphatic filariasis.
Keywords: Lymphatic filariasis, Brugia malayi, BmALT-2, Gene gun
1. Introduction
Lymphatic filariasis is a parasitic infection that affects over 120 million people worldwide and causes a chronic infection that leads to severe impairment, loss of productivity and social stigma [1]. There are no vaccines available to control this infection. However, several vaccine candidates have been identified by our group and others. Among these the Brugia malayi abundant larval transcript-2 (BmALT-2) is a leading vaccine candidate [2].
The ALT-2 gene family is present in all filarial parasites and the gene product has no known similarity to proteins from non-filarial organisms [3]. The gene is highly stage specific with more than 3% of all ESTs identified from B. malayi L3s belonging to BmALT-2. The ALT products are also conserved among the filarial parasites and thought to play an important role in the establishment of infection. Presence of anti-BmALT-2 antibodies in the sera of putatively immune individuals, but not in the infected or non-immune individuals [4] suggest the potential of BmALT-2 an attractive prophylactic vaccine candidate. Multiple studies validated the vaccine-efficacy of BmALT-2 [5–7].
DNA based vaccines are relatively simple and inexpensive to produce [8]. Following DNA vaccination, the protein of interest is expressed in the skin cells [9]. Antigens of filarial parasite such as chitinase [10], paramyosin [11], glutathione-S-transferase [12], tropomyosin [13] OvB20 [13], ALT-2 [5] and SXP-1 [5] have been successfully developed as experimental DNA vaccines.
A major drawback of DNA vaccine is that only low levels of immune responses can be generated even with increasing doses of the DNA. This response may be largely influenced by the route of DNA administration [14, 15]. Most common route of DNA vaccine administration is the intradermal injection. Alternative non-invasive DNA delivery method include gene gun or electroporation [16]. Gene gun-based DNA vaccination have been tested using filarial antigens such as paramyosin, heat shock protein70 and intermediate filament protein [17]. Unfortunately, these studies evaluated only antibody responses following gene gun delivery of the antigens. None of the studies evaluated protective responses. Therefore, in this study we evaluated the protective responses generated following gene gun delivery of BmALT-2 DNA and compared that to intradermal delivery.
2. Materials and methods
2.1 Animals and parasites
Balb/c mice purchased from Charles River laboratories (Wilmington, MA) were used in these studies and animal use protocol was approved by IACUC committee of the University of Illinois Rockford. B. malayi third stage infective larvae (L3) were obtained from NIH/NIAID Filariasis research reagent resource center.
2.2 Plasmids
Codon optimized B. malayi ALT-2 was synthesized at Genscript (Piscataway, NJ) and was PCR amplified using gene specific primers as described previously [6]. BmALT-2GFPpVAX plasmid expressing green fluorescent protein (GFP) was constructed by inserting GFP from pAcGFP1 plasmid (Clontech, Mountain View, CA) at EcoR1and XhoI sites of the BmALT-2pVAX plasmid. Empty pVAX vectors served as controls. After confirming the sequences, plasmids were maintained and propagated in Escherichia coli TOP10F’ cells and purified using endotoxin free plasmid extraction kit (Qiagen, Valencia CA). Purified plasmids did not have any detectable levels of endotoxin as determined by the ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit (Genscript).
2.3 Recombinant BmALT-2 expression and production of antiBmALT-2 antibodies
Recombinant BmALT-2 protein (rBmALT-2) was prepared as described previously [6]. Endotoxin levels were less than 1 EU/mg as determined by LAL assay. Ten Balb/c mice were injected subcutaneously with 4 doses of 15µg of rBmALT-2 in Imject® alum (Thermo Fisher Scientific, Rockford, IL) at 2 weeks interval and serum was collected for antibodies.
2.4 Preparation of gene gun cartridges
A Helios Gene Gun™ (BioRad, Hercules, CA) was used for the biolistic vaccination and cartridges were prepared according to the method described by O'Brien [18]. Briefly, 100µl of 0.05M spermidine was added to varying amounts of 1µm gold microcarriers, and mixed thoroughly by sonicating in water bath for 20 seconds. Required amount of BmALT-2pVAX or BmALT-2GFPpVAX or empty pVAX plasmid was added to the gold/spermidine mixture and finally co-precipitated by the addition of 100µl of 1M CaCl2 while vortexing. The gold/DNA precipitate was then resuspended in Poly Vinyl Pyrrolidine (PVP) in ethanol and loaded onto pre-dried tefzel tubing (BioRad). Optimization studies showed that 0.1mg/ml PVP allowed maximum binding of gold carrying DNA to the tubing as well as low residual DNA in cartridges after shooting (<5% of total DNA bound). Optimized 0.5 inch cartridges were prepared with a DNA loading ratio (DLR) of 2 and a microcarrier loading quantity (MLQ) of 0.5 mg, yielding a cartridge having 1µg plasmid in 0.5mg gold carrier.
