Abstract
Studies have suggested that manipulations of the central melanocortin circuitry by pharmacological agents produce robust effects on the regulation of body weight and glucose homeostasis. In this review, we discuss recent findings from genetic mouse models that have further established the physiological relevance of this circuitry in the context of glucose and energy balance. In addition, we will discuss distinct neuronal populations that respond to central melanocortins to regulate food intake, energy expenditure, insulin sensitivity, and insulin secretion, respectively. Finally, multiple hormonal and neural cues (e.g., leptin, estrogen, and serotonin) that use the melanocortin systems to regulate energy and glucose homeostasis will be reviewed. These findings suggest that targeting the specific branches of melanocortin circuits may be potential avenues to combat the current obesity and diabetes epidemics.
Keywords: melanocortins, leptin, estrogen, serotonin, body weight
Introduction
The use of genetic mouse models has catalyzed substantial advances in the understanding about how the central nervous system (CNS) provides a coordinated control of energy and glucose homeostasis. While numerous molecules in the brain and neural structures play key roles in regulating energy and glucose balance and deserve attention, the current review will focus on the central melanocortin system. As illustrated below, the physiological significance of the central melanocortin system has gone beyond the regulation of feeding and body weight. Current evidence indicates that central melanocortins also regulate insulin sensitivity and glucose homeostasis through distinct CNS populations expressing melanocortin receptors. We will also review evidence supporting the role of the melanocortin system as the key mediator for multiple metabolic cues, such as leptin, estrogens, and serotonin.
The central melanocortin system
The central melanocortin system comprises neurons that produce endogenous melanocortins and the downstream neurons that express melanocortin receptors.1-3 The melanocortin neurons include those expressing pro-opiomelanocortin (POMC) and those expressing neuropeptide Y (NPY) and agoutirelated peptide (AgRP), which are both located in the arcuate nucleus (ARC). While POMC neurons synthesize and secrete an anorexigenic peptide, α-melanocyte–stimulating hormone (α-MSH), to activate melanocortin receptors, NPY/AgRP neurons release orexigenic peptides, NPY, and AgRP.1-3 Notably, AgRP is the endogenous antagonist of the melanocortin receptors.1-3 POMC and NPY/AgRP populations have been long believed to be the primary central regulators of energy homeostasis.4, 5
The anorexigenic property of POMC neurons has been well established, as the deletion of POMC gene causes hyperphagia and obesity.6 However, neither single deletion of AgRP or NPY nor double deletion of AgRP and NPY in mice leads to abnormality in food intake and body weight.7 These results are not in agreement with pharmacological studies showing that treatment of AgRP or NPY leads to an increase in food intake.8, 9 It has been argued that the lack of phenotypes in animals with gene mutations at the embryonic stage could result from possiblegenetic compensations during early development. To circumvent this issue and establish the role of NPY/AgRP neurons in the control of body weight, several groups have used distinct genetic mouse models to achieve selective ablation of NPY/AgRP neurons during adulthood. For example, Palmiter and colleagues used a mouse model with the Creinducible diphtheria toxin receptor (DTR). Crossing these mice with AgRP-Cre transgenic mice generated mice with DTR expressed only in AgRP-expressing cells. Injections of diphtheria toxin into these mice results in selective ablation of NPY/AgRP neurons. They found that ablation of NPY/AgRP neurons during adulthood leads to rapid decreases in food intake and body weight.10 Similarly, Barsh and colleagues crossed AgRP-Cre transgenic mice to a loxP-flanked mitochondrial transcription factor A (Tfam) allele to selectively delete Tfam from AgRP cells, which causes progressive loss of this population as animals grow.11 These mice with NPY/AgRP ablation display modest lean phenotypes.11 Finally, targeted expression of a neurotoxic ataxin-3 to AgRP-expressing neurons resulted in loss of the NPY/AgRP neurons and decreases in food intake and body weight.12 Results from these three different models with genetic ablation of NPY/AgRP neurons all support a physiological role of these neurons in promoting feeding and body weight gain. This notion has been further supported by recent studies using genetic tools to selectively manipulate electrophysiological properties of NPY/AgRP neurons. For example, Aponte and colleagues generated mice expressing the light-activated cation channel, channelrhodopsin-2 (ChR2), only in NPY/AgRP neurons.13 Light stimulation in these mice induces rapid activation of NPY/AgRP neurons, which results in increased feeding.13 Similarly, Krashes and colleagues used Designer-Receptors-Exclusively-Activated-by-Designer-Drugs (DREADD)14, 15 to rapidly depolarize or hyperpolarize NPY/AgRP neurons in mice.16 While depolarization of NPY/AgRP neurons promotes eating, hyperpolarization of these neurons inhibits eating.16 Collectively, these genetic mouse models with NPY/AgRP ablation or stimulation/inhibition during adulthood demonstrate a physiological role of NPY/AgRP neurons in the control of energy homeostasis. The discrepancy between these recent studies10-13, 16 and those with early embryonic gene deletion7 may indicate that other neuronal populations (e.g., POMC neurons) undergo adaptive changes to compensate for the loss of NPY/AgRP during early development.
It is important to note that NPY/AgRP ablation or stimulation/inhibition models cannot rule out the possibility that other neuropeptides or neurotransmitters released by these neurons may contribute to the regulation of energy homeostasis. Indeed, Aponte and colleagues demonstrated that increased feeding induced by activation of NPY/AgRP neurons does not require the melanocortin receptors,13 suggesting that these neurons may release neurotransmitters other than AgRP to regulate feeding. NPY released from these NPY/AgRP neurons could certainly be one of these neurotransmitters. Alternatively, NPY/AgRP neurons also release GABA, a classic neurotransmitter that has been implicated in the control of body weight.10, 17 To evaluate the physiological relevance of GABA release from NPY/AgRP neurons, Tong and colleagues generated a mouse model carrying loxP-flanked alleles encoding the vesicular GABA transporter (VGAT), which is required for presynaptic release of GABA.18 Crossing these loxed VGAT mice with knockin AgRP-IRES-Cre mice produced mice with selective deletion of VGAT in NPY/AgRP neurons. Mice lacking VGAT in NPY/AgRP neurons have reduced GABA release, and these mice display reduced body weight primarily due to increased energy expenditure.19 These findings provide genetic evidence that GABA release from NPY/AgRP neurons is required to maintain normal energy homeostasis. It has been suggested that NPY/AgRP neurons provide a direct GABAergic input to POMC neurons, which forms a neural network to mediate actions of various metabolic signals.10, 17, 20, 21 This notion is further supported by the observations that the inhibitory postsynaptic currents (both at the basal condition and after ghrelin treatment) recorded from identified POMC neurons are significantly attenuated in mice lacking VGAT in NPY/AgRP neurons. In addition, the orexigenic effects of ghrelin are significantly blunted in these mutant mice.19 In addition to this GABAergic AgRP-POMC network, recent evidence from the Palmiter group demonstrated that GABA released from NPY/AgRP neurons also acts on neurons in the parabrachial nucleus to maintain normal feeding behavior.22
Neurons expressing melanocortin receptors receive inputs from POMC neurons and NPY/AgRPneurons to regulate energy and glucose homeostasis. In particular, melanocortin receptor 3 and 4 (MC3Rs and MC4Rs) have been demonstrated to be the most relevant melanocortin receptors in the context of energy and glucose homeostasis.5, 23, 24 For example, MC3Rs are required to mediate melanocortin actions on energy expenditure, as MC3R knockout mice show decreased energy expenditure and increased sensitivity to diet-induced obesity.23, 25 Effects of MC3Rs on food intake are not yet fully understood. Initial characterization of MC3R knockout mice showed that mutants eat less when fed with chow, and no difference in food intake was observed when fed with a high-fat diet (HFD).23, 25 However, Butler and colleagues recently reported that MC3R knockout mice eat more during the light cycle, but not in the dark cycle.26 This suggests that MC3Rs may be involved in the circadian control of feeding behavior. Similarly, Butler and colleagues recently demonstrated that MC3R knockout mice, when subjected to a light-cycle feeding paradigm, exhibit hyperinsulinemia and glucose intolerance compared to wild-type controls.27 These findings support the notion that MC3Rs in the brain are important for the circadian control of glucose and energy homeostasis.
