Abstract
BACKGROUND
Patients were recruited from the Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease, located within the University of Pittsburgh Medical Center. Idiopathic pulmonary fibrosis results in scarring of the lung and respiratory failure, and has a median survival of 3 to 5 years from the time of diagnosis. The purpose of this study was to determine whether patients with idiopathic pulmonary fibrosis and their care partners could be more optimally managed by a disease-management intervention entitled “Program to Reduce Idiopathic Pulmonary Fibrosis Symptoms and Improve Management,” which nurses delivered using the format of a support group. We hypothesized that participation would improve perceptions of health-related quality of life (HRQoL) and decrease symptom burden.
METHODS
Subjects were 42 participants randomized to an experimental (10 patient/care partner dyads) or control (11 patient/care partner dyads) group. Experimental group participants attended the 6-week program, and controls received usual care. Before and after the program, all participants completed questionnaires designed to assess symptom burden and HRQoL. Patients and care partners in the intervention group were also interviewed in their home to elicit information on their experience after participating in the Program to Reduce Idiopathic Pulmonary Fibrosis Symptoms and Improve Management.
RESULTS
After the intervention, experimental group patients rated their HRQoL less positively (P = .038) and tended to report more anxiety (P = .077) compared with controls. Care partners rated their stress at a lower level (P = .018) compared with controls. Course evaluations were uniformly positive. Post-study qualitative interviews with experimental group participants suggested benefits not exemplified by these scores. Patient participants felt less isolated, were able to put their disease into perspective, and valued participating in research and helping others.
CONCLUSION
Further exploration of the impact of disease-management interventions in patients with advanced lung disease and their care partners is needed using both qualitative and quantitative methodology. Disease-management interventions have the potential to positively affect patients with advanced lung disease and their care partners.
Interstitial lung disease (ILD) is a condition that results in the progressive inability to maintain normal blood oxygen levels because of impaired transfer of gas across the alveolar-capillary membrane.1 More than 150 clinical diagnostic entities are associated with ILD.2,3 Idiopathic pulmonary fibrosis (IPF), also known pathologically as usual interstitial pneumonia, is one of the most common forms of ILD. The prevalence of IPF has been estimated to range from 4.0 per 100,000 persons aged 18 to 34 years to 227.2 per 100,000 persons aged ≥75 years.4 IPF is often viewed as having uniformly poor survival.4–7 However, the clinical course varies widely with some patients dying within 1 year of diagnosis and others living much longer.8–12 The reason for this difference is unknown.8,12 There is currently no therapy that reverses or cures the lung damage.5,13 Lung transplantation remains the only available treatment if the patient meets established criteria.5,9
Patients diagnosed with IPF share problems common to any chronic illness, a life change that can affect emotional response in a manner disproportionate to physical disability.14 As with other chronic illnesses, the progressive disability associated with IPF affects both the patient and the care partner. The emotional consequences can be particularly devastating, given the lack of effective therapy. De Vries and colleagues15 reported that approximately 25% of patients with IPF experience depressive symptoms. The burden of illness is commonly shared by a care partner who may also experience significant burden as the illness progresses. For this reason, care partners have been termed “the hidden patient.”16
These findings and similar reports from other studies suggest potential benefit from an intervention incorporating palliative care approaches for the patient and care partner.17–22 Although commonly integrated into the management of patients with cancer, the use of palliative care is limited in patients with lung disease.23 The goal of palliative care is “to prevent and relieve suffering and to support the best possible quality of life for patients and their families, regardless of the stage of the disease or the need for other therapies.”24 Palliation may be the only therapeutic option for patients with advanced cancer, chronic obstructive pulmonary disease, cystic fibrosis, and IPF.25 The concepts integral to palliative care seem particularly appropriate for patients with IPF, whose mortality rate exceeds that of many cancers.26
Although the stress of the diagnosis of a chronic medical illness typically induces anxiety and depression, these symptoms may diminish if there is hope for a cure. When the prognosis is unknown or the condition is likely to be terminal, anxiety and depression can become more severe and prolonged.27 Patients with IPF face challenges to their functional independence, self-esteem, and quality of life as a result of a progressive decrease in exercise capacity. Activities of daily living require extra planning and time. “Cough becomes a dreaded ordeal, and onset of exacerbations or new symptoms reawakens the fear of respiratory crisis or death.”28 Faced with these stressors, patients with IPF are at high risk for developing anxiety and depression. Because care providers typically focus on treatment options, prognosis, and relief of physical discomfort, they may not actively solicit concerns arising from anxiety and depressive symptoms from the patient or care providers.27
Studies have shown depression and anxiety are common in patients with chronic health problems, but little research has been done in patients with IPF.28 Although anxiety and depression are inevitable in patients with advanced lung diseases, both are highly responsive to treatment with medications and cognitive behavioral therapy.28 If depression and anxiety are present, they are likely to affect health-related quality of life (HRQoL). Ratings of HRQoL provide a means of quantifying, in a standardized manner, how chronic illness affects daily life, health, and well-being.29,30 A limited literature has evaluated HRQoL in patients with IPF.15,17,18,19,31,20,21,32,33 In these studies, patients with IPF consistently demonstrated impaired HRQoL affecting both physical and psychologic function. Dyspnea was an important factor influencing HRQoL,12 as was poor sleep quality34 and forced vital capacity (FVC).12,15,17–22,31–35
Several studies have demonstrated that a psychoeducational intervention can benefit those who have serious illness accompanied by stress and depression and can improve their psychologic well-being.35–38 Although much of the literature focuses on those who experience a long-term illness trajectory and their caregivers, the psychosocial impact of a psychoeducational intervention targeted early in the onset of a potentially life-ending illness, such as IPF, has not been examined.