2.5 BmALT-2GFP expression in COS-1 cells and in the skin of mice
COS-1 cells (ATCC, Manassas, VA) cultured at 85% confluence were bombarded with BmALT-2GFPpVAX DNA using the Gene Gun™ at 50, 100 and 150 psi helium pressures [19]. After 48 hours GFP expression was visualized under a fluorescent microscope. Transfection using Lipofectamine™2000 (Invitrogen) was used as a control.
For in vivo experiments, 5µg BmALT-2GFPpVAX plasmids were bombarded into the shaved abdominal skin of mouse using the gene gun at 200, 300 and 400psi helium pressures [17]. 48hrs later the pre-marked skin site was collected and 5µm thick serial sections prepared in a cryotome (Damon/IEC Minotome, Elkhart, IN). GFP fluorescence was observed under a fluorescent microscope.
We collected the skin and lateral inguinal lymph nodes from DNA transfected mice and probed for BmALT-2 mRNA by PCR using gene specific primers. BmALT-2 protein expression was evaluated in the skin homogenate by immunoblot using polyclonal mouse antiBmALT-2 antibodies.
2.6 Dose standardization studies using BmALT-2 DNA in mice
Cartridges were prepared with 2.5, 5 and 7.5µg of DNA in 0.5mg gold carriers by varying the DNA Loading Ratio (DLR). 5 mice each were vaccinated using gene gun as above with 2.5, 5, 7.5 and 15µg (2×7.5µg) BmALT-2pVAX plasmid. All animals were boosted with the same DNA dose after 28 days and blood was collected by retro orbital bleeding. Presence of antiBmALT-2 antibody was detected in serum using indirect ELISA as described previously [20].
2.7 Biolistic` gene gun vaccination protocol in mice
Balb/c mice were divided into 5 groups consisting of 5 animals each. Group 1 animals received 5µg of pVAX vector via gene gun and served as controls (5µg pVAX GG). Group 2 animals received 5ug BmALT-2pVAX via gene gun (5µg BmALT-2 GG). Group 3 were vaccinated intradermally with 5 µg of BmALT-2pVAX (5µg BmALT-2 i.d.) and Group 4 were vaccinated intradermally with 100µg of BmALT-2pVAX (100µg BmALT-2 i.d.) respectively. Group 5 (100µg pVAX i.d.) served as controls and received 100µg of pVAX vector injected intradermally. All animals received 4 doses of the vaccine at 4 week intervals.
2.8 Parasite challenge studies using micropore chambers
One week after the last immunization, 20 B. malayi L3 was surgically implanted into the peritoneal cavity of mice in a micropore chamber as described previously [21]. Chambers were removed after 48 hours and examined microscopically for cell adherence and death of parasites. The parasite was considered dead if it was not motile, limpid and had several adherent cells on its surface. Results were expressed as percentage protection in vaccinated animals over the controls.
2.9 In vitro antibody-dependent cellular cytotoxicity (ADCC) assay
An ADCC assay was performed as described previously to determine if the antiBmALT-2 antibodies from vaccinated animals can participate in the killing of B. malayi L3s [22]. Larval viability was determined under a light microscope 48 h after incubation. Parasites that were limpid and straight with no movements were counted as non-viable. If they were still limpid and straight for the next 8 hours at 37°C, they were counted as dead. The live larvae remained active. Results were expressed as percentage of immobile or dead parasites to the total number of parasites recovered within each experiment [23].
2.10 Immune correlation of protection
Anti-BmALT-2 IgG antibody responses were analyzed in the sera of vaccinated mice using an indirect ELISA as described previously [4]. Levels of secreted cytokines in the culture supernatants of splenocytes (1×106 cells/ml in 24 well plates) stimulated for 72 hrs with rBmALT-2(1µg/ml) were measured using the BD™ Cytometric Bead Array Mouse Th1/Th2/Th17 Cytokine kit (San Jose, CA). Unstimulated cells served as controls. The amount of cytokine present was calculated from the standards provided and analyzed with the FCAP Array™ software.
2.11. Statistical analysis
Statistical analyses were performed using Graphpad® Prism 5. Comparisons between multiple groups were made with one-way ANOVA analyses with Tukey’s test. Probability values (p) of <0.05 were considered statistically significant.
3. Results
3.1 BmALT-2 is expressed in COS-1 cells and the mouse skin after gene gun delivery
GFP expression was present in BmALT-2GFPpVAX transfected COS-1 cells 48hrs after gen gun delivery with 150psi (Figure 1B) and 100psi (Figure 1C). No GFP expression was observed in cells transfected with pVAX (Figure 1D). COS-1 cells transfected with BmALT-2GFPpVAX using Liopfectamine also expressed GFP (Figure 1A). These findings showed that excellent transfection efficiency can be obtained following in vitro gene gun delivery of 1µg of BmALT-2GFPpVAX using100 psi helium pressure.
Figure 1.