MC4Rs are also involved in the regulation of energy and glucose homeostasis. For example, mutations in MC4R gene in mice5 or humans28, 29 lead to obesity. Data obtained from MC4R knockout mice indicate that central MC4R signals contribute to the control of body weight balance by regulating both food intake and energy expenditure.5, 30, 31 Recent evidence also demonstrated that the central melanocortin system directly controls peripheral lipid and glucose metabolism, effects that are independent of its role in food intake and energy expenditure.32 An interesting question is which MC4R populations in the CNS are responsible for each of these distinct functions, given that MC4Rs are expressed in multiple relevant CNS sites.33-35 The Lowell and Elmquist laboratories developed a loxTB MC4R null mouse model whose MC4R expression is globally disrupted by a loxP-flanked transcriptional blocking cassette (loxTB) inserted into the MC4R gene.36 These loxTB MC4R null mice display hyperphagia, lower energy expenditure, obesity, hyperinsulinemia, and hyperglycemia,36 phenotypes identical to those seen in the conventional MC4R knockout mice.5 Uniquely, the loxTB cassette can be removed by the Cre-recombinase, which results in reactivation of MC4R expression. The loxTB MC4R null mice were crossed with SIM1-Cre transgenic mice to restore MC4R expression only in SIM1 neurons in the paraventricular nucleus of the hypothalamus (PVH) and the amygdala.36 This manipulation markedly improves the obesity seen in loxTB MC4R null mice. Notably, the hyperphagia is completely rescued, while reduced energy expenditure, hyperglycemia, and hyperinsulinemia are unaffected.36 These findings demonstrate that MC4Rs expressed by SIM1 neurons in the PVH and the amygdala control food intake, but not energy expenditure and glucose/insulin balance.
We have recently crossed the loxTB MC4R null mice with the ChAT-IRES-Cre and Phox2b-Cre mice, respectively.37 The ChAT-IRES-Cre restored MC4R expression in all cholinergic neurons, which include both the sympathetic preganglionic neurons in the intermediolateral column (IML) and the parasympathetic preganglionic neurons in the dorsal motor nucleus of the vagus (DMV); the Phox2b-Cre led to selective reexpression of MC4Rs in autonomic control neurons, including the parasympathetic preganglionic neurons in the DMV.37 We found that while MC4Rs in cholinergic neurons (both sympathetic and parasympathetic neurons) are sufficient to increase energy expenditure and partially rescue the obesity seen in loxTB MC4R mice, reexpression of MC4Rs in Phox2b neurons (parasympathetic neurons) does not significantly affect energy expenditure and body weight.37 Therefore, these findings suggest that MC4Rs expressed in the sympathetic preganglionic neurons in the IML are important for the control of energy expenditure. Further, we found that reexpression of MC4Rs in cholinergic neurons attenuates both hyperglycemia and hyperinsulinemia.37 In addition, euglycemic–hyperinsulinemic clamp studies revealed that hepatic insulin action and insulin-mediated suppression of hepatic glucose production are improved in mice with MC4Rs reexpressed in cholinergic neurons.37 In contrast, restoration of MC4Rs in Phox2b neurons only attenuates hyperinsulinemia, but has fewer effects on glucose levels.37 Based on these findings, we suggest that MC4Rs expressed in the sympathetic preganglionic neurons in the IML are involved in the regulation of insulin sensitivity in liver and hepatic glucose production, whereas MC4Rsexpressed by parasympathetic neurons in the DMV may control insulin secretion from the pancreas.
Leptin
Leptin is a circulating adipokine that plays critical roles in the regulation of body mass and body composition.38 Leptin contributes to the regulation of body weight by influencing both food intake39, 40 and energy expenditure.41-43 Biological actions of leptin are thought to be primarily mediated by the long-form leptin receptor (also known as LEPR-B).44 Accumulating evidence indicates that leptin produces antiobesity effects by acting via LEPR-B in the brain.45 For example, CNS-specific deletion of LEPR-B results in marked obesity.46, 47 In contrast, transgenic, brain-specific reconstitution of LEPR-B in LEPR-deficient (db/db) mice ameliorates obesity.48-50
LEPR-B is abundantly expressed in several sites within the hypothalamus, including the ARC, the dorsomedial nucleus of the hypothalamus, the lateral hypothalamic area, and the ventromedial hypothalamic nucleus (VMH).51-56 Taking advantage of the Cre-loxP genetic animal models that allow manipulations of LEPR-B in a cell- or site-specific manner, the relative importance of leptin action at these different sites is beginning to be understood.
The melanocortin pathway is downstream of leptin actions. Particularly, leptin directly depolaizes POMC neurons.21, 57 Furthermore, fasted rodents (a condition of reduced leptin levels) and leptin-deficient (ob/ob) mice both have decreased hypothalamic POMC mRNA content, which can be normalized by exogenous leptin administration.58-60 These findings support the possibility that leptin acts on LEPR-B expressed by POMC neurons to regulate body weight balance. To directly test this possibility, we have previously crossed the loxPflanked LEPR-B allele with the POMC-Cre transgene, which resulted in selective deletion of endogenous LEPR-B from POMC neurons.20 We demonstrated that this deletion causes modest obesity primarily because of decreased energy expenditure.20, 61 Bjørbæk and colleagues have recently crossed actindriven loxTB LEPR-B alleles with the POMC-Cre transgene, which results in reexpression of LEPR-B in all POMC neurons (including those that do not endogenously express LEPR-B) on the db/db background.62 The reexpression leads to a modest reduction in body weight.62 Collectively, the deletion and reexpression models provide consistent evidence to support that POMC neurons are one physiologically relevant site that mediates leptin actions on body weight control. Of note, while selective deletion of LEPR-R from POMC neurons does not significantly affect food intake,20, 61 reexpression of LEPR-R in all POMC neurons partially rescue hyperphagia seen in db/db mice.62 This discrepancy can be interpreted to suggest that LEPR-B is only expressed in POMC neurons that regulate energy expenditure but are not major regulators of food intake. Alternatively, the lack of feeding phenotype in deletion models is due to compensatory effects of other brain regions expressing LEPR-B.