The purpose of this study was to test the ability of the Program to Reduce Idiopathic Pulmonary Fibrosis Symptoms and Improve Management (PRISIM) to decrease symptom burden, decrease stress, and improve perceptions of HRQoL for patients with IPF and their care partners. We hypothesized that, compared with usual care, the PRISIM intervention would decrease symptom burden for patients and decrease stress and improve perceptions of HRQoL for patients and caregivers.
MATERIALS AND METHODS
Design
This study used a quantitatively driven, concurrent nested mixed-method design with the experimental group receiving the (PRISIM) intervention and the control group receiving usual care only. The study enrolled 42 participants. Ten patient/care partner dyads were randomized to the intervention group, and 11 patient/care partner dyads were randomized to usual care. All patients/care partners completed questionnaires designed to measure anxiety, depression, perceived stress, and HRQoL before and after completion of the intervention. Patients completed a questionnaire to assess dyspnea at the same intervals. In addition, patient/care partners randomized to the intervention group were interviewed in their homes after completion of the intervention.
Participants were randomized to group after baseline questionnaires were completed. A permutated blocked design was used to ensure that equal numbers of patients with moderate (FVC 55%–70% predicted) and severe (FVC < 55% predicted) disease were assigned to each group. The study received institutional review board approval, and all participants provided informed consent.
Setting and sample
Patients were recruited from a university-based ILD program. Patients were required 1) to be aged more than 21 years; 2) to be able to read and understand English; 3) to be diagnosed with IPF; and 4) to have an FVC reflecting moderate (FVC 55%–70% predicted) or severe (FVC < 55% predicted) disease. Care partners were required 1) to be aged more than 21 years; 2) to be able to read and understand English; and 3) to live with or care for the patient with IPF. Both patient and care partner had to consent to study participation. Because the study was designed to obtain pilot data for a future study, sample size was not based on a power calculation.
Intervention
The intervention consisted of 6 weekly group sessions attended by patients and care partners. The content for the sessions was developed collaboratively by a pulmonary clinical nurse specialist whose practice involved patients with IPF, a psychiatric clinical specialist with training as a cognitive behavioral therapist, and an advanced care planning instructor. The sessions included the following: “What is IPF and How to Live with it?” (Session 1), which reviewed the causes, pathophysiology, and treatment of IPF; “Gaining Control of Your Moods and Feelings: You Feel the Way You Think” (Session 2), which discussed basic principles of cognitive behavior techniques and cognitive distortions; “Gaining Control of Your Moods and Feelings: What Can You Do about Depression” (Session 3), which discussed concepts of stress and depression and interrelationships with illness; “Putting Your Life in Order: What Do I Do Now?” (Session 4), which addressed planning for uncertainty and concerns related to terminal illness, communicating with clinicians, coping, and planning to one’s affairs; “Living with IPF” (Session 5), which discussed symptom management, energy conservation, oxygen therapy, and the importance of exercise; and “Wrap-up and Review Group” (Session 6), which focused on informal discussion and review. Each session lasted 2 hours. Both groups received a copy of the book Feeling Good: The New Mood Therapy39 to read at leisure (controls) or use in group exercises (experimental group).
Patients randomized to usual care were seen by members of the clinical care team consisting of a pulmonary clinical nurse specialist and physicians with expertise in the management of IPF, typically at intervals of every 3 to 6 months. The pulmonary clinical nurse specialist was available by phone to answer questions and conducted a monthly support group for those wanting to attend. Psychologic counseling was provided, if indicated, but was not offered on a routine basis.
Instruments
Dyspnea was measured using the University of California at San Diego Shortness of Breath Questionnaire,40 which asked subjects to rate severity of shortness of breath on a 6-point scale (0 = not at all to 5 = maximal or unable to do) during 21 activities of daily living associated with varying exertion. Scores were obtained by summing responses on all items (range 0–120). Internal consistency and validity have been well established.40
Anxiety was measured using the Beck Anxiety Inventory (BAI), a standardized, 21-item tool41 that uses a 4-point scale (0 = absent/not at all disturbing to 3 = I could barely stand it) to measure anxiety. Scores were obtained by summing the 21 items (range 0–63). A score of 0 to 7 indicated no anxiety, 8 to 15 indicated mild anxiety, 16 to 25 indicated moderate anxiety, and greater than 26 indicated severe anxiety.41 Reported mean scores for psychiatric outpatients with anxiety disorders range from 17 to 29,42 and means for community samples ranged from 7 to 11. Reliability and validity have been well established.43
Depression was measured using the Beck Depression Inventory-II (BDI-II),44 a 21-item well-validated self-report instrument designed to measure the severity of depression in adults and adolescents 13 years or older.44 The BDI-II is a revised version of the original instrument. A score of 0 to 13 suggests minimal depression, 14 to 19 suggests mild depression, 20 to 28 suggests moderate depression, and 29 to 63 suggests severe depression.45 Reliability and validity have been well established.44
The Perceived Stress Scale46 was designed to measure the degree to which subjects find their lives unpredictable, uncontrollable, and overloading. Respondents were asked to indicate how they feel or thought in the last month using the option 0 (never) to 4 (very often). Total scores range from 0 to 40, with higher scores indicating more stress. Internal consistency and validity have been well established with a diverse population.47–49
HRQoL was measured using the Short Form (SF)-36 (Version 2), a widely used generic scale.18,50 Scores range from 0 (maximum impairment) to 100 (no impairment). The 8 domains can be grouped into a physical score that includes physical functioning, role physical, bodily pain, and general health, and a mental score that includes vitality, social functioning, role emotional, and mental health.51 The physical score and mental score were normalized to responses from the general population (mean score = 50). Validity and reliability have been well demonstrated.52
The battery required approximately 1 hour to complete. Demographic data were obtained from the patient and care partner. Diagnosis and the most recent pulmonary function value (FVC) were obtained from the medical record. Pulmonary function tests were obtained at 3- to 6-month intervals or more frequently, if indicated.
