Abstract
Recently, we demonstrated a novel role for gastrointestinal mast cells (MCs) in the early events that lead to the generation of Th2 immunity to helminth infection.1 Mice lacking MCs (KitW/KitW-v and KitW-Sh) showed a significant inhibition of Th2 cell priming following infection with the parasitic helminth Heligmosomoides polygyrus bakeri (Hp). We showed that MCs degranulate during the early stages of infection when the helminth larvae invade the small intestinal tissue. Furthermore, MC degranulation was required for the enhanced expression and production of the tissue-derived cytokines IL-25, IL-33 and TSLP, which are required for the optimal orchestration and priming of type 2 immunity. In this addendum we aim to address several questions raised by our findings — in particular, the mechanisms through which MCs may recognize helminth exposure in the early stages of infection and by which they may enhance expression of critical tissue cytokines thus, enabling Th2 priming. Furthermore, we will discuss these findings in the context of recently described novel innate immune cells, such as type 2 hematopoietic progenitors and type 2 innate lymphoid cells.
Keywords: helminth, mast cell, IL-25, IL-33, progenitor, type 2 innate lymphoid cells, mucosal immunity
Introduction
Infections with gastrointestinal helminths remain highly prevalent, particularly in developing countries, with over 1 billion humans estimated to be infected worldwide.2 Helminth infection results in nutrient malabsorption, intestinal inflammation, impaired growth and development and reduced educational performance in the host.2 Conversely, helminth infections have been shown to play key roles in the suppression and modulation of inappropriate inflammation, and loss of helminths as a result of increased hygiene or drug treatments results in an increased rate of autoimmune, inflammatory and allergic disorders (reviewed in ref. 3). Furthermore, experimental treatments with live helminths are currently undergoing clinical trials to test their efficacy as novel therapeutics in a variety of human disease settings.3 Thus, understanding the pathways that generate, modulate and regulate the host immune response during helminth infection is of significant clinical relevance.
Mast cells (MCs) are a potent arm of the innate immune system and can be found in barrier tissues throughout the body. MCs are induced by cytokines such as stem cell factor (SCF), IL-3, IL-4 and IL-9 and accumulate in the inflamed tissue. In response to a wide range of infections MCs rapidly release a multitude of inflammatory mediators including histamine, proteases and cytokines (reviewed in ref. 4). During gastrointestinal helminth infections MCs are traditionally considered a key effector cell during late stage T helper 2 (Th2) associated inflammation, where they cross-link antigen specific IgE via the surface high affinity FcεRI and degranulate. The effector role for MCs during helminth infection has been shown to be important for the expulsion of many helminth species from the gastrointestinal tract (reviewed in ref. 5). In contrast, the innate IgE-independent role of tissue resident MCs during the early stages of helminth exposure is relatively poorly understood. MCs possess a wide range of receptors that enable them to innately sense pathogen associated molecules and to respond rapidly. Recent studies have suggested that tissue resident MCs provide critical adjuvant signals following antigen exposure that amplifies the priming of the adaptive immune response.6 In particular, rapid MC degranulation and histamine release immediately following allergen exposure was required for the induction of early inflammation and migration of dendritic cells to the lymph node to prime adaptive immune responses in a model of contact hypersensitivity.6
Similarly, in our recent study we demonstrated that within the first days of Hp infection in the small intestine MCs degranulate in an IgE-independent manner and that MC mediator release was required for the upregulation of tissue-derived cytokines (IL-25, IL-33 and TSLP) and optimal migration of dendritic cells required for Th2 cell priming.1 However, to date the mechanisms through which MCs sense worm infection and the specific mediators released by MCs that mediate enhanced orchestration of tissue cytokine production and type 2 immunity are unknown. Below, we speculate on some of the candidate pathways through which MCs may mediate their effects during gastrointestinal helminth infection.
How Do Mast Cells Sense Gastrointestinal Helminth Infection?