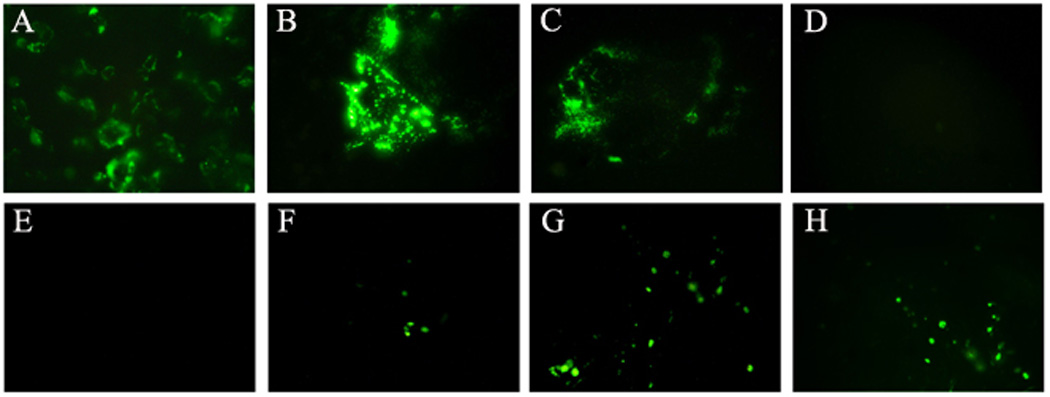
GFP expression in COS-1 cells (A–D) and mouse skin (E–H) after 48hrs of transfection with BmALT-2GFPpVAX plasmid. COS-1 cells were transfected with 1µg of BmALT-2GFPpVAX using gene gun at 150 Psi (B) and 100 Psi(C) helium pressures. Empty pVAX plasmid was used as negative control (D) while transfection using Lipofectamine was used as positive control (A). GFP expression was observed in skin sections of mice transfected with 5µg BmALT-2GFPpVAX using gene gun at 200psi (F), 300psi (G) and 400psi (H) helium pressure after 48 hours, while normal skin had no fluorescence (E).
GFP expression was also present at the skin site of mice 48hrs after gene gun vaccination. The intensity of GFP expression increased with increased helium pressures (Figure 1F–H). No GFP expression was observed in control mice (Figure 1E). Since 400 psi helium pressure was found to be optimal for the biolistic vaccination in mice skin, we used this delivery parameter in all our in vivo vaccination trials.
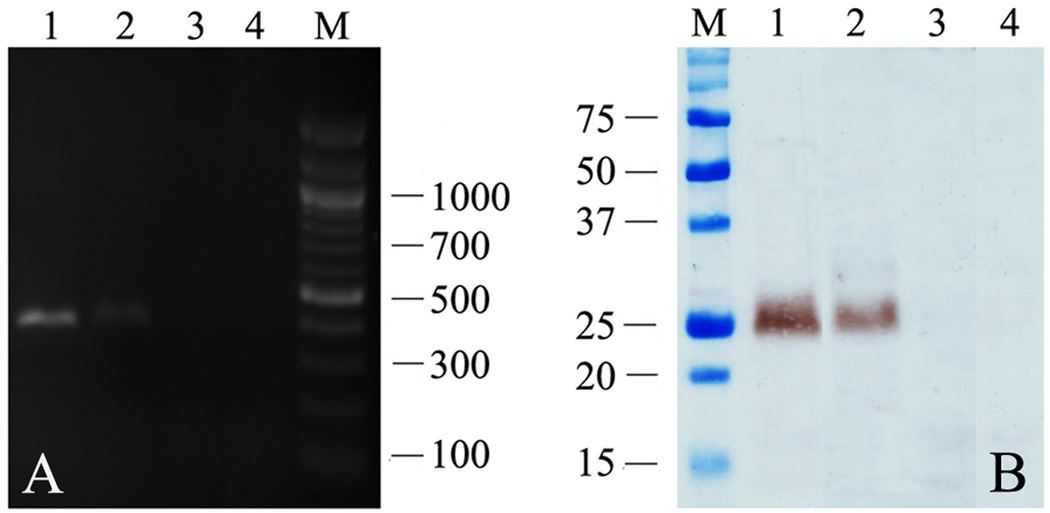
A 384 bp amplicon representing BmALT-2 mRNA transcripts was present in the skin site and skin draining lymph nodes of mice vaccinated with BmALT-2pVAX using gene gun (Figure 2A). Subsequent immunoblot analysis confirmed that a 25kDa band comparable to the size of rBmALT-2 was evident in the skin homogenates of gene gun vaccinated mice (Figure 2B).
Figure 2.
Confirmation of mRNA (A) and protein (B) expression of BmALT-2 in the skin and lymph nodes of BmALT-2pVAX gene gun vaccinated mice. Lanes 1 and 2 represent the skin and node samples respectively from BmALT-2pVAX gene gun vaccinated mice while lanes 3 and 4 represent the skin and node samples respectively from control pVAX gene gun vaccinated mice.