Interviews
Each member of the experimental group dyad was interviewed separately in their home by one member of the research team (KOL) after completion of the intervention. These interviews were open-ended, for example, “tell me about participation in the PRISIM intervention,” which allowed for data collection that reflected the perspectives of each member of the dyad regarding their experience. Responses guided future questions and became more focused as the interviews progressed. Qualitative methods were incorporated into the study to address the conflicting results in the quantitative portion of the study. We were interested in learning what explained the finding that the intervention had a negative impact on the patients, but a positive impact on the care partners.
DATA ANALYSIS
Quantitative analysis
Baseline demographic and medical profile data were compared using chi-square and t tests, as appropriate. Because differences in intervention and control groups were found in baseline scores for anxiety and the physical aspect of HRQoL, questionnaire responses were analyzed using analysis of covariance (ANCOVA). Theoretically, baseline differences should not occur with randomization. Because such differences were present, ANCOVA was used to control for these initial differences. The Statistical Package for the Social Sciences v14.0 (SPSS Inc, Chicago, IL) was used for all data analyses. A P value of less than .05 was chosen to indicate statistical significance.
Qualitative analysis
Verbatim transcripts were analyzed line by line initially and then more generally by a qualitative research expert (EO). Data analysis consisted of open coding, wherein codes or concepts were developed that reflected the meaning in the transcripts. These codes were then analyzed through a process referred to as axial coding. Eventually, using a process referred to as selective coding, only certain codes were determined to be supported by the ongoing data collection, and those codes were then analyzed in more depth and integrated into an explanatory framework.
Findings
Of the 42 subjects, 2 patients and 3 care partners did not complete data collection. Two dyads did not continue because the patient died or received a lung transplant. The third care partner failed to return after randomization. All were enrolled in the control group. Reasons for attrition were lung transplantation and death. Data are reported for the remaining 37 participants (Table I). No statistically significant differences were found between the intervention and the control group in regard to demographic variables or FVC. The majority (58%) of patients had scores indicating mild to severe anxiety, compared with 21% of care partners. Four patients reported scores consistent with mild depression (n = 2) or moderate (n = 2) depression; all were currently receiving treatment. No care partners reported depressive symptoms.
Table I.
Demographic and medical characteristics of participants who provided pre- and post-intervention data (n = 37)a
| Variable | Patients
|
Care partners
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 21) | Experimental (n = 10) | Control (n = 11) | df | Fisher’s exact test | Total (n = 20) | Experimental (n = 10) | Control (n = 10) | df | Fisher’s exact test | |
| Age, y | 66.19 ± 10.93 | 65.2 ± 10.28 | 67.09 ± 11.90 | n/a | n/a | 65.05 ± 10.67 | 63.3 ± 12.7 | 67 ± 8.58 | n/a | n/a |
| Gender, % male | 76.2% | 33.33% (n = 7) | 42.9% (n = 9) | 1 | .6351 | 23.8% | 19.04% (n = 4) | 4.76% (n = 1) | 1 | .14 |
| Caucasian, % | 95.2% | 47.6% (n = 10) | 47.6% (n = 10) | 1 | 1.0 | 95.24% | 100% (n = 10) | 90% (n = 9) | 1 | 1.0 |
| Diagnosis | ||||||||||
| biopsy % | 57.1% | 14.29% | 42.86% | 1 | .03a | n/a | n/a | n/a | n/a | n/a |
| HRCT | 42.9% | 33.33% | 9.52% | |||||||
| Lung function, % pred (n = 20) | ||||||||||
| FVC > 55% | 70% | 40% | 30% | 1 | .665 | n/a | n/a | n/a | n/a | n/a |
| FVC 50%–55% | 15% | 5% | 10% | |||||||
| FVC < 50% | 15% | 5% | 10% | |||||||
| Depression, % prior or current | 19.05% | 9.52% | 9.52% | 1 | 1.0 | 27.78% | 16.67% | 11.11% | 1 | 1.0 |
| Dropout, % yes | 4.76% | 0% | 4.76% | 1 | 1.0 | 19.05% | 4.76% | 14.29% | 1 | .14 |
n/a, not applicable; df, degrees of freedom; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; HRCT, high-resolution computed tomography.