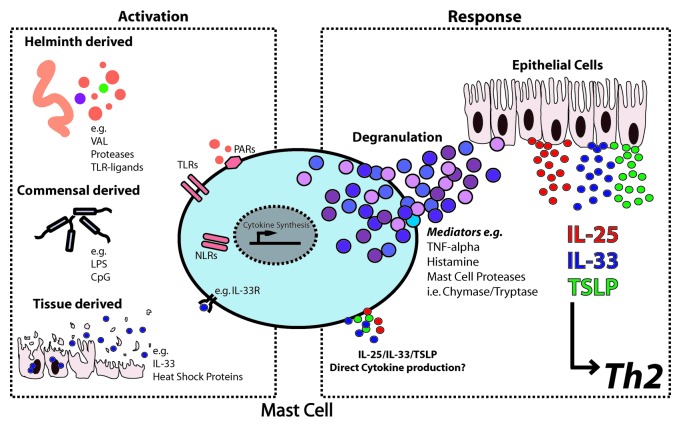
MCs, like many cells of the innate immune system, are equipped with a wide range of pathogen sensing receptors that allow them to recognize danger and are often referred to as “sentinels” of the immune system (Fig. 1; left panel). In our recent study we showed that MCs degranulate within the first days of a helminth infection in an IgE-independent manner — but the way in which MCs recognize intestinal worm infections are unknown. Attempts to delineate these pathways are complicated because helminth infections in the gastrointestinal mucosa also inevitably lead to exposure of intestinal MCs to signals derived from the abundant commensal bacteria. As such, we hypothesize that MCs may (1) recognize helminth derived products directly; (2) recognize invading commensal bacteria signals with concurrent bystander effects on the anti-helminth response; (3) require dual signals from both commensals and helminths. In addition to pathogen derived signals, infection also leads to significant tissue damage and thus, intestinal MCs are also likely to be exposed to a range of danger signals.
Figure 1. Potential mechanisms of IgE-independent mast cell activation and tissue-derived cytokine induction during intestinal helminth infection. Activation (Left panel): Helinth derived antigens, immunomodulators and proteases along with concurrent stimulation by commensal derived molecules and/or dangers signals can be recognized by mast cells through a variety or receptors including Toll-like receptors (TLRs), NOD-like receptors (NLRs) and “alarmin” receptors (e.g., IL-33R). Response (Right panel): Following stimulation mast cells respond via degranulation and/or the synthesis of cytokines, possibly include IL-25, IL-33 and TSLP. Moreover, mast cell inflammatory mediators have the ability to cross talk with other cells, such as epithelial cells, to induce the production of tissue-derived cytokines that are ultimately required for the optimal orchestration, amplification and priming of Th2 responses toward gastrointestinal helminths.
Helminth excretory/secretory (E/S) products represent an abundant source of stimulatory molecules that can be recognized by MCs. For example, it was recently shown that Hp E/S is dominated by a group of venom-allergen like proteins (VAL) and similar proteins are found to be produced by a wide range of parasitic worms.7 Interestingly, one of the main roles of MCs in barrier tissues has been shown to be to recognize venom proteins from pathogens and to release mediators that inhibit their toxicity to the host.8 Similarly, helminths secrete a variety of proteases that can be recognized by protease-activated receptors (PARs) on MCs, resulting in degranulation. Many parasitic helminths have evolved protease inhibitors in order to facilitate their persistence in the host via the degradation of proteases, although little is known about the role of MCs in recognizing helminth derived proteases.9
In addition MCs express a wide range of toll-like receptors (TLRs) which sense pathogen derived molecules.10 TLR recognition of signals derived from the commensal flora have a critical role in maintaining immune homeostasis and TLR ligands also provide adjuvant signals following tissue damage.11 Interestingly, although TLRs are traditionally considered to recognize microbial signals there is also evidence that the host may recognize stimulatory helminth molecules through TLRs and helminths can co-opt TLR signaling for immunomodulation.12 Signaling via TLR-4 appears to be required for the generation of inflammation following infection with Trichuris muris, suggesting that TLR-4 signaling plays a role in the induction of immunity to helminths.13 Co-stimulation of MCs via TLR-4 also enhances their ability to promote Th2 responses and eosinophilia and thus, TLR-4 co-stimulation may be important in the context of helminth infection.14
MCs also express NOD-like receptors (NLRs) — a family of cytoplasmic proteins involved in sensing microbial stimuli and implicated in the induction and amplification of immunity. Activation of MCs with NOD agonists results in the release of pro-inflammatory cytokines and inflammation in the intestine.15 MC activation via NOD receptors has been linked to the development of inflammation during Crohn’s disease and NOD2 expressing MCs are found in high numbers in the intestines of inflammatory bowel disease patients.16 Interestingly, it has been shown that NOD ligands are not only derived from microbial pathogens but also from parasitic helminths and that these ligands help to drive Th2 immunity.17 Recognition of NOD ligands is required for optimal Th2 priming via the regulation of TSLP and subsequent modulation of dendritic cells to express OX40-L.18 Although direct recognition of NOD ligands by stromal cells was found to be sufficient for TSLP production and Th2 priming, signaling through the hematopoietic compartment was needed for optimal responses.19 In this study, dendritic cells were found to be the major hematopoietic cell population required. However, it is still possible that MCs may contribute to optimal Th2 orchestration via NOD signaling in other settings.