Indeed, leptin has been shown to act on a subset of neurons in the VMH, namely steroidogenic factor 1 (SF1) neurons. SF1 is a transcription factor that is expressed exclusively in the VMH within the brain.63 Deletion of SF1 in mice disrupts VMH structure64 and leads to obesity.65 We found that leptin directly depolarizes SF1 neurons via LEPR-B-mediated mechanisms.66 In addition, selective deletion of LEPR-B in SF1 neurons produces modest obesity,66, 67 indicating that LEPR-B expressed by SF1 neurons is also required to maintain normal body weight. Interestingly, mice lacking LEPR-B only in SF1 neurons show impaired thermogenic responses to acute HFD challenge,66 suggesting that leptin actions via SF1 neurons are required to mediate the appropriate thermogenic responses to overnutrition.
Actions of leptin in the CNS have been implicated in glycemic control (as reviewed in Refs. 68 and 69). Many of these leptin regulations on glucose homeostasis appear to be mediated by LEPR-B expressed in the hypothalamus. For example, it has been shown that while partial restoration of LEPR-B in the ARC on the LEPR-B null background only modestly decreases body weight, such manipulation remarkably improves hyperinsulinemia and completely normalizes hyperglycemia seen in LEPR-B null mice.70 Similarly, LEPR-B reexpression in all POMC neurons on the db/db background produces robust improvements in the glucose/insulin profile, while body weight is only modestly reduced.62 Moreover, SF1-specific deletion of LEPR-B causes insulin resistance before onset of obesity.67 Consistent with findings that acute infusion of leptin in the third cerebral ventricle markedly inhibits liver glycogenolysis and suppresses glucose productionin rodents,71 phenotypes observed in these genetic mouse models support that leptin actions in the ARC (e.g., POMC neurons) and in SF1 neurons are required to regulate insulin sensitivity and glucose homeostasis andthat these effects are likely independent of leptin effects on body weight and adiposity.
In addition to the aforementioned hypothalamic neurons, emerging evidence suggests that leptin may also act on extra-hypothalamic sites to regulate feeding behavior and body weight balance. For example, Hayes and colleagues have recently shown that knock-down of LEPR-B in the nucleus of solitary tract (NTS) and area postrema (AP) via stereotaxic injections of AAV-shRNA leads to increased susceptibility to diet-induced obesity.72 Development of obesity in these animals is not due to alterations in energy expenditure, but rather to impaired satiation and increased food intake.72 Consistently, Scott and colleagues. demonstrated that selective deletion of LEPR-B in the NTS produces hyperphagia in mice.73 Thus, these results suggest that LEPR-B expressed in the NTS/AP is required to mediate the anorexigenic effects of leptin.
Leptin modulates multiple intracellular signaling cascades that in turn lead to alteration of gene expression profiles and changes of neuronal electrophysiological activity. As LEPR-B is a member of the class I cytokine receptor superfamily that commonly activates Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway,44 the JAK/STAT3 pathway is thought to be a central mediator of leptin actions. Indeed, leptin rapidly induces tyrosine phosphorylation on both JAK2 and STAT3 in the hypothalamus. Knock-in mice in which the STAT3 binding site on LEPR-B (Tyr 1138) is mutated display hyperphagic and obese phenotypes as db/db mice that completely lack LEPR-B.74 In addition, deletion of STAT3 from the entire population of the leptin-responsive cells results in hyperphagia and obesity.75 Interestingly, no deficits in reproduction and glucose homeostasis were observed in these mouse lines, suggesting that other signaling pathways rather than STAT3 may be involved.
Leptin also modulates other signaling pathways, including the PI3K/Akt pathway,76-79 the mTOR/S6K pathway,80, 81 and the AMPK pathway.82 Leptin increases the levels of PIP3, 83, 84 a catalyzed product of PI3K. Pharmacological and genetic studies have shown that the PI3K pathway is required to mediate leptin actions to acutely suppress food intake85 and to depolarize POMC neurons.76-78 The hypothalamic mTOR/S6K pathway is another indispensable signaling mechanism mediating leptin anorexigenic actions.86 Leptin inhibits 5′-AMP-activated protein kinase (AMPK) in the hypothalamus. Expression of the constitutively active form of AMPK attenuates leptin’s anorexigenic effects.82 However, the molecular links between LEPR-B and PI3K, mTOR/S6K, or AMPK are not fully understood.
Leptin signaling is negatively regulated by suppressor of cytokine signaling-3 (SOCS-3)87 and protein tyrosine phosphatase 1B (PTP1B).88, 89 SOCS-3 and PTP1B are increased within the hypothalamus in a state of excess nutrition/obesity.89-92 Overexpression of SOCS-3 or PTP1B in vitro attenuates leptin-induced STAT3 activation.88, 89 Mice with brain-specific SOCS-3 deletion are protected from diet-induced obesity and leptin resistance.93, 94 PTP1B knockout mice are likewise resistant to dietinduced obesity.88, 89, 95 Deletion of SOCS3 from POMC neurons results in increased leptin sensitivity and improves glucose homeostasis despite normal body weight gain.96 In addition, mice lacking PTP1B only in POMC neurons show reduced body weight, enhanced leptin sensitivity, and increased energy expenditure.97 Interestingly, a direct comparison among brain-specific knockout mice of SOCS-3, PTP1B, or both suggests that brain functions of SOCS-3 and PTP1B do not completely overlap in terms of control of energy homeostasis.98 Collectively, SOCS-3 and PTP1B contribute to cellular leptin resistance.
Estrogens
Ovarian estrogens exert important antiobesity effects in women and female mammals. For example, lower levels of estrogens in postmenopausal women are associated with an increased risk for developing obesity.99-101 Ovariectomized (OVX) animals with reduced estrogen signaling develop obesity and hyperadiposity.102-104 Although OVX induces a transient increase in food intake, the hyperphagia does not seem to account for the development of obesity.104 Further, OVX rats gain weight to a similar extent when they are pair-fed compared to estradioltreated rats,105, 106 suggesting that endogenous estrogens regulate body weight homeostasis primarily by modulating energy expenditure. However, estradiol replacement was shown to decrease food intake and increase energy expenditure in rodents,107 indicating that exogenous estrogens may promote a negative energy balance by influencing both energy intake and energy expenditure. Importantly, estrogens are also thought to play a role in regulating fat distribution. For example, female humans and rodents distribute relatively more fat in subcutaneous depot, while males have more fat stored in visceral depot, which is more likely to cause metabolic syndromes such as insulin resistance.108-110 Estrogens appear to account for this sexual dimorphism because the differences in the fat distribution between premenopausal females and age-matched men are abolished between postmenopausal females and age-matched men.111
Estrogen receptor-α (ERα), one of the estrogen receptors, is believed to mediate most estrogenic effects on energy homeostasis. For example, female mice with a targeted deletion in the ERα gene (ERαKO) develop obesity and hyperadiposity, primarily due to decreased energy expenditure.112 Although no hyperphagia is observed in ERαKO mice,112-114 ERα is clearly required to mediate normal satiation process because estradiol-induced hypophagia and CCK-induced satiation in wild-type mice are blocked in ERαKO mice.114
ERα is expressed in brain regions implicated in the regulation of energy balance. These include the PVH, medial preoptic area (MPOA), ARC, VMH, and NTS, etc.115, 116 In earlier attempts to determine the effects of estrogen on food intake and body weight in these CNS regions, intranuclear microinjections and lesions were often used. However, due to the inherent difficulty in precisely placing cannulae or producing lesions in small but complex brain regions, findings obtained from these studies are difficult to reproduce and interpret.117-122
Recently, the role of estrogens and ERα in the VMH in the regulation of energy balance has been reexamined using the ERα silencing approach.123 In this study, ERα in the VMH is knocked down with an AAV-shRNA.123 Animals with impaired ERα signaling in the VMH are less sensitive to estradiol-induced weight loss and develop obesity characteristic of increased visceral fat.123 The obesity syndrome is likely caused by decreased physical activity and impaired diet-induced thermogenesis, whereas food intake of these animals is not directly affected.123 We recently generated mice with ERα deleted in VMH SF1 neurons.124 We found that deletion of ERα in VMH SF1 neurons in female mice, while not affecting food intake, significantly reduces basal metabolic rate and diet-induced thermogenesis, which consequently results in increased body weight and hyperadiposity.124 Interestingly, a significant increase in visceral fat deposition (versus subcutaneous fat deposition) was observed in these mutant females.124 Finally, we showed that the decreased energy expenditure and increased visceral fat distribution in mice lacking ERα in SF1 neurons presumably results from decreased sympathetic tone (as demonstrated by decreased plasma norepinephrine levels).124 Our findings are largely consistent with those obtained from the VMH-specific ERα knock-down model. Collectively, these results support the hypothesis that ERα signaling in VMH neurons (e.g., SF1 neurons) plays an important role in regulating energy expenditure and fat distribution.