Variables are mean ± standard deviation unless otherwise indicated.
Patient response
When post-intervention comparisons were made using ANCOVA, there were no statistically significant differences in mean scores on the Shortness of Breath Questionnaire (P = .972), Perceived Stress Scale (P = .531), BDI (P = .894), or SF-36 Mental Component (P = .772) (Table II). The mean difference for BAI scores approached statistical significance (P = .077) (Fig 1). Mean scores for the SF-36 physical component exhibited a statistically significant difference (P = .038), reflecting lower (more negative) scores for intervention patients (Fig 2). Thus, contrary to expectations, findings suggested a negative impact of the intervention on perceptions of physical HRQoL and a tendency for greater anxiety.
Table II.
Adjusted mean scores reflecting symptom burden, stress, and perceptions of health-related quality of life by intervention and control group participants (n = 37)a
| Patients
|
Care partners
|
|||
|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |
| SOBQ | 49.51 ± 22.64 | 49.88 ± 22.64 | ||
| Beck Anxiety | 15.13 ± 6.92 | 8.56 ± 6.95 | 2.22 ± 4.28 | 4.72 ± 4.32 |
| Beck Depression | 9.71 ± 4.34 | 9.44 ± 4.35 | 4.33 ± 3.29 | 4.84 ± 3.29 |
| Perceived Stress | 19.32 ± 3.64 | 18.20 ± 3.65 | 17.61 ± 2.68 | 20.99 ± 2.69 |
| SF-36 Physical | 31.06 ± 4.61 | 36.04 ± 4.63 | 50.12 ± 5.72 | 51.31 ± 5.72 |
| SF-36 Mental | 55.98 ± 2.71 | 55.61± 2.71 | 50.52 ± 1.67 | 50.05 ± 1.67 |
SOBQ, Shortness of Breath Questionnaire; SF-36, Short Form-36.
Values are mean ± standard deviation.
Fig 1.
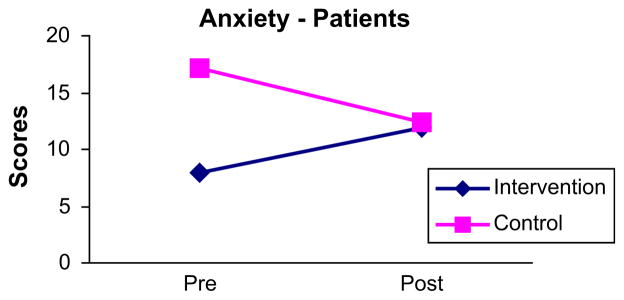
Change in patient’s pre- and post-intervention scores for BAI.
Fig 2.
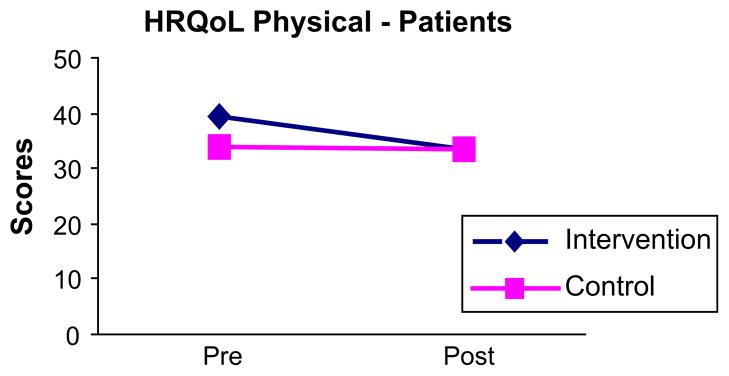
Change in patient’s pre- and post-intervention scores for SF-36 Physical Component Score. HRQoL = health-related quality of life.
Care partner response
When post-intervention comparisons were made using ANCOVA, there were no statistically significant differences between the intervention group and the control group for mean scores on the BAI (P = .260), BDI (P = .751), SF-36 Physical (P = .669), or SF-36 Mental (P = .565). Mean scores on the Perceived Stress Scale were significantly lower (P = .018) for the intervention group (Fig 3). Thus, findings suggested no impact on anxiety, depressive symptoms, or HRQoL, but a positive impact on perceived stress.
Fig 3.
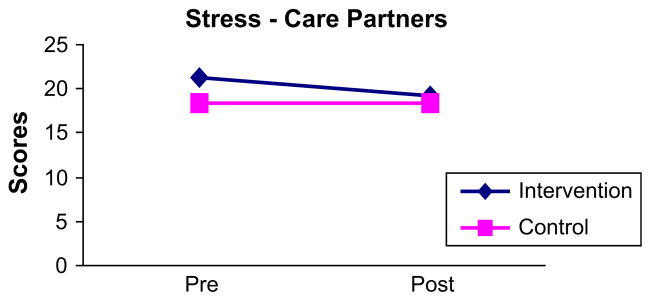
Change in care partner’s pre- and post-intervention scores for the Perceived Stress Scale.