Interestingly, MCs could theoretically regulate tissue-derived cytokines via a positive feedback mechanism. For example, IL-33 is constitutively expressed and acts as an “alarmin” at epithelial barriers. Under steady-state conditions IL-33 regulates gene expression in the nucleus via interactions with NF-κB,20,21 however, upon tissue damage it is released following and MCs appear to be one of the main targets of IL-33.20,22 MCs express the IL-33R (ST-2 + IL-1RAcP) and become activated upon IL-33 stimulation resulting in the release of inflammatory mediators.23 Thus, one possibility to be explored is that MCs sense signals derived from tissue damage such as IL-33 during the initial stages of helminth invasion and enhance tissue cytokine expression, including IL-33 itself, via a novel positive feedback mechanism.
How Do Mast Cells Contribute to Enhanced Tissue-Derived Cytokine Production?
Tissue-derived cytokines such as IL-25, IL-33 and TSLP have been shown to have essential roles during helminth infection, and mice lacking any of the three cytokines show abrogated priming of Th2 cells and reduced anti-parasitic responses.24-26 All three cytokines are produced by epithelial cells at mucosal barriers,27 although many other cell sources for these cytokines have been reported. As addressed above tissue-derived cytokines can be constitutively expressed in the tissues although their expression is rapidly upregulated upon infection. Indeed, we found an enhanced expression of these cytokines during the early stages of Hp infection in WT mice, which was absent in MC deficient mice.1 The mechanism(s) through which MCs can enhance the production of tissue-derived cytokines are poorly defined.
It has been reported that MCs are able to produce all three cytokines,28-30 thus, one possibility is that MCs themselves contribute to the enhanced expression of these factors following Hp infection. However, it is unlikely that MCs — a relatively rare population in the steady-state/early-infected intestine — produce sufficient quantities of these cytokines alone. Another possibility is that activation and/or degranulation of MCs results in the release of cytokines and inflammatory mediators that cross talk with bystander cells (e.g., epithelial cells) in the intestine to upregulate the production of IL-25, IL-33 and TSLP. Indeed in line with our findings it was previously reported that induction of TSLP in airway epithelial cells is abolished in mice lacking MCs during allergic rhinitis.31
MCs are able to produce a plethora of mediators upon their activation (reviewed in ref. 10). MCs are characterized by their high number of granules that contain pre-stored inflammatory mediators, although MCs may also synthesize many products upon stimulation. Thus, MCs are highly immunocompetent and produce a range of stimulatory molecules with the potential to activate bystander cells and induce tissue-derived cytokines (Fig. 1; right panel). For example, MC derived TNF-α attracts inflammatory cells including T cells, neutrophils and DCs to barrier tissues during the early stages of infection and potently induces lymph node hypertrophy.32-34 MC derived TNF-α is required for the generation of optimal Th2 immunity and worm expulsion during Trichinella spiralis infection and generation of Th2 responses in a mouse model of asthma.35,36 Moreover, TNF-α can regulate expression of IL-25,37 IL-3338 and TSLP39 in human and murine diseases. TNF-α acts in synergy with either pro-inflammatory or type 2 cytokines to regulate expression of tissue-derived cytokines and it is likely important that the predominant cytokine milieu will determine the impact of TNF-α in this regard.