3.2 Gene gun vaccination induced antibody production
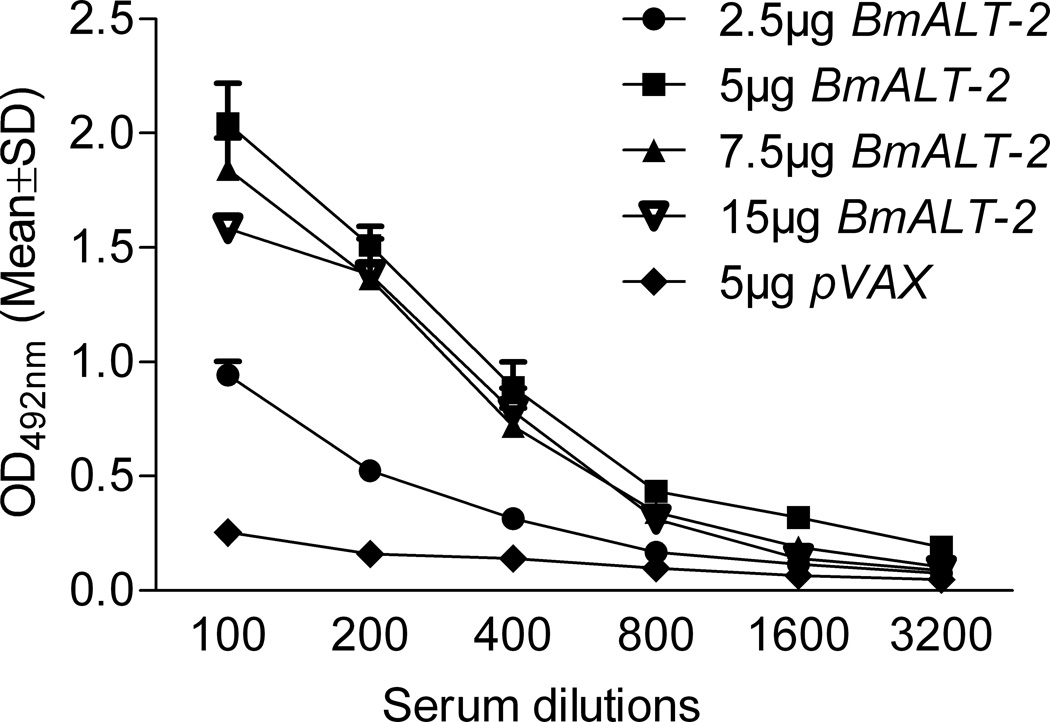
Anti-BmALT-2 IgG antibodies were present in the serum of Balb/c mice vaccinated with BmALT-2pVAX plasmid using gene gun (Figure 3). Compared to intradermal route of delivery, the gene gun immunization approach elicited significantly high titer of IgG antibodies in mice (Supplementary figure 1). The IgG levels remained significantly high (P<0.01) at all dilutions of the DNA vaccine (5, 7.5 and 15µg of DNA) compared to pVAX control (Figure 3). Since 5µg of DNA vaccination was sufficient to produce significantly high IgG antibody titers we used this dose in the in vivo protection studies.
Figure 3.
BmALT-2 specific IgG levels in the serum of Balb/c mice vaccinated with 2.5, 5, 7.5 and 15µg of BmALT-2pVAX plasmid using a gene gun. The IgG levels were determined by indirect ELISA from serum obtained after two gene gun vaccinations. IgG levels remained significantly high (P<0.01) at all dilutions in mice vaccinated with 5, 7.5 and 15µg of DNA vaccine against the control given 5µg of pVAX vaccination using gene gun. N=5.
3.3 Gene gun vaccination offered protection against infective larvae
About 25% of L3 were dead (P<0.001) in the micropore chambers implanted in the peritoneal cavity of mice vaccinated with 5 µg of BmALT-2pVAX using gene gun. Comparable results were obtained in mice given 100µg BmALT-2pVAX intradermally (Table 1). However, in mice vaccinated intradermally with 5µg of BmALT-2pVAX only 5% of L3 were dead. In the control animals all the recovered infective larvae were alive with no cell adherence.
Table 1.
Protection against B. malayi infective larvae in mice vaccinated with ALT-2pVAX using gene gun and intradermal delivery methods.
| Groups | Percentage protection | |
|---|---|---|
| In vitro ADCC assaya | In vivo micropore chamber assayb | |
| 5µg pVAX GG | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 5µg BmALT-2 GG | 33.8 ± 5.3*** | 24.8 ± 3.4*** |
| 5µg BmALT-2 i.d. | 6.3 ± 8.8 | 5.5 ± 3.8 |
| 100µg BmALT-2 i.d. | 38.2 ± 2.6*** | 25.3 ± 6.2*** |
| 100µg pVAX i.d. | 0.0 ± 0.0 | 0.0 ± 0.0 |
ADCC assay was performed by incubating 50 µl of pooled mice sera (n = 5) samples with 0.5×105 normal peritoneal exudates cells and 10 B. malayi L3 at 37°C for 48 hrs. Sera from animals in each group were pooled and used in triplicates for the assay.