Qualitative analysis
The interviews included 19 experimental group participants. One care partner was interviewed alone because the patient died before scheduling. Three common themes emerged (Table III). Participants in PRISIM “didn’t feel isolated,” felt “able to put my disease into perspective,” and “felt it was important to participate in research to help others.” They recognized “you aren’t the only one that has a problem” which “gave comfort” and provided an “improved mental picture.” PRISIM was viewed as “very beneficial” and led to perceptions that one was “not as bad off as you think you are.” There was also a sense that participation “might help us and maybe we could help somebody else.”
Table III.
Themes and verbatim extracts from the qualitative evaluation
| Emergent themes | Verbatim extracts |
|---|---|
| Did not feel isolated when participating in a disease-management program | “Going to the course introduced me to a lot of people just like me. The experience was fabulous. I appreciated being accepted into the class. I figure if they can do it, then so can I.” (P) |
| “It really gave me comfort to know there were other people in the same situation I was.” (P) | |
| “I met a lot of nice people; it was good for us, it was good for me.” (P) | |
| “I think his (pt) overall mental picture improved because he knows that there are other people that have the same disease.” (CP) | |
| “You recognize you aren’t the only one that has a problem.” (P) | |
| “There’s such camaraderie when you go through something like that and you’re all facing the same problems.” (P) | |
| “First and foremost, it’s always good to be around people that have the same illness who share the same concerns, your same fears, your same outlook, what is to be perceived in the future for yourself, everybody comes from different aspects of life, different ages, and the opportunity is there to express your innermost feelings or figure out how you can address your issues in life.” (P) | |
| “Well, originally when I first became ill, I felt very isolated and when I went to the PRISIM I saw other people in the same condition and even worse, and I felt really good because I had companions or people that knew what my problem was.” (P) | |
| “Beneficial to be with other people that had IPF.” (P) | |
| Able to put individual disease into perspective | “We were told that the disease was very rare, and then to actually come together and see that there were other people with the same disease, was very beneficial.” (CP) |
| “I really didn’t realize how fortunate I was.” (P) | |
| “I just think that it made them not feel sorry for themselves and made them thankful because they’d hear some other people that were a little bit worse, and made them thankful.” (CP) | |
| “When you see other people you think, I’m not so bad.” It just goes to show that you’re not as bad off as you think you are.” (P) | |
| “I have a much better attitude and I’m not afraid, and I’ve learned to live with it. They’ve helped me through the prism program with the depression and learning to live with it and seeing other people and making friends with them and they’re doing fine, so I can do fine.” (P) | |
| Felt that it was important to participate in research to help others with the disease | “Just thought that might be something that could help us and maybe we could help somebody else, too.” (P) |
P, patient statement; CP, care partner statement; PRISIM, Program to Reduce Idiopathic Pulmonary Fibrosis Symptoms and Improve Management; IPF, idiopathic pulmonary fibrosis.
DISCUSSION
To our knowledge, this study is the first to test the ability of an intervention to decrease symptom burden and improve perceptions of HRQoL for patients with IPF and their care partners. Contrary to expectations, ratings of physical HRQoL were more negative at the conclusion of the intervention and tended to reflect more anxiety. As anticipated, care partners in the intervention group experienced less stress after participation in PRISIM.
Although these findings suggested increased distress, participants repeatedly voiced appreciation for being able to participate in PRISIM. Qualitative themes supported feelings of less isolation, a more balanced view of the illness, and personal satisfaction from being able to participate in research. Although there was some increase in distress, there were also benefits.
This outcome is perhaps not surprising. PRISIM was constructed to assist in coping with depressive symptoms and encourage end-of-life decision-making, as well as increase knowledge of the disease. Although it is possible to avoid such discussions, the potential rapid progression of IPF suggests that a more proactive approach is necessary. Nine months after study entry, 7 patients (33%) had received lung transplants and 6 patients (29%) had died.
When faced with a terminal illness or a life-changing event such as a lung transplant, frank disclosure can encourage discussion regarding terminal care preferences.53 Unfortunately, such discussions are often avoided.54,55 From a study of 105 patients with chronic obstructive pulmonary disease, Heffner and colleagues55 reported that most patients (94.3%) expressed worries about their health, although few (42%) had completed an advance directive. Although most (98.9%) wanted discussions about end-of-life decision-making, few (19%) had such discussions and few (14.3%) thought their physician understood their preferences. As a consequence, patients were left to wonder what they might experience and clinicians were without clear knowledge of their preferences. Not discussing such issues can lead to distress that may not be recognized. Our findings reinforce the importance of directly addressing such topics and carefully listening to the patient and care partner.
Hopwood and Stephens56 studied psychologic distress in patients with advanced lung cancer and reported a prevalence of the affective disorder varying from 23% to 47%. Depression was found in patients with more advanced stages of malignancy, more severe illness, and poorer physical status. Patients with lung cancer are similar to patients with IPF in regard to their prognosis and limited life expectancy. In 41 patients with IPF, De Vries and colleagues15 reported that approximately 25% experienced depressive symptoms. Our sample reported a lower incidence. This finding was potentially influenced by the support available to all patients, including a clinical nurse specialist, support group, and active research program.