As well as recognizing foreign proteases (see above) MCs are themselves an abundant source of proteases with immunostimulatory capacities (e.g., chymase and tryptase) that signal via protease-activated receptors i.e., PAR-2.4,10,40 Release of MC proteases during helminth infection has been shown to alter epithelial barrier function whereas conversely, absence of MC proteases can result in reduced Th2 responses or delayed onset of immunity.41,42 Protease signaling via protease-activated receptor 2 (PAR-2) has recently been shown to be required for release of IL-25 and TSLP by epithelial cells during Trichinella spiralis infection and airway allergic responses.43,44 Thus, MC derived proteases represent prime candidates for the cross talk between MCs and epithelial cells and the induction of tissue-derived cytokines.
Control of Type 2 Innate Populations Via a Mast Cell-IL-25/IL-33 Axis?
Fully differentiated mature MCs are only found in the tissues that are populated by bone marrow derived populations of MC-precursors that circulate in the blood. The exact signals that attract MC-precursors to the tissues and stimulate them to undergo differentiation are not fully elucidated, although SCF and IL-3 are known to play critical roles. As detailed above we identified a role for MCs in regulating the tissue-derived cytokines IL-25 and IL-33 in the intestine during the early stages of helminth infection. These cytokines have been shown to play critical roles in orchestrating innate type 2 immunity, including the recruitment of Lin- progenitors with the capacity to differentiate to MCs.45 In our recent study we observed the expansion of a Lin- cell population in the small intestine during Hp infection in WT mice, but not MC deficient mice, that expressed the hematopoietic markers CD34 and Sca-1. When these cells were isolated and cultured with SCF and IL-3 they upregulated the expression of the c-kit and FcεRI, indicative of a MC phenotype.1 In line with previous reports these progenitors could be partially restored in MC deficient mice (that lack tissue IL-25 following infection) upon treatment with exogenous rIL-25. Thus, this leads to the intriguing hypothesis that MC degranulation following infection plays an important role in repopulating barrier tissues with MC progenitors by the regulation of an IL-25 driven axis.
In addition, many recent studies have identified populations of type 2 innate lymphoid cells (iLCs) in the lymph node and tissues that produce IL-13 during helminth infection and allergy. Expansion of these cell types is also critically regulated by IL-25 and/or IL-33 production in the inflamed tissue and these cells have all been characterized by their lack of conventional immune lineage markers (Lin-), as well as their expression of a range of other surface markers including IL-33R (ST-2), the IL-7Rα subunit (CD127), the activation molecule ICOS and intermediate expression of c-kit, among others. In several independent studies a range of similar cell types were identified and differentially termed Natural Helper Cell (NHC),46 Nuocyte47 or Innate helper 2 cell.48 Collectively these cells are referred to as type 2 iLCs as they share the ability to produce type 2 cytokines but also share similarities with pro-inflammatory innate lymphoid lineages that produce IFN-γ, IL-17A and IL-22.49
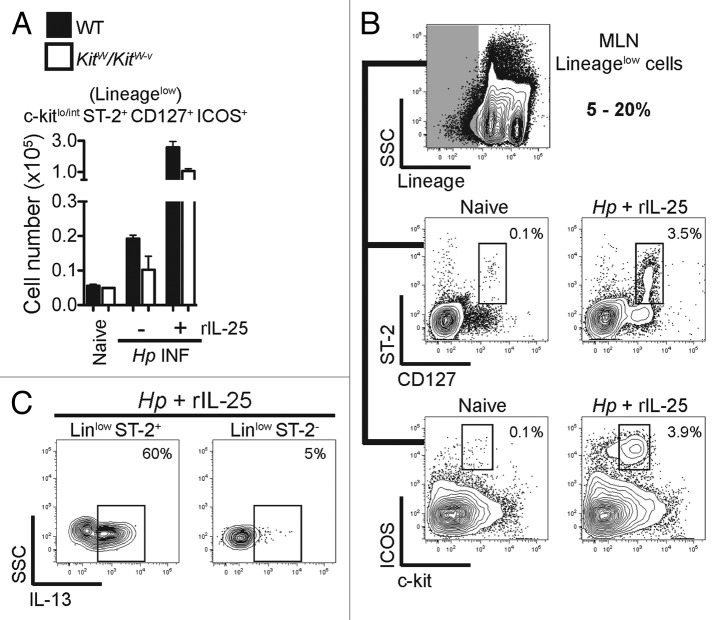