In vivo micropore chamber assay was performed by surgically implanting 20 B. malayi L3 into the peritoneal cavity of each mouse (N = 5). 48 hrs after implantation, chambers were removed and larval viability and death determined. All infective larvae recovered in the controls were alive.
Significant protection (P<0.001) as compared to control groups.
We also performed an in vitro ADCC assay to support the protective ability of anti-ALT-2 antibodies generated following gene gun or intradermal vaccine delivery. These findings showed that sera samples from 5µg BmALT-2pVAX gene gun vaccinated mice provided 34% protection (P<0.001) over controls. This was again comparable to the protection obtained using sera samples from 100µg BmALT-2pVAX i.d. vaccinated mice (38% protection (P<0.001)). However, when sera samples from 5µg BmALT-2pVAX i.d. vaccinated mice were used in the ADCC only 6% protection could be achieved (Table 1). These findings collectively show that the gene gun based vaccination can generate protective responses with 20 fold less amounts of DNA.
3.4 Gene gun vaccination induced high IgG1 responses
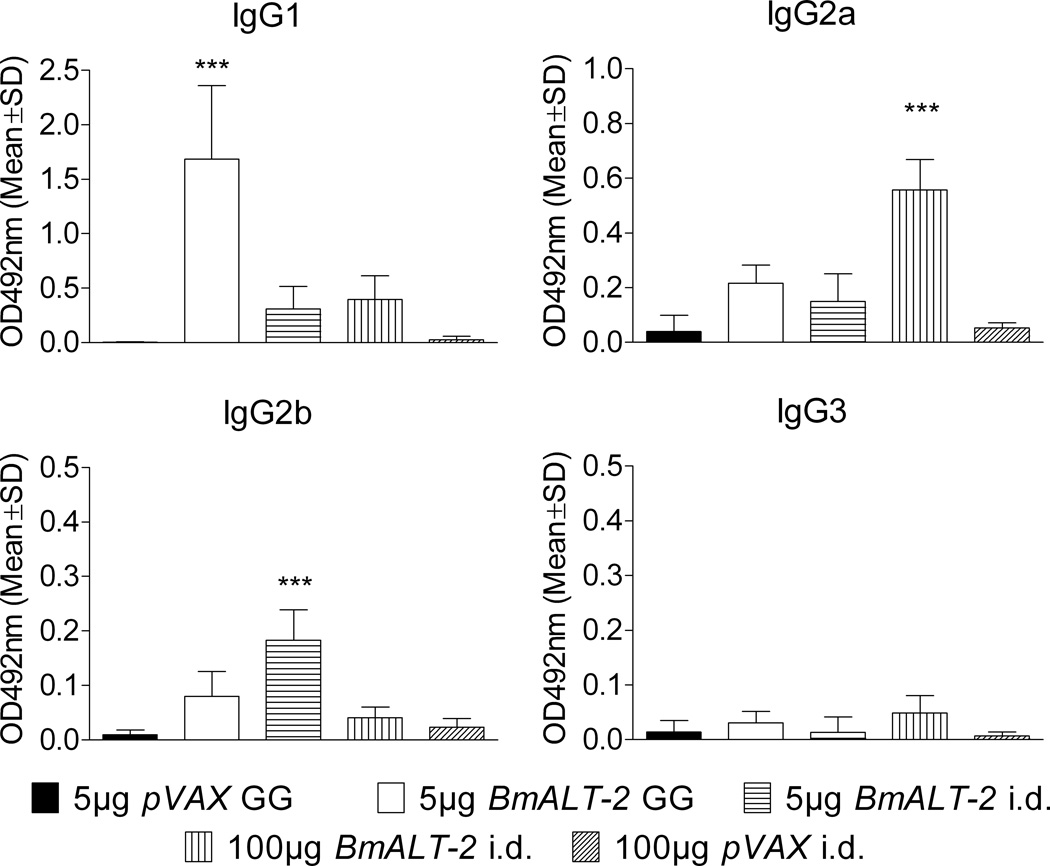
a comparison of the isotype of antibody responses (Figure 4) showed that gene gun delivery method predominantly induced an IgG1 and IgG2a anti-BmALT-2 antibody responses (P<0.001), whereas, intradermal delivery method mainly induced an IgG2a and IgG2b anti-BmALT-2 antibody responses (P<0.001). Anti-ALT-2 IgG3 antibody levels were not significantly elevated in any of the vaccinated groups.
Figure 4.
BmALT-2 specific IgG1, IgG2a, IgG2b and IgG3 antibody isotype profiles in the serum of mice vaccinated with BmALT-2pVAX using gene gun and intradermal delivery methods. The bars represent the mean optical density at 492nm of five mice determined by indirect ELISA with a serum dilution of 1:200. ***P<0.001.