A recent study demonstrated that NPY/AgRP neurons are required to mediate the anorexigenic effects of estrogens. In this study, Xu and colleagues showed that hypothalamic expression of NPY and AgRP in wild-type mice is tightly regulated across the estrus cycle, with the lowest levels during the estrus, which coincides with the plasma estrogen peak and feeding nadir.125 They further showed that central estradiol administration suppresses fastinginduced c-Fos activation in NPY/AgRP neurons and blunts the refeeding response.125 Importantly, the cyclic changes in food intake and estradiolinduced anorexia are blunted in mice with degenerated NPY/AgRP neurons.125 This study indicates that NPY/AgRP neurons are functionally required for the cyclic changes in feeding across estrous cycles. Surprisingly, these authors also found that ERα is not expressed in NPY/AgRP neurons,125 suggesting that estrogen may regulate these neurons indirectly via presynaptic neurons that express ERα (e.g., POMC neurons).
Indeed, POMC neurons coexpress ERα.124, 126, 127 In addition, estrogens regulate excitability of POMC neurons. Using electron microcopy, Horvath and colleagues have reported that the number of excitatory synaptic inputs to ARC POMC neurons rises as mice enter proestrus when estrogen levels arehigh.107 Further, central estradiol administration rapidly increases the excitatory synapses on POMC neurons, an effect that is also reflected by increasedminiature excitatory postsynaptic current recorded from POMC neurons.107 These synaptological rearrangements in POMC neurons are tightly paralleled with the effects of estradiol on food intake and body weight.107 Similarly, Ronnekleiv and colleagues reported that estradiol administration in hypothalamic slices activates POMC neurons by rapidly uncoupling GABAB receptors from the G protein–gated inwardly rectifying K+ channels.128 Importantly, we recently demonstrated that female mice lacking ERα in POMC neurons only develop hyperphagia.124 These observations, together with the findings from the Horvath and Ronnekleiv groups, indicate that estrogen/ERα signals inPOMC neurons are physiologically relevant in the regulation of food intake.124
Serotonin
Central serotonin (5-HT) systems play critical roles in the suppression of feeding. Brain 5-HT is primarily synthesized by neurons in the dorsal raphe nucleus (DRN) in the midbrain, which have projections to the hypothalamus.129 5-HT release from the DRN is rapidly enhanced after each meal,130 suggesting that increased 5-HT bioavailability may participate in the regulation of feeding behavior. Indeed, fenfluramine, a pharmacological agent that increases serotonin content by stimulating synaptic release of serotonin and blocking its reuptake into presynaptic terminals,131 shows a potent anorexigenic activity in rodents and humans.132-134 Conversely, treatments that suppress central serotoninergic signaling produce hyperphagia and weight gain in humans and rodents.135-138 At least 14 serotonin receptors have been cloned, and many of these receptors have been implicated in the regulation of food intake and body weight.139 In particular, the 5-HT2C receptor (5-HT2CR), which is exclusively expressed in the CNS,139 has been shown to mediate a significant portion of the anorexigenic effects of central serotonin systems. For example, relatively selective 5-HT2CR agonists, including mCPP, promote satiety and produce hypophagia. These effects are blocked by 5-HT2CR antagonists or in 5-HT2CR knockout animals.140-144 Notably, deletion of 5-HT2CRs causes hyperphagia and obesity in mice,144, 145 indicating that the endogenous 5-HT2CR signal is a physiological regulator of feeding. In addition, mutations in the 5-HT2CR gene have been recently linked to several obesity conditions seen in humans. For instance, commonly used atypical antipsychotic drugs (e.g., clozapine and olanzapine) have been reported to cause serious weight gain, which may be associated with their 5-HT2CR antagonist properties and with polymorphisms in 5-HT2CR gene.146, 147 Furthermore, a splicing variant of 5-HT2CR with impaired function has been suggested to contribute to hyperphagia and obesity in patients with Prader-Willi syndrome.148
Recent studies have demonstrated that 5-HT2CRs are also involved in glycemic control, actions that are independent of their effects on food intake and body weight. For example, deletion of 5-HT2CRs in ob/ob mice leads to synergistic impairment of glucose balance, while such double deletion does not lead to more severe obese phenotypes compared to ob/ob mice.149 In addition, mCPP administration at a subthreshold dose (1 mg/kg), which does not affect food intake and body weight, ameliorates insulin resistance and glucose intolerance in mice with diet-induced obesity.150 Collectively, these findings indicate that both endogenous 5-HT2CRs and exogenous drugs that activate 5-HT2CRs exert antiobesity and antidiabetic effects.
5-HT2CRs are widely expressed in the brain,151 and the physiological relevant sites of 5-HT2CRs that regulate body weight and glucose balance are difficult to identify due to the lack of commercially available 5-HT2CR–selective drugs. Using neuroanatomy, electrophysiology, and genetic mouse models, several groups have demonstrated that POMC neurons are one of the physiologically important targets of 5-HT2CR signals in the context of energy homeostasis. For example, POMC neurons coexpress 5-HT2CRs152 and receive inputs from 5-HT–immunoreactive nerve terminals from the DRN.153 These anatomical findings are further supported by electrophysiological studies showing that 5-HT compounds, including fenfluramine and mCPP, activate POMC neurons, effects that are blocked by 5-HT2CR antagonists.152, 154 In addition, 5-HT2CR agonists increase POMC expression in the ARC.150, 155 Collectively, these findings indicate that a 5-HT2CR–melanocortin circuit may provide the anatomical basis to mediate the anorexigenic actions of 5-HT compounds (e.g., fenfluramine).