Given the expression of IL-25 and IL-33 following Hp infection we hypothesized that type 2 iLCs may expand in the draining lymph node during the early stages of infection. Administration of exogenous rIL-25 to Hp infected mice during the first four days of Hp infection elicited a population of Lin- cells in the MLN, whereas only a negligible increase in the same cells could be seen during Hp infection alone in WT mice compared with naïve WT mice and MC deficient mice that lack upregulated expression of IL-25 and IL-33 (Fig. 2A). rIL-25 induced Lin- cells expressed ST-2, CD127, ICOS and were c-kitint in line with previous descriptions of type 2 innate lymphoid cells (Fig. 2A and B). The population resembling type 2 iLCs were also found to express high levels of IL-13, in contrast to other Lin- cells in the lymph node (Fig. 2C), suggesting these cells truly resemble type 2 iLCS. Interestingly, we reported that administration of exogenous IL-25 in WT mice during Hp infection resulted in significant worm expulsion in this otherwise chronic model of helminth infection. Thus, it is possible that high numbers of type 2 iLCs can drive expulsion during Hp infection as reported in other infections such as Nippostrongylus brasiliensis.47,48
Figure 2. Type 2 innate lymphoid cells are rare in the MLN during the early stages of Hp infection but can be induced by exogenous rIL-25 treatment. C57BL/6 mice were infected with 200 Hp L3 and Mesenteric Lymph Node (MLN) derived lymphocytes isolated and stained for a population resembling type 2 innate lymphoid cells via flow cytometry. A) Total numbers of type 2 innate lymphoid cells were quantified at day 6 post infection (p.i.) in the MLN of WT (black bars) and mast cell deficient KitW/KitW-v mice (white bars) following Hp infection alone or Hp infection with rIL-25 treatment (0.4μg i.p. on days 0–4 p.i.). B) Cells were gated as low/negative for a lineage cocktail (CD3/CD4/CD19/CD11b/CD11c) (shaded gate) and expressed the surface markers ST-2 (IL-33R), CD127 (IL-7Rα), ICOS and were c-kit low/int. C) Expression of IL-13 in Lineage low/negative gated ST-2+ or ST-2- populations in Hp infected, rIL-25 treated mice. Data are representative of two independent experiments with n = 4–6 mice per group.
Thus, it is not yet clear why we could detect a significant induction of Lin- MC progenitors in Hp infected WT mice that expressed both endogenous IL-25 and IL-33 but only a small induction of type 2 iLC cells. One possibility is that a higher threshold of IL-25 expression is required for the induction of type 2 iLC populations than is needed for the recruitment of the MC-progenitor population. Indeed the concentrations of exogenous rIL-25 that were systemically administered are fairly high (0.4μg/injection) and it is not yet clear if this truly resembles physiological expression levels. Another possibility is that the action of endogenous IL-25 and IL-33 elicited during Hp infection is restricted to the tissues and has only limited systemic effects. Alternatively, induction of type 2 iLCs may be selectively dependent on other cellular sources of IL-25 and IL-33 outside of the inflamed tissue. For example, radio-resistant cells in the spleen can produce IL-33 following viral infection in the lung.50 Furthermore, as reconstitution of MC deficient mice with IL-25 also restored the expression of IL-33 and TSLP, we cannot rule out that the recruitment of such progenitor is not directly dependent upon IL-25 itself, in contrast to type 2 iLCs, and rather on the downstream induction of other signals. Further work is needed to delineate the pathways that orchestrate these responses in the tissues and periphery following helminth infections.