3.5 Gene gun vaccination promoted Th2 cytokine responses in splenocytes
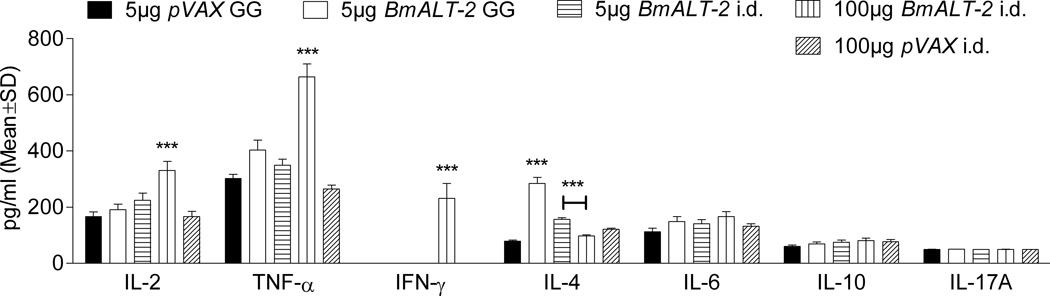
In response to stimulation with rBmALT-2, significant (P<0.001) amounts of IL-4 was produced by spleen cells from mice vaccinated with 5µg ALT-2pVAX using gene gun (Figure 5), whereas, spleen cells from mice vaccinated intradermally with 100µg BmALT-2pVAX secreted significant (P<0.001) amounts of IFN-γ, IL-12 and TNF-α compared to controls. Levels of IL-6, IL-10 and IL-17A remained unchanged in all the groups. These results show that a Th2 type responses predominated following gene gun vaccination, whereas, a Th1 type response predominated following intradermal vaccination.
Figure 5.
Cytokine profile of spleen cells from mice vaccinated with BmALT-2pVAX using gene gun or intradermal delivery methods. A cytokine bead array was used to determine the level of each cytokine in the culture supernatants of 1 × 105 spleen cells stimulated with 1 µg/ml of rBmALT-2. The supernatants were incubated with equal volumes of capture beads and PE detection solution after which the florescence was read in a BD™ FACS Calibur instrument. ***P<0.001.
4. Discussion
In this study, we compared the protective responses elicited following delivery of BmALT-2 DNA vaccine using the conventional intradermal route versus the gene gun delivery method. Our results show that the gene gun delivery method is more efficient and requires only 20 fold less antigen to achieve the same result as the intradermal vaccine route.
BmALT-2 is the most abundantly expressed protein in the cuticle of L3 stage and is believed to be important for the parasite molting process. Antibody mediated neutralization of BmALT-2 appears to predispose the larvae to lethal attack by immune cells and finally to death of the L3. Thus, BmALT-2 appears to be an important protein for the survival of the parasite in the host. Given the significant potential of BmALT-2 as a DNA vaccine candidate for lymphatic filariasis, we chose to compare vaccination with BmALT-2 using two different DNA delivery approaches, gene gun method and intra dermal delivery method.
Initial experiments in COS-1 cells and mouse skin confirmed that BmALT-2 protein can be expressed in mammalian cells following gene gun delivery of BmALT-2 DNA. Gene gun vaccination delivers the plasmid to the dermal layers of the mouse skin [15] where it is believed to directly transfect the antigen presenting cells and transport the antigen to draining lymph nodes [24–26]. We were able to demonstrate BmALT-2 mRNA transcripts and protein in the draining lymph nodes of gene gun vaccinated mice 48hours after vaccination.
Serum anti-BmALT-2 IgG antibodies generated following gene gun delivery was predominantly of IgG1 isotype, whereas, intradermal delivery of BmALT-2 elicited largely an IgG2a isotype response. Similar isotype switching to IgG1 after gene gun vaccination has been reported earlier by Li et al. for B. malayi paramyosin [17]. Previous studies with Onchocerca volvulus chitinase DNA also showed that a clear IgG1 predominance in antibody isotype development following gene gun vaccination [10].