The physiological relevance of this 5-HT2CR–melanocortin circuitry is established using genetic mouse models. First of all, we showed that the anorexigenic action of fenfluramine is blunted in Ay mice101 or MC4R knockout mice,156 suggesting that the intact central melanocortin system is required to mediate the pharmacological actions of 5-HT. Recently, we generated a loxTB 5-HT2CR null mouse model in which expression of 5-HT2CRs is disrupted globally by inserting a loxP-flanked transcriptional blocker cassette.157 Crossing these loxTB 5-HT2CR null mice with transgenic POMC-Cre mice produced 2C/POMC mice in which 5-HT2CR is expressed only in POMC neurons. We found that loxTB 5-HT2CR null mice predictably develop hyperphagia and obesity and show attenuated anorexigenic responses to fenfluramine and mCPP.158 All of these deficiencies are normalized in 2C/POMC mice.158 Notably, energy expenditure is not affected in either loxTB 5-HT2CR null mice or 2C/POMC mice.158 These results highlight the physiological functions of the 5-HT2CR–melanocortin circuitry in the control of food intake and body weight. We recently demonstrated that while the anorexigenic effects of fenfluramine are abolished in mice with global MC4R deficiency, these effects can be restored in mice with MC4Rs reexpressed only in SIM1 neurons in the PVH and the amygdala.159 These observations further support the model that 5-HT compounds (e.g., fenfluramine) act on 5-HT2CRs expressed by POMC neurons to stimulate secretion of α-MSH, which in turn activates MC4Rs expressed by SIM1 neurons in the PVH and amygdala to suppress food intake.
The 5-HT2CR–melanocortin circuitry is also physiologically relevant in the regulation of insulin sensitivity and glucose homeostasis. For example, it has been shown that mCPP improves glucose tolerance and insulin sensitivity in wild-type mice with diet-induced obesity, while such antidiabetic effects are blunted in mice lacking MC4Rs.150 In addition, young loxTB 5-HT2CR null mice develop insulin resistance in the liver, phenotypes that are independent of hyperphagia and obesity.160 Notably, insulin resistance is normalized by reexpression of 5-HT2CRs only in POMC neurons (2C/POMC mice).160 In addition, we demonstrated that while the global deletion of 5-HT2CRs abolishes antidiabetic effects of mCPP, such effects are restored in 2C/POMC mice.160 Collectively, these findings demonstrate that 5-HT2CRs expressed by POMC neurons are physiological regulators of insulin sensitivity and glucose homeostasis.
In addition to 5-HT2CRs, 5-HT1B receptors (5-HT1BRs) are another important target of 5-HT action on feeding. Specifically, high-affinity 5-HT1BR agonists and fenfluramine produce substantial reductions in food intake, effects that are attenuated by pharmacological blockade 5-HT1BRs.161-163 In addition, 5-HT1BR knockout mice show increased body weight and food intake164 and are less sensitive to fenfluramine-induced anorexia.165 5-HT1BRs are widely expressed in the brain, with particularly high levels in the olfactory tubercle, caudate putamen, cortex, hypothalamus, hippocampal formation, thalamus, DRN, and cerebellum.156, 166, 167 NPY/AgRP neurons in the ARC may be one of the physiologically relevant targets of 5-HT1BRs to regulate food intake. First, we demonstrated that 5-HT–positive terminals establish synaptic contacts on both cell body and axon terminals of NPY/AgRP neurons,105 and 5-HT1BRs are expressed by a subset of NPY/AgRP neurons.105 We further showed that 5-HT and selective 5-HT1BR agonists hyperpolarize NPY neurons and decrease their firing rate, effects that are blocked by the 5-HT1BR antagonist.105 Given that NPY/AgRP neurons provide a strong inhibitory GABAergic projection to POMC neurons19, 21, 168 and that 5-HT1BRs expressed on axon terminals have been demonstrated to suppress GABA release,169, 170 the direct inhibitory effects of 5-HT1BRs on NPY/AgRP neurons may lead to indirect activation (disinhibition) of POMC neurons. Indeed, we observed that fenfluramine and 5-HT1BR agonists potently suppress the inhibitory postsynaptic currents in POMC neurons. Finally, we showed that the anorexigenic effects of fenfluramine and the 5-HT1BR agonist are blunted in MC4R knockout mice or Ay mice.105 Collectively, these findings support a model that 5-HT directly inhibits NPY/AgRP neurons via 5-HT1BRs. This action indirectly activates POMC neurons, and this 5-HT1BR–NPY/AgRP–POMC circuitry may at least partly mediate the anorexigenic effects of 5-HT.
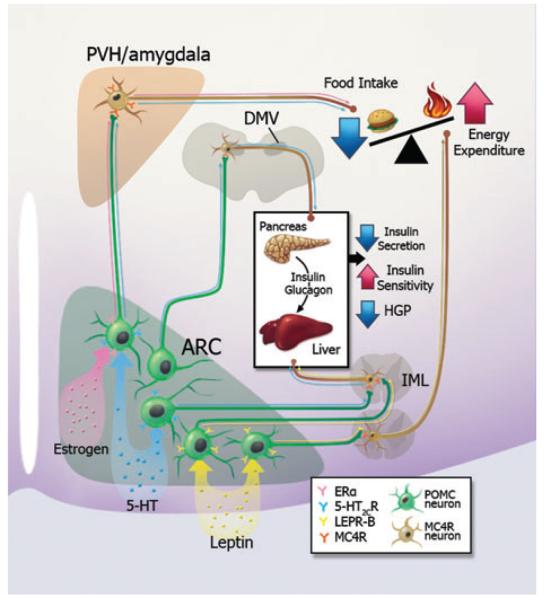
As discussed above, current evidence indicates that the central melanocortin pathway plays essential roles in multiple aspects of energy and glucose homeostasis. These include food intake, energy expenditure, insulin sensitivity, and insulin secretion. Remarkably, many of these functions are mediated by distinct CNS regions expressing melanocortin receptors (Fig. 1). Further, multiple hormones and/or neurotransmitters, including leptin, serotonin, and estrogen, have been shown to directly act on POMC neurons to regulate energy and glucose balance (Fig. 1). Interestingly, the physiological functions of these signals, while all acting through POMC neurons, are not necessarily identical. For example, leptin directly acts on POMC neurons to regulate energy expenditure and glucose homeostasis.20 On the other hand, estrogens, via actions on ERα in POMC neurons, suppress food intake, but do not directly regulate energy expenditure.124 5-HT acts on 5-HT2CRs in POMC neurons to regulate both feeding158 and insulin sensitivity,160 but does not affect energy expenditure. Collectively, these results suggest that several subsets of POMC neurons exist that project to and act on distinct downstream MC4R populations to exert different functions. Multiple metabolic cues may be integrated by distinct or partially overlapping POMC subsets. Supporting this notion, we have recently found that acute electrophysiological responses to leptin, insulin, and 5-HT2CR agonists are largely segregated in distinct subsets of POMC neurons.57, 171
Figure 1.
A schematic model for functional segregation of the central melanocortin system. Current evidence suggests that several subsets of POMC neurons exist in the ARC that project to and act on distinct downstream MC4R populations to suppress food intake, to increase energy expenditure, to decrease insulin secretion, or to increase insulin sensitivity, respectively. Multiple metabolic cues, including estrogens, 5-HT, and leptin, directly act on distinct or partially overlapping POMC subsets to regulate different aspects of energy and glucose homeostasis.