Cognitive behavioral techniques have been widely used to managed depressive symptoms after a diagnosis of Alzheimer’s,57,58 end-stage lung disease,58 arthritis,59 or coronary artery disease,58 and in older adults.10 Cognitive behavioral interventions can benefit those who experience major depression, as well as those who experience depressive symptoms and anxiety.50,60 Patients with recurrent depression who were maintained at the same dose of their antidepressant medication, but received 6 cognitive therapy sessions, had a lower relapse rate than those who only had their antidepressant medication dose increased and did not receive cognitive therapy.60 The Program to Encourage Active, Rewarding Lives of Seniors study, which tested a cognitive behavioral intervention in patients with minor depression, found that patients who enrolled in the program had a 50% reduction in depressive symptoms, 43% had complete remission from depression, and 36% had improved HRQoL.61 No studies were identified that studied the impact of cognitive behavioral techniques in patients with IPF.
On the basis of qualitative data, the main benefits of PRISIM seemed to relate to feeling less isolated and better able to view IPF and its symptoms in perspective. The instruments selected to measure study outcomes focused on changes in symptom burden, perceived stress, and HRQoL and may not have captured changes that occurred as a result of the intervention. As an example, we did not measure self-efficacy (confidence) in dealing with this illness. It is possible that findings regarding the intervention would have differed if the study included different outcome measures or a longer follow-up interval. A longer follow-up was not judged feasible because 9 months after study entry, 7 patients had received lung transplants and 6 patients had died.
LIMITATIONS
The study was conducted to obtain pilot data for a future study, the sample size was small, and the study was underpowered to detect a difference between groups. Findings need to be confirmed in a larger sample. The requirement to attend multiple sessions was a barrier to recruitment, and an Internet-based intervention may have been more appealing. However, our format had the advantage of promoting discussion among participants and group leaders. Of note, some of the participants traveled 2 to 3 hours to attend the session. Participants were recruited from a center that specialized in management of patients with ILD. Both groups therefore likely received support/education although not in the same intense manner. Patients with IPF or their care partners without access to such support may have responded differently to the intervention. Because of variability in disease progression and time since onset of symptoms and referral, patients were assigned to a group on the basis of disease severity, rather than time since diagnosis. Using time since diagnosis as the basis for group assignment may have resulted in a different outcome. The rapid progression of IPF also served as a limitation. Nine months after study entry, 13 patients (62%) either received lung transplants or died. This outcome reinforces the need to develop interventions that prepare patients for end-of-life decision-making and dealing with symptom burden but limited our ability to conduct a more extended evaluation of the program.
CONCLUSIONS
PRISIM was innovative in its attempt to combine information about disease management with information included in palliative care programs and strategies designed to elicit discussion of preferences in end-of-life decision-making. The program enrolled the care partner and patient in an attempt to enhance sharing on difficult to discuss topics. Although questionnaire responses indicated the intervention tended to negatively affect participants, qualitative data suggested positive benefits. Further exploration of the impact of disease-management interventions in patients with advanced lung disease and their care partners is needed using both qualitative and quantitative methodology.
Acknowledgments
Funding for this study was provided by the Fairbanks-Horix Foundation.
Footnotes
Work was performed at the University of Pittsburgh Medical Center.
Drs Lindell, Olshansky, Song, Zullo, Gibson, Kaminski, and Hoffman have no conflict of interest.
References
- 1.Raghu G. Interstitial lung diseases: a clinical overview and general approach. In: Fishman Elias AJA, Fishman JA, Grippi MA, Kaiser LR, Senior RM, editors. Fishman’s pulmonary diseases and disorders. New York: McGraw-Hill; 1998. pp. 1037–53. [Google Scholar]
- 2.Ryu JH, Daniels CE, Hartman TE, Yi ES. Diagnosis of interstitial lung diseases. Mayo Clin Proc. 2007;82:976–86. doi: 10.4065/82.8.976. [DOI] [PubMed] [Google Scholar]
- 3.Hay J. Interstitial pulmonary fibrosis. In: Murray JA, Nadel JA, editors. Textbook of respiratory medicine. Philadelphia: WB Saunders; 1988. pp. 1079–96. [Google Scholar]
- 4.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–6. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 5.King TE., Jr Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med. 2005;172:268–79. doi: 10.1164/rccm.200503-483OE. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society, American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 7.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 8.Dauber JH, Gibson KF, Kaminski N. Interferon-gamma 1b in idiopathic pulmonary fibrosis: what we know and what must we learn. Am J Respir Crit Care Med. 2004;170:107–8. doi: 10.1164/rccm.2405001. [DOI] [PubMed] [Google Scholar]