Impact and Outlook
MCs are distributed throughout barrier tissues and are highly immunocompetent cells that express the required machinery to sense pathogenic stimuli and to rapidly respond via release of a multitude of inflammatory mediators. MCs have been described to have key early roles in the induction and amplification of immune responses in a wide range of diseases and increasing evidence suggests one way in which they contribute to this process is via the regulation of tissue-derived cytokines such as IL-25, IL-33 and TSLP. The precise mechanisms through which MCs may fulfill this function during intestinal helminth infections are yet to be elucidated. Future work should focus on dissecting the contribution of MCs as sensors and amplifiers during the early stages of tissue immune responses. Recently developed inducible MC specific knockout mice should allow for significant advances in this field.
Acknowledgments
We would like to acknowledge Martin Metz, Emilia Danilowicz-Luebert, Sebastian Rausch and all other co-authors for their vital input into the original manuscript. We further acknowledge Max Loehning who provided the anti-ST-2 antibody used in Figure 2. Research in the laboratory of S.H. was supported by funding from the DFG (SFB650 and Ha2545/3-1) and the Broad Foundation.
Glossary
Abbreviations:
- MC
mast cell
- Hp
Heligmosomoides polgyrus bakeri
- iLC
innate lymphoid cell
- lin
lineage marker cocktail
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/21507
References
- 1.Hepworth MR, Daniłowicz-Luebert E, Rausch S, Metz M, Klotz C, Maurer M, et al. Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proc Natl Acad Sci U S A. 2012;109:6644–9. doi: 10.1073/pnas.1112268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 3.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 4.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–42. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 5.Pennock JL, Grencis RK. The mast cell and gut nematodes: damage and defence. Chem Immunol Allergy. 2006;90:128–40. doi: 10.1159/000088885. [DOI] [PubMed] [Google Scholar]
- 6.Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Köhler A, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–84. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Hewitson JP, Harcus Y, Murray J, van Agtmaal M, Filbey KJ, Grainger JR, et al. Proteomic analysis of secretory products from the model gastrointestinal nematode Heligmosomoides polygyrus reveals dominance of venom allergen-like (VAL) proteins. J Proteomics. 2011;74:1573–94. doi: 10.1016/j.jprot.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–30. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 9.Klotz C, Ziegler T, Daniłowicz-Luebert E, Hartmann S. Cystatins of parasitic organisms. Adv Exp Med Biol. 2011;712:208–21. doi: 10.1007/978-1-4419-8414-2_13. [DOI] [PubMed] [Google Scholar]
- 10.Galli SJ, Maurer M, Lantz CS. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999;11:53–9. doi: 10.1016/S0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 11.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–49. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, Al-Riyami L, et al. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J Immunol. 2005;174:284–93. doi: 10.4049/jimmunol.174.1.284. [DOI] [PubMed] [Google Scholar]
- 13.Helmby H, Grencis RK. Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection. Eur J Immunol. 2003;33:2974–9. doi: 10.1002/eji.200324264. [DOI] [PubMed] [Google Scholar]
- 14.Nigo YI, Yamashita M, Hirahara K, Shinnakasu R, Inami M, Kimura M, et al. Regulation of allergic airway inflammation through Toll-like receptor 4-mediated modification of mast cell function. Proc Natl Acad Sci U S A. 2006;103:2286–91. doi: 10.1073/pnas.0510685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng BS, He SH, Zheng PY, Wu L, Yang PC. Mast cells play a crucial role in Staphylococcus aureus peptidoglycan-induced diarrhea. Am J Pathol. 2007;171:537–47. doi: 10.2353/ajpath.2007.061274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okumura S, Yuki K, Kobayashi R, Okamura S, Ohmori K, Saito H, et al. Hyperexpression of NOD2 in intestinal mast cells of Crohn’s disease patients: preferential expression of inflammatory cell-recruiting molecules via NOD2 in mast cells. Clin Immunol. 2009;130:175–85. doi: 10.1016/j.clim.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci U S A. 2010;107:20459–64. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magalhaes JG, Fritz JH, Le Bourhis L, Sellge G, Travassos LH, Selvanantham T, et al. Nod2-dependent Th2 polarization of antigen-specific immunity. J Immunol. 2008;181:7925–35. doi: 10.4049/jimmunol.181.11.7925. [DOI] [PubMed] [Google Scholar]