In conclusion, the past two decades have been an exciting time in the field of obesity and diabetes research. We have witnessed an explosion of knowledge regarding the control of energy balance and glucose homeostasis. This includes genetic, pharmacological, and neuroanatomic studies. While the increase in our knowledge is impressive, it is somewhat disappointing that the number of treatments for obesity and its complications have not kept up with the pace of discovery. Hopefully, the ever-increasing knowledge base will lead to rational strategies in the years that follow to deal with the increasing incidences of obesity and diabetes.
Acknowledgments
We thank Ms. Xiaorui Zhang for the illustration. YX is supported by R00DK085330, R01DK093587, P30 DK079638–03, the Naman Family Fund for Basic Research, and the Curtis Hankamer Basic Research Fund; JKE is supported by RL1 DK081185, R37DK53301, and R01DK071320; MF is supported by the American Heart Association.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 2.Cone RD. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 3.Williams DL, Schwartz MW. The melanocortin system as a central integrator of direct and indirect controls of food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R2–R3. doi: 10.1152/ajpregu.00226.2005. [DOI] [PubMed] [Google Scholar]
- 4.Cone RD. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol. Metab. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 5.Huszar D, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 6.Yaswen L, et al. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 7.Qian S, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ollmann MM, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 9.Pierroz DD, et al. Chronic administration of neuropeptide Y into the lateral ventricle inhibits both the pituitary-testicular axis and growth hormone and insulin-like growth factor I secretion in intact adult male rats. Endocrinology. 1996;137:3–12. doi: 10.1210/endo.137.1.8536627. [DOI] [PubMed] [Google Scholar]
- 10.Gropp E, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 11.Xu AW, et al. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3:e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bewick GA, et al. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J. 2005;19:1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- 13.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander GM, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson SM, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat. Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flier JS. AgRP in energy balance: will the real AgRP please stand up? Cell Metab. 2006;3:83–85. doi: 10.1016/j.cmet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Wojcik SM, et al. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Tong Q, et al. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balthasar N, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen AS, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 24.Fan W, et al. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141:3072–3079. doi: 10.1210/endo.141.9.7665. [DOI] [PubMed] [Google Scholar]
- 25.Butler AA, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 26.Butler AA. The melanocortin system and energy balance. Peptides. 2006;27:281–290. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton GM, et al. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle. FASEB J. 2010;24:862–872. doi: 10.1096/fj.09-142000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaisse C, et al. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat. Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 29.Yeo GS, et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 30.Chen AS, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 31.Ste Marie L, et al. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc. Natl. Acad. Sci. USA. 2000;97:12–339. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogueiras R, et al. The central melanocortin system directly controls peripheral lipid metabolism. J. Clin. Invest. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishi T, et al. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J. Comp. Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, et al. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J. Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mountjoy KG, et al. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 36.Balthasar N, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 37.Rossi J, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leibel RL, Chung WK, Chua SC., Jr The molecular genetics of rodent single gene obesities. J. Biol. Chem. 1997;272:31,937–31,940. doi: 10.1074/jbc.272.51.31937. [DOI] [PubMed] [Google Scholar]
- 39.Alingh Prins A, et al. Daily rhythms of feeding in the genetically obese and lean Zucker rats. Physiol. Behav. 1986;38:423–426. doi: 10.1016/0031-9384(86)90115-0. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin CL, Baile CA. Ontogeny of feeding behavior in the Zucker obese rat. Physiol. Behav. 1981;26:607–612. doi: 10.1016/0031-9384(81)90132-3. [DOI] [PubMed] [Google Scholar]
- 41.Trayhurn P, Thurlby PL, James WP. Thermogenic defect in pre-obese ob/ob mice. Nature. 1977;266:60–62. doi: 10.1038/266060a0. [DOI] [PubMed] [Google Scholar]
- 42.Dauncey MJ. Activity-induced thermogenesis in lean and genetically obese (ob/ob) mice. Experientia. 1986;42:547–549. doi: 10.1007/BF01946696. [DOI] [PubMed] [Google Scholar]
- 43.Dauncey MJ, Brown D. Role of activity-induced thermogenesis in twenty-four hour energy expenditure of lean and genetically obese (ob/ob) mice. Q. J. Exp. Physiol. 1987;72:549–559. doi: 10.1113/expphysiol.1987.sp003096. [DOI] [PubMed] [Google Scholar]
- 44.Tartaglia LA. The leptin receptor. J. Biol. Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 45.Halaas JL, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen P, et al. Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMinn JE, et al. Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am. J. Physiol. Endocrinol. Metab. 2005;289:E403–E411. doi: 10.1152/ajpendo.00535.2004. [DOI] [PubMed] [Google Scholar]
- 48.Kowalski TJ, et al. Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 2001;50:425–435. doi: 10.2337/diabetes.50.2.425. [DOI] [PubMed] [Google Scholar]
- 49.Chua SC, Jr, et al. Transgenic complementation of leptin receptor deficiency. II. Increased leptin receptor transgene dose effects on obesity/diabetes and fertility/lactation in lepr-db/db mice. Am. J. Physiol. Endocrinol. Metab. 2004;286:E384–E392. doi: 10.1152/ajpendo.00349.2003. [DOI] [PubMed] [Google Scholar]
- 50.de Luca C, et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J. Clin. Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elmquist JK, et al. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138:839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- 52.Elmquist JK, et al. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc. Natl. Acad. Sci. USA. 1998;95:741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fei H, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl. Acad. Sci. USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mercer JG, et al. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz MW, et al. Identification of targets of leptin action in rat hypothalamus. J. Clin. Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott MM, et al. Leptin targets in the mouse brain. J. Comp. Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams KW, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J. Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mizuno TM, et al. Hypothalamic proopiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz MW, et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 60.Thornton JE, et al. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 1997;138:5063–5066. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- 61.Hill JW, et al. Direct insulin and leptin action on proopiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huo L, et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9:537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ikeda Y, et al. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol. Endocrinol. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- 64.Dellovade TL, et al. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J. Comp. Neurol. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 65.Majdic G, et al. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 66.Dhillon H, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 67.Bingham NC, et al. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149:2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol. Rev. 2011;91:389–411. doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalra SP. Pivotal role of leptin-hypothalamus signaling in the etiology of diabetes uncovered by gene therapy: a new therapeutic intervention? Gene Ther. 2011;18:319–325. doi: 10.1038/gt.2010.164. [DOI] [PubMed] [Google Scholar]