- 9.Lindell KO, Jacobs SS. Idiopathic pulmonary fibrosis. Am J Nurs. 2003;103:32–42. doi: 10.1097/00000446-200304000-00016. quiz 43. [DOI] [PubMed] [Google Scholar]
- 10.Selman M, Carrillo G, Estrada A, Mejia M, Becerril C, Cisneros J, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS ONE. 2007;2:e482. doi: 10.1371/journal.pone.0000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez A, Rogers RM, Dauber JH. The prognosis of idiopathic pulmonary fibrosis. Am J Respir Cell Molec Biol. 2003;29(3 Suppl):S19–26. [PubMed] [Google Scholar]
- 12.Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE, Jr, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636–43. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson KF, Kaminski N. The mechanisms of idiopathic pulmonary fibrosis; can we see the elephant? Drug Discov Today Dis Mech. 2004;1:117–22. [Google Scholar]
- 14.Lewis KS. Emotional adjustment to a chronic illness. Lippincotts Prim Care Pract. 1998;2:38–51. [PubMed] [Google Scholar]
- 15.De Vries J, Kessels BL, Drent M. Quality of life of idiopathic pulmonary fibrosis patients. Eur Respir J. 2001;17:954–61. doi: 10.1183/09031936.01.17509540. [DOI] [PubMed] [Google Scholar]
- 16.Roche V. The hidden patient: addressing the caregiver. Am J Med Sci. 2009;337:199–204. doi: 10.1097/MAJ.0b013e31818b114d. [DOI] [PubMed] [Google Scholar]
- 17.Chang JA, Curtis JR, Patrick DL, Raghu G. Assessment of health-related quality of life in patients with interstitial lung disease. Chest. 1999;116:1175–82. doi: 10.1378/chest.116.5.1175. [DOI] [PubMed] [Google Scholar]
- 18.Martinez TY, Pereira CA, dos Santos ML, Ciconelli RM, Guimaraes SM, Martinez JA. Evaluation of the short-form 36-item questionnaire to measure health-related quality of life in patients with idiopathic pulmonary fibrosis. Chest. 2000;117:1627–32. doi: 10.1378/chest.117.6.1627. [DOI] [PubMed] [Google Scholar]
- 19.Clark M, Cooper B, Singh S, Cooper M, Carr A, Hubbard R. A survey of nocturnal hypoxaemia and health related quality of life in patients with cryptogenic fibrosing alveolitis. Thorax. 2001;56:482–6. doi: 10.1136/thorax.56.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jastrzebski D, Kozielski J, Banas A, Cebula T, Gumola A, Ziora D, Krzywiecki A. Quality of life during one-year observation of patients with idiopathic pulmonary fibrosis awaiting lung transplantation. J Physiol Pharmacol. 2005;56(Suppl 4):99–105. [PubMed] [Google Scholar]
- 21.Swigris JJ, Kuschner WG, Jacobs SS, Wilson SR, Gould MK. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax. 2005;60:588–94. doi: 10.1136/thx.2004.035220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swigris JJ, Gould MK, Wilson SR. Health-related quality of life among patients with idiopathic pulmonary fibrosis. Chest. 2005;127:284–94. doi: 10.1378/chest.127.1.284. [DOI] [PubMed] [Google Scholar]
- 23.Lynn J, Goldstein NE. Advance care planning for fatal chronic illness: avoiding commonplace errors and unwarranted suffering. Ann Intern Med. 2003;138:812–8. doi: 10.7326/0003-4819-138-10-200305200-00009. [DOI] [PubMed] [Google Scholar]
- 24.National Consensus Project for Quality Palliative Care. Clinical Practice Guidelines for quality palliative care, executive summary. J Palliat Med. 2004;7:611–27. doi: 10.1089/jpm.2004.7.611. [DOI] [PubMed] [Google Scholar]
- 25.Hansen-Flaschen J. Advanced lung disease. Palliation and terminal care. Clin Chest Med. 1997;18:645–55. doi: 10.1016/s0272-5231(05)70407-x. [DOI] [PubMed] [Google Scholar]
- 26.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–84. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 27.Pasacreta J, Minarik PA, Nield-Anderson L. Anxiety and depression. In: Ferrell BC, Coyle N, editors. Textbook of palliative nursing. 2. New York: Oxford University Press; 2006. [Google Scholar]
- 28.Wingate BJ, Hansen-Flaschen J. Anxiety and depression in advanced lung disease. Clin Chest Med. 1997;18:495–505. doi: 10.1016/s0272-5231(05)70397-x. [DOI] [PubMed] [Google Scholar]
- 29.McSweeney AJ. Health status measurement in chronic obstructive pulmonary disease. Dis Mon. 1995;41:11–7. [Google Scholar]
- 30.Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax. 2001;56:880–7. doi: 10.1136/thorax.56.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swigris JJ, Stewart AL, Gould MK, Wilson SR. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61. doi: 10.1186/1477-7525-3-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Ogawa T, Watanabe F, Nishimura K. Health-related quality of life in patients with idiopathic pulmonary fibrosis. What is the main contributing factor? Respir Med. 2005;99:408–14. doi: 10.1016/j.rmed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Tomioka H, Imanaka K, Hashimoto K, Iwasaki H. Health-related quality of life in patients with idiopathic pulmonary fibrosis—cross-sectional and longitudinal study. Intern Med. 2007;46:1533–42. doi: 10.2169/internalmedicine.46.6218. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan V, McCormack MC, Mathai SC, Agarwal S, Richardson B, Horton, et al. Sleep quality and health-related quality of life in idiopathic pulmonary fibrosis. Chest. 2008;134:693–8. doi: 10.1378/chest.08-0173. [DOI] [PubMed] [Google Scholar]