In mice, B cell differentiation and regulation of IgG isotypes are under the control of the T cell products with IL-4 and IL-12 directing IgG subclass switching of IgG1 and IgG2a, respectively [27]. Consistent with this observation, we showed that spleen cells from mice vaccinated with gene gun predominantly secreted IL-4 in response to rBmALT-2 potentially promoting IgG1 class switch. However, spleen cells from intradermally vaccinated mice predominantly secreted IFN-γ, IL-12 and TNF-α in response to rBmALT-2 potentially promoting IgG2a class switch. This was in line with the earlier work on BmALT-2 DNA vaccine where an IFN- γ dominated response was generated following intradermal injection [6]. Since these cytokines broadly represent the Th1 and Th2 patterns of the immune response [28], our findings suggest that the Th2 biased responses generated following gene gun delivery of BmALT-2 DNA vaccine is equally efficient as a protective response against B. malayi L3 compared to the Th1 biased response generated following intradermal delivery of BmALT-2 DNA
About 25% protection was obtained following immunization of mice with 5µg of BmALT-2 DNA using gene gun approach. The degree of protection conferred following intradermal iimmunization with 100 µg of BmALT-2 DNA is approximately 26%. Thus, gene gun immunization conferred comparable levels of protection with 20 fold less DNA dose. In this study maximum vaccine-induced protection obtained was only about 26%. Although this is not a significantly high protection, in previous studies maximum protection afforded by DNA vaccination was only around 30–37% [6, 7]. When combined with protein vaccination as a DNA prime protein boost vaccination regimen, we were able to get as high as 64% protection following vaccination with BmALT-2 [6]. In these prime boost approach regimen we delivered 100 µg of BmALT-2 DNA via intradermal vaccination. Further studies will determine if gene gun delivery of 5 µg of BmALT-2 DNA is sufficient to provide the necessary priming for the protein vaccination. . Two major advantages of gene gun based DNA delivery over intradermal delivery are (1) same level of protection can be achieved with 20 fold less amount of the DNA vaccine and (2) most importantly gene gun based delivery is minimally non-invasive and easy to administer. Introduction of new technologies such as Powdermed-Pfizer will make the gene gun a practical approach for delivery of DNA vaccines to remote villages in endemic areas.
In summary, our findings show that biolistic vaccination of BmALT-2 DNA vaccine provided significant protection against challenge infections in vaccinated animals. The degree of protection was comparable to intradermal delivery of BmALT-2 but with 20 fold less DNA. Thus, gene gun delivery of BmALT-2 DNA vaccine offers an effective and safer needleless alternative to conventional intradermal injections. Further optimization of gene gun with multiple vaccine candidates and prime boost approach will make this delivery method a highly viable vaccination tool for lymphatic filariasis control in the field.
Supplementary Material
The highlights of the present work.
Protective responses to a DNA vaccine were compared after 2 delivery methods.
Brugia malayi Abundant larval Transcript -2 DNA was used as the vaccine candidate.
Gene gun delivery of BmALT-2 DNA vaccine was optimized in this study.
Findings show that gene gun delivery of BmALT-2 DNA elicits significant protection.
Gene gun approach required only 20 fold less DNA compared to intradermal approach.
Acknowledgements
We would like to thank John Javeherian and Lisa Foti for technical assistance with the animal experiments . B. malayi third stage infective larvae (L3) were obtained from NIH/NIAID Filariasis research reagent resource center, College of Veterinary Medicine, University of Georgia, Athens, GA. This study was supported by the NIH grant AI064745.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zeldenryk LM, Gray M, Speare R, Gordon S, Melrose W. The emerging story of disability associated with lymphatic filariasis: a critical review. PLoS Negl Trop Dis. 2011;5(12):e1366. doi: 10.1371/journal.pntd.0001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory WF, Atmadja AK, Allen JE, Maizels RM. The abundant larval transcript-1 and -2 genes of Brugia malayi encode stage-specific candidate vaccine antigens for filariasis. Infect Immun. 2000;68(7):4174–4179. doi: 10.1128/iai.68.7.4174-4179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez-Escobar N, Bennett C, Prieto-Lafuente L, Aebischer T, Blackburn CC, Maizels RM. Heterologous expression of the filarial nematode alt gene products reveals their potential to inhibit immune function. BMC Biol. 2005;3:8. doi: 10.1186/1741-7007-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gnanasekar M, Rao KV, He YX, Mishra PK, Nutman TB, Kaliraj P, et al. Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi. Infect Immun. 2004;72(8):4707–4715. doi: 10.1128/IAI.72.8.4707-4715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramachandran S, Kumar MP, Rami RM, Chinnaiah HB, Nutman T, Kaliraj P, et al. The larval specific lymphatic filarial ALT-2: induction of protection using protein or DNA vaccination. Microbiol Immunol. 2004;48(12):945–955. doi: 10.1111/j.1348-0421.2004.tb03624.x. [DOI] [PubMed] [Google Scholar]