- 70.Coppari R, et al. The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 71.Pocai A, et al. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005;54:3182–3189. doi: 10.2337/diabetes.54.11.3182. [DOI] [PubMed] [Google Scholar]
- 72.Hayes MR, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scott MM, et al. Leptin receptor expression in hind-brain Glp-1 neurons regulates food intake and energy balance in mice. J. Clin. Invest. 2011;121:2413–2421. doi: 10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bates SH, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 75.Piper ML, et al. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol. Endocrinol. 2008;22:751–759. doi: 10.1210/me.2007-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Plum L, et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to dietsensitive obesity. J. Clin. Invest. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niswender KD, et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 78.Hill JW, et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J. Clin. Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukuda M, et al. Monitoring FoxO1 localization in chemically identified neurons. J. Neurosci. 2008;28:13,640–13,648. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cota D, et al. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J. Neurosci. 2008;28:7202–7208. doi: 10.1523/JNEUROSCI.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8:459–467. doi: 10.1016/j.cmet.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Minokoshi Y, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 83.Metlakunta AS, Sahu M, Sahu A. Hypothalamic phosphatidylinositol 3-kinase pathway of leptin signaling is impaired during the development of diet-induced obesity in FVB/N mice. Endocrinology. 2008;149:1121–1128. doi: 10.1210/en.2007-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu AW, et al. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J. Clin. Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Y, et al. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab. 12:88–95. doi: 10.1016/j.cmet.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cota D, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 87.Bjorbaek C, et al. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 88.Cook WS, Unger RH. Protein tyrosine phosphatase 1B: a potential leptin resistance factor of obesity. Dev. Cell. 2002;2:385–387. doi: 10.1016/s1534-5807(02)00158-2. [DOI] [PubMed] [Google Scholar]
- 89.Zabolotny JM, et al. PTP1B regulates leptin signal transduction in vivo. Dev. Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 90.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 91.Enriori PJ, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 92.White CL, et al. HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and independent mechanisms. Am. J. Physiol. Endocrinol. Metab. 2008;296:E291–E299. doi: 10.1152/ajpendo.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mori H, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 94.Howard JK, et al. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploin-sufficiency of Socs3. Nat. Med. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 95.Bence KK, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat. Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 96.Kievit P, et al. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 97.Banno R, et al. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J. Clin. Invest. 2010;120:720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Briancon N, et al. Combined neural inactivation of suppressor of cytokine signaling-3 and protein-tyrosine phosphatase-1B reveals additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes. 2010;59:3074–3084. doi: 10.2337/db10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carr MC. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 100.Flegal KM, et al. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 101.Freedman DS, et al. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA. 2002;288:1758–1761. doi: 10.1001/jama.288.14.1758. [DOI] [PubMed] [Google Scholar]
- 102.Drewett RF. Sexual behaviour and sexual motivation in the female rat. Nature. 1973;242:476–477. doi: 10.1038/242476a0. [DOI] [PubMed] [Google Scholar]
- 103.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol. Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 104.Wallen WJ, Belanger MP, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J. Nutr. 2001;131:2351–2357. doi: 10.1093/jn/131.9.2351. [DOI] [PubMed] [Google Scholar]
- 105.Roy EJ, Wade GN. Role of food intake in estradiol-induced body weight changes in female rats. Horm. Behav. 1977;8:265–274. doi: 10.1016/0018-506x(77)90001-0. [DOI] [PubMed] [Google Scholar]
- 106.Mueller K, Hsiao S. Estrus- and ovariectomy-induced body weight changes: evidence for two estrogenic mechanisms. J. Comp. Physiol. Psychol. 1980;94:1126–1134. doi: 10.1037/h0077746. [DOI] [PubMed] [Google Scholar]
- 107.Gao Q, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat. Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 108.Bjorntorp P. Hormonal control of regional fat distribution. Hum. Reprod. 1997;1(12 Suppl):21–25. doi: 10.1093/humrep/12.suppl_1.21. [DOI] [PubMed] [Google Scholar]
- 109.Bjorntorp P. Obesity. Lancet. 1997;350:423–426. doi: 10.1016/S0140-6736(97)04503-0. [DOI] [PubMed] [Google Scholar]
- 110.Bjorntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition. 1997;13:795–803. doi: 10.1016/s0899-9007(97)00191-3. [DOI] [PubMed] [Google Scholar]
- 111.Kotani K, et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int. J. Obes. Relat. Metab. Disord. 1994;18:207–212. [PubMed] [Google Scholar]
- 112.Heine PA, et al. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. USA. 2000;97:12,729–12,734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ohlsson C, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem. Biophys. Res. Commun. 2000;278:640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 114.Geary N, et al. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 115.Osterlund M, et al. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res. Mol. Brain Res. 1998;54:175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- 116.Merchenthaler I, et al. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J. Comp. Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 117.Butera PC, Beikirch RJ. Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res. 1989;491:266–273. doi: 10.1016/0006-8993(89)90062-0. [DOI] [PubMed] [Google Scholar]
- 118.Palmer K, Gray JM. Central vs. peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiol. Behav. 1986;37:187–189. doi: 10.1016/0031-9384(86)90404-x. [DOI] [PubMed] [Google Scholar]
- 119.Butera PC, Willard DM, Raymond SA. Effects of PVN lesions on the responsiveness of female rats to estradiol. Brain Res. 1992;576:304–310. doi: 10.1016/0006-8993(92)90694-5. [DOI] [PubMed] [Google Scholar]
- 120.Hrupka BJ, Smith GP, Geary N. Hypothalamic implants of dilute estradiol fail to reduce feeding in ovariectomized rats. Physiol. Behav. 2002;77:233–241. doi: 10.1016/s0031-9384(02)00857-0. [DOI] [PubMed] [Google Scholar]
- 121.Dagnault A, Richard D. Lesions of hypothalamic paraventricular nuclei do not prevent the effect of estradiol on energy and fat balance. Am. J. Physiol. 1994;267:E32–E38. doi: 10.1152/ajpendo.1994.267.1.E32. [DOI] [PubMed] [Google Scholar]
- 122.Wade GN, Zucker I. Modulation of food intake and locomotor activity in female rats by diencephalic hormone implants. J. Comp. Physiol. Psychol. 1970;72:328–336. doi: 10.1037/h0029461. [DOI] [PubMed] [Google Scholar]
- 123.Musatov S, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu Y, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc. Natl. Acad. Sci. USA. 2009;106:15–932. doi: 10.1073/pnas.0904747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miller MM, et al. Effects of age and long-term ovariectomy on the estrogen-receptor containing subpopulations of beta-endorphin-immunoreactive neurons in the arcuate nucleus of female C57BL/6J mice. Neuroendocrinology. 1995;61:542–551. doi: 10.1159/000126878. [DOI] [PubMed] [Google Scholar]