- 19.Magalhaes JG, Rubino SJ, Travassos LH, Le Bourhis L, Duan W, Sellge G, et al. Nucleotide oligomerization domain-containing proteins instruct T cell helper type 2 immunity through stromal activation. Proc Natl Acad Sci U S A. 2011;108:14896–901. doi: 10.1073/pnas.1015063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488–95. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 21.Ali S, Mohs A, Thomas M, Klare J, Ross R, Schmitz ML, et al. The dual function cytokine IL-33 interacts with the transcription factor NF-κB to dampen NF-κB-stimulated gene transcription. J Immunol. 2011;187:1609–16. doi: 10.4049/jimmunol.1003080. [DOI] [PubMed] [Google Scholar]
- 22.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enoksson M, Lyberg K, Möller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C. Mast cells as sensors of cell injury through IL-33 recognition. J Immunol. 2011;186:2523–8. doi: 10.4049/jimmunol.1003383. [DOI] [PubMed] [Google Scholar]
- 24.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–9. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 25.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–67. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–9. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–90. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, et al. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–6. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- 30.Okayama Y, Okumura S, Sagara H, Yuki K, Sasaki T, Watanabe N, et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur Respir J. 2009;34:425–35. doi: 10.1183/09031936.00121008. [DOI] [PubMed] [Google Scholar]
- 31.Miyata M, Hatsushika K, Ando T, Shimokawa N, Ohnuma Y, Katoh R, et al. Mast cell regulation of epithelial TSLP expression plays an important role in the development of allergic rhinitis. Eur J Immunol. 2008;38:1487–92. doi: 10.1002/eji.200737809. [DOI] [PubMed] [Google Scholar]
- 32.Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–12. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 33.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–48. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 34.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 35.Ierna MX, Scales HE, Saunders KL, Lawrence CE. Mast cell production of IL-4 and TNF may be required for protective and pathological responses in gastrointestinal helminth infection. Mucosal Immunol. 2008;1:147–55. doi: 10.1038/mi.2007.16. [DOI] [PubMed] [Google Scholar]
- 36.Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 37.Fina D, Franzè E, Rovedatti L, Corazza GR, Biancone L, Sileri PP, et al. Interleukin-25 production is differently regulated by TNF-α and TGF-β1 in the human gut. Mucosal Immunol. 2011;4:239–44. doi: 10.1038/mi.2010.68. [DOI] [PubMed] [Google Scholar]
- 38.Verri WA, Jr., Souto FO, Vieira SM, Almeida SC, Fukada SY, Xu D, et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis. 2010;69:1697–703. doi: 10.1136/ard.2009.122655. [DOI] [PubMed] [Google Scholar]
- 39.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, et al. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–7. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 40.Jacob C, Yang PC, Darmoul D, Amadesi S, Saito T, Cottrell GS, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280:31936–48. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence CE, Paterson YY, Wright SH, Knight PA, Miller HR. Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse. Gastroenterology. 2004;127:155–65. doi: 10.1053/j.gastro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 42.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A. 2003;100:7761–6. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park MK, Cho MK, Kang SA, Park HK, Kim YS, Kim KU, et al. Protease-activated receptor 2 is involved in Th2 responses against Trichinella spiralis infection. Korean J Parasitol. 2011;49:235–43. doi: 10.3347/kjp.2011.49.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–34. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr., Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–6. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 47.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearson C, Uhlig HH, Powrie F. Lymphoid microenvironments and innate lymphoid cells in the gut. Trends Immunol. 2012;33:289–96. doi: 10.1016/j.it.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Bonilla WV, Fröhlich A, Senn K, Kallert S, Fernandez M, Johnson S, et al. The alarmin interleukin-33 drives protective antiviral CD8⁺ T cell responses. Science. 2012;335:984–9. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]