- 6.Thirugnanam S, Pandiaraja P, Ramaswamy K, Murugan V, Gnanasekar M, Nandakumar K, et al. Brugia malayi: comparison of protective immune responses induced by Bm-alt-2 DNA, recombinant Bm-ALT-2 protein and prime-boost vaccine regimens in a jird model. Exp Parasitol. 2007;116(4):483–491. doi: 10.1016/j.exppara.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand SB, Murugan V, Prabhu PR, Anandharaman V, Reddy MV, Kaliraj P. Comparison of immunogenicity, protective efficacy of single and cocktail DNA vaccine of Brugia malayi abundant larval transcript (ALT-2) and thioredoxin peroxidase (TPX) in mice. Acta Trop. 2008;107(2):106–112. doi: 10.1016/j.actatropica.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Kyelem D, Biswas G, Bockarie MJ, Bradley MH, El-Setouhy M, Fischer PU, et al. Determinants of success in national programs to eliminate lymphatic filariasis: a perspective identifying essential elements and research needs. Am J Trop Med Hyg. 2008;79(4):480–484. [PMC free article] [PubMed] [Google Scholar]
- 9.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356(6365):152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 10.Harrison RA, Wu Y, Egerton G, Bianco AE. DNA immunisation with Onchocerca volvulus chitinase induces partial protection against challenge infection with L3 larvae in mice. Vaccine. 1999;18(7–8):647–655. doi: 10.1016/s0264-410x(99)00274-1. [DOI] [PubMed] [Google Scholar]
- 11.Li BW, Zhang S, Curtis KC, Weil GJ. Immune responses to Brugia malayi paramyosin in rodents after DNA vaccination. Vaccine. 1999;18(1–2):76–81. doi: 10.1016/s0264-410x(99)00182-6. [DOI] [PubMed] [Google Scholar]
- 12.Catmull J, Wilson ME, Kirchhoff LV, Metwali A, Donelson JE. Induction of specific cell-mediated immunity in mice by oral immunization with Salmonella expressing Onchocerca volvulus glutathione S-transferase. Vaccine. 1999;17(1):31–39. doi: 10.1016/s0264-410x(98)00147-9. [DOI] [PubMed] [Google Scholar]
- 13.Harrison RA, Bianco AE. DNA immunization with Onchocerca volvulus genes, Ov-tmy-1 and OvB20: serological and parasitological outcomes following intramuscular or GeneGun delivery in a mouse model of onchocerciasis. Parasite Immunol. 2000;22(5):249–257. doi: 10.1046/j.1365-3024.2000.00304.x. [DOI] [PubMed] [Google Scholar]
- 14.Boyle JS, Silva A, Brady JL, Lew AM. DNA immunization: induction of higher avidity antibody and effect of route on T cell cytotoxicity. Proc Natl Acad Sci U S A. 1997;94(26):14626–14631. doi: 10.1073/pnas.94.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci U S A. 1993;90(24):11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang NS, Burkholder J, Roberts B, Martinell B, McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proc Natl Acad Sci U S A. 1990;87(24):9568–9572. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li BW, Rush A, Zhang SR, Curtis KC, Weil GJ. Antibody responses to Brugia malayi antigens induced by DNA vaccination. Filaria J. 2004;3(1):1. doi: 10.1186/1475-2883-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien JA, Lummis SC. Biolistic transfection of neuronal cultures using a hand-held gene gun. Nat Protoc. 2006;1(2):977–981. doi: 10.1038/nprot.2006.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida M, Li XW, Mertens P, Alpar HO. Transfection by particle bombardment: delivery of plasmid DNA into mammalian cells using gene gun. Biochim Biophys Acta. 2009;1790(8):754–764. doi: 10.1016/j.bbagen.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Joseph SK, Verma SK, Sahoo MK, Dixit S, Verma AK, Kushwaha V, et al. Sensitization with anti-inflammatory BmAFI of Brugia malayi allows L3 development in the hostile peritoneal cavity of Mastomys coucha. Acta Trop. 2011;120(3):191–205. doi: 10.1016/j.actatropica.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Abraham D, Grieve RB, Holy JM, Christensen BM. Immunity to larval Brugia malayi in BALB/c mice: protective immunity and inhibition of larval development. Am J Trop Med Hyg. 1989;40(6):598–604. doi: 10.4269/ajtmh.1989.40.598. [DOI] [PubMed] [Google Scholar]
- 22.Chandrashekar R, Rao UR, Parab PB, Subrahmanyam D. Brugia malayi: serum dependent cell-mediated reactions to microfilariae. Southeast Asian J Trop Med Public Health. 1985;16(1):15–21. [PubMed] [Google Scholar]
- 23.Veerapathran A, Dakshinamoorthy G, Gnanasekar M, Reddy MV, Kalyanasundaram R. Evaluation of Wuchereria bancrofti GST as a vaccine candidate for lymphatic filariasis. PLoS Negl Trop Dis. 2009;3(6):e457. doi: 10.1371/journal.pntd.0000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raz E, Carson DA, Parker SE, Parr TB, Abai AM, Aichinger G, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci U S A. 1994;91(20):9519–9523. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinman DM, Sechler JM, Conover J, Gu M, Rosenberg AS. Contribution of cells at the site of DNA vaccination to the generation of antigen-specific immunity and memory. J Immunol. 1998;160(5):2388–2392. [PubMed] [Google Scholar]
- 26.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2(10):1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 27.Coffman RL, Seymour BW, Lebman DA, Hiraki DD, Christiansen JA, Shrader B, et al. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 28.Hodgkin PD, Yamashita LC, Seymour B, Coffman RL, Kehry MR. Membranes from both Th1 and Th2 T cell clones stimulate B cell proliferation and prepare B cells for lymphokine-induced differentiation to secrete Ig. J Immunol. 1991;147(11):3696–3702. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.