- 35.Spiegel D, Bloom JR, Yalom I. Group support for patients with metastatic cancer. A randomized outcome study. Arch Gen Psychiatry. 1981;38:527–33. doi: 10.1001/archpsyc.1980.01780300039004. [DOI] [PubMed] [Google Scholar]
- 36.Connor SR. Denial in terminal illness: to intervene or not to intervene. Hosp J. 1992;8:1–15. doi: 10.1080/0742-969x.1992.11882739. [DOI] [PubMed] [Google Scholar]
- 37.Greenstein M, Breitbart W. Cancer and the experience of meaning: a group psychotherapy program for people with cancer. Am J Psychother. 2000;54:486–500. doi: 10.1176/appi.psychotherapy.2000.54.4.486. [DOI] [PubMed] [Google Scholar]
- 38.Butler LD, Koopman C, Cordova MJ, Garlan RW, DiMiceli S, Spiegel D. Psychological distress and pain significantly increase before death in metastatic breast cancer patients. Psychosom Med. 2003;65:416–26. doi: 10.1097/01.psy.0000041472.77692.c6. [DOI] [PubMed] [Google Scholar]
- 39.Burns D. Feeling good: the new mood therapy. New York: Avon Books; 1999. [Google Scholar]
- 40.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire, 113. San Diego. Chest: University of California; 1998. pp. 619–24. [DOI] [PubMed] [Google Scholar]
- 41.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1998;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 42.Fydrich T, Dowdall D, Chambless DL. Reliability and validity of the Beck Anxiety Inventory. J Anxiety Disord. 1992;6:55–61. [Google Scholar]
- 43.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 44.Beck AT, Steer RA, Brown GK. Beck Depression Inventory manual. 2. New York: The Psychological Corporation, Harcourt Brace and Company; 2002. [Google Scholar]
- 45.Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the Beck Depression Inventory. A review. Psychopathology. 1998;31:160–8. doi: 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- 46.Cohen S, Hoberman H. Positive events and social support as buffers of life change stress. J Appl Soc Psychol. 1983;13:99–125. [Google Scholar]
- 47.Farabaugh AH, Mischoulon D, Fava M, Green C, Guyker W, Alpert J. The potential relationship between levels of perceived stress and subtypes of major depressive disorder (MDD) Acta Psychiatr Scand. 2004;110:465–70. doi: 10.1111/j.1600-0447.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- 48.Golden-Kreutz DM, Browne MW, Frierson GM, Andersen BL. Assessing stress in cancer patients: a second-order factor analysis model for the Perceived Stress Scale. Assessment. 2004;11:216–23. doi: 10.1177/1073191104267398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen CH, Tseng YF, Chou FH, Wang SY. Effects of support group intervention in postnatally distressed women. A controlled study in Taiwan. J Psychosom Res. 2000;49:395–9. doi: 10.1016/s0022-3999(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 50.van Dulmen AM, Fennis JF, Bleijenberg G. Improved prognosis of functional abdominal complaints by attending to psychic factors. Ned Tijdschr Geneeskd. 1998;142:641–5. [PubMed] [Google Scholar]
- 51.Ware JE, Kosinski MA, Keller SD. SF-36 Physical and Mental Health Summary scales: a user’s manual. Boston, MA: The Health Institute. New England Medical Center; 1994. [Google Scholar]
- 52.Boueri FM, Bucher-Bartelson BL, Glenn KA, Make BJ. Quality of life measured with a generic instrument (Short Form-36) improves following pulmonary rehabilitation in patients with COPD. Chest. 2001;119:77–84. doi: 10.1378/chest.119.1.77. [DOI] [PubMed] [Google Scholar]
- 53.Hansen-Flaschen JH. Palliative home care for advanced lung disease. Respir Care. 2000;45:1478–89. [PubMed] [Google Scholar]
- 54.Rubenfeld GD, Curtis JR. Palliative respiratory care. Respir Care. 2000;45:1318–9. [PubMed] [Google Scholar]
- 55.Heffner JE, Fahy B, Hilling L, Barbieri C. Attitudes regarding advance directives among patients in pulmonary rehabilitation. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1735–40. doi: 10.1164/ajrccm.154.6.8970363. [DOI] [PubMed] [Google Scholar]
- 56.Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18:893–903. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- 57.Gitlin LN, Belle SH, Burgio LD, Czaja SJ, Mahoney D, Gallagher-Thompson D. Effect of multicomponent interventions on caregiver burden and depression: the REACH multisite initiative at 6-month follow-up. Psychol Aging. 2003;18:361–74. doi: 10.1037/0882-7974.18.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Napolitano MA, Babyak MA, Palmer S, Tapson V, Davis RD, Blumenthal JA. Effects of a telephone-based psychosocial intervention for patients awaiting lung transplantation. Chest. 2002;122:1176–84. doi: 10.1378/chest.122.4.1176. [DOI] [PubMed] [Google Scholar]
- 59.Lin EH, Katon W, Von Korff M, Tang L, Williams JW, Jr, Kroenke K. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA. 2003;290:2428–9. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- 60.Fava GA, Ruini C, Rafanelli C, Grandi S. Cognitive behavior approach to loss of clinical effect during long-term antidepressant treatment: a pilot study. Am J Psychiatry. 2002;159:2094–5. doi: 10.1176/appi.ajp.159.12.2094. [DOI] [PubMed] [Google Scholar]
- 61.Ciechanowski P, Wagner E, Schmaling K, Schwartz S, Williams B, Diehr P. Community-integrated home-based depression treatment in older adults: a randomized controlled trial. JAMA. 2004;291:1569–77. doi: 10.1001/jama.291.13.1569. [DOI] [PubMed] [Google Scholar]