- 127.de Souza FS, et al. The estrogen receptor alpha colocalizes with proopiomelanocortin in hypothalamic neurons and binds to a conserved motif present in the neuron-specific enhancer nPE2. Eur. J. Pharmacol. 2011;660:181–187. doi: 10.1016/j.ejphar.2010.10.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Malyala A, et al. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J. Comp. Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- 129.Lechin F, van der Dijs B, Hernandez-Adrian G. Dorsal raphe vs. median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: relevance for neuropharmacological therapy. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:565–585. doi: 10.1016/j.pnpbp.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 130.De Fanti BA, Hamilton JS, Horwitz BA. Meal-induced changes in extracellular 5-HT in medial hypothalamus of lean (Fa/Fa) and obese (fa/fa) Zucker rats. Brain Res. 2001;902:164–170. doi: 10.1016/s0006-8993(01)02371-x. [DOI] [PubMed] [Google Scholar]
- 131.Rowland NE, Carlton J. Neurobiology of an anorectic drug: fenfluramine. Prog. Neurobiol. 1986;27:13–62. doi: 10.1016/0301-0082(86)90011-0. [DOI] [PubMed] [Google Scholar]
- 132.Foltin RW, Moran TH. Food intake in baboons: effects of a long-acting cholecystokinin analog. Appetite. 1989;12:145–152. doi: 10.1016/0195-6663(89)90103-7. [DOI] [PubMed] [Google Scholar]
- 133.McGuirk J, et al. Differential effects of d-fenfluramine, l-fenfluramine and d-amphetamine on the microstructure of human eating behaviour. Behav. Pharmacol. 1991;2:113–119. [PubMed] [Google Scholar]
- 134.Rogers PJ, Blundell JE. Effect of anorexic drugs on food intake and the micro-structure of eating in human subjects. Psychopharmacology (Berl) 1979;66:159–165. doi: 10.1007/BF00427624. [DOI] [PubMed] [Google Scholar]
- 135.Blundell JE, Leshem MB. Central action of anorexic agents: effects of amphetamine and fenfluramine in rats with lateral hypothalamic lesions. Eur. J. Pharmacol. 1974;28:81–88. doi: 10.1016/0014-2999(74)90115-0. [DOI] [PubMed] [Google Scholar]
- 136.Geyer MA, et al. Behavioral studies following lesions of the mesolimbic and mesostriatal serotonergic pathways. Brain Res. 1976;106:257–269. doi: 10.1016/0006-8993(76)91024-6. [DOI] [PubMed] [Google Scholar]
- 137.Ghosh MN, Parvathy S. The effect of cyproheptadine on water and food intake and on body weight in the fasted adult and weanling rats. Br. J. Pharmacol. 1973;48:328P–329P. [PMC free article] [PubMed] [Google Scholar]
- 138.Saller CF, Stricker EM. Hyperphagia and increased growth in rats after intraventricular injection of 5,7-dihydroxytryptamine. Science. 1976;192:385–387. doi: 10.1126/science.1257774. [DOI] [PubMed] [Google Scholar]
- 139.Vickers SP, Dourish CT. Serotonin receptor ligands and the treatment of obesity. Curr. Opin. Investig. Drugs. 2004;5:377–388. [PubMed] [Google Scholar]
- 140.Vickers SP, et al. Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology (Berl) 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- 141.Kennett GA, Curzon G. Potencies of antagonists indicate that 5-HT1C receptors mediate 1-3(chlorophenyl)piperazine-induced hypophagia. Br. J. Pharmacol. 1991;103:2016–2020. doi: 10.1111/j.1476-5381.1991.tb12369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kennett GA, et al. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- 143.Clifton PG, Lee MD, Dourish CT. Similarities in the action of Ro 60-0175, a 5-HT2C receptor agonist and d-fenfluramine on feeding patterns in the rat. Psychopharmacology (Berl) 2000;152:256–267. doi: 10.1007/s002130000504. [DOI] [PubMed] [Google Scholar]
- 144.Tecott LH, et al. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 145.Nonogaki K, et al. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat. Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- 146.Templeman LA, et al. Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet. Genomics. 2005;15:195–200. doi: 10.1097/01213011-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 147.Reynolds GP, Zhang ZJ, Zhang XB. Association of antipsychotic drug-induced weight gain with a 5-HT2C receptor gene polymorphism. Lancet. 2002;359:2086–2087. doi: 10.1016/S0140-6736(02)08913-4. [DOI] [PubMed] [Google Scholar]
- 148.Kishore S, Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science. 2006;311:230–232. doi: 10.1126/science.1118265. [DOI] [PubMed] [Google Scholar]
- 149.Wade JM, et al. Synergistic impairment of glucose homeostasis in ob/ob mice lacking functional serotonin 2C receptors. Endocrinology. 2008;149:955–961. doi: 10.1210/en.2007-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhou L, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Molineaux SM, et al. 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc. Natl. Acad. Sci. USA. 1989;86:6793–6797. doi: 10.1073/pnas.86.17.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heisler LK, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 153.Kiss J, Leranth C, Halasz B. Serotoninergic endings on VIP-neurons in the suprachiasmatic nucleus and on ACTH-neurons in the arcuate nucleus of the rat hypothalamus. A combination of high resolution autoradiography and electron microscopic immunocytochemistry. Neurosci. Lett. 1984;44:119–124. doi: 10.1016/0304-3940(84)90068-5. [DOI] [PubMed] [Google Scholar]
- 154.Qiu J, et al. Serotonin 5-hydroxytryptamine2C receptor signaling in hypothalamic proopiomelanocortin neurons: role in energy homeostasis in females. Mol. Pharmacol. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- 155.Lam DD, et al. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Heisler LK, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 157.Zigman JM, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu Y, et al. 5-HT2CRs expressed by proopiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Y, et al. A serotonin and melanocortin circuit mediates d-fenfluramine anorexia. J. Neurosci. 2010;30:14,630–14,634. doi: 10.1523/JNEUROSCI.5412-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu Y, et al. 5-HT(2C)Rs expressed by proopiomelanocortin neurons regulate insulin sensitivity in liver. Nat. Neurosci. 2010;13:1457–1459. doi: 10.1038/nn.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Halford JC, Blundell JE. The 5-HT1B receptor agonist CP-94,253 reduces food intake and preserves the behavioural satiety sequence. Physiol. Behav. 1996;60:933–939. doi: 10.1016/0031-9384(96)00073-x. [DOI] [PubMed] [Google Scholar]
- 162.Lee MD, Simansky KJ. CP-94, 253: a selective serotonin1B (5-HT1B) agonist that promotes satiety. Psychopharmacology (Berl) 1997;131:264–270. doi: 10.1007/s002130050292. [DOI] [PubMed] [Google Scholar]
- 163.Lee MD, et al. Infusion of the serotonin1B (5-HT1B) agonist CP-93,129 into the parabrachial nucleus potently and selectively reduces food intake in rats. Psychopharmacology (Berl) 1998;136:304–307. doi: 10.1007/s002130050570. [DOI] [PubMed] [Google Scholar]
- 164.Bouwknecht JA, et al. Male and female 5-HT(1B) receptor knockout mice have higher body weights than wildtypes. Physiol. Behav. 2001;74:507–516. doi: 10.1016/s0031-9384(01)00589-3. [DOI] [PubMed] [Google Scholar]
- 165.Lucas JJ, et al. Absence of fenfluramine-induced anorexia and reduced c-Fos induction in the hypothalamus and central amygdaloid complex of serotonin 1B receptor knock-out mice. J. Neurosci. 1998;18:5537–5544. doi: 10.1523/JNEUROSCI.18-14-05537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bonaventure P, et al. Detailed mapping of serotonin 5-HT1B and 5-HT1D receptor messenger RNA and ligand binding sites in guinea-pig brain and trigeminal ganglion: clues for function. Neuroscience. 1998;82:469–484. doi: 10.1016/s0306-4522(97)00302-3. [DOI] [PubMed] [Google Scholar]
- 167.Bruinvels AT, et al. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 168.Roseberry AG, et al. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron. 2004;41:711–722. doi: 10.1016/s0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- 169.Chadha A, et al. The 5HT(1B) receptor agonist, CP-93129, inhibits [(3)H]-GABA release from rat globus pallidus slices and reverses akinesia following intrapallidal injection in the reserpine-treated rat. Br. J. Pharmacol. 2000;130:1927–1932. doi: 10.1038/sj.bjp.0703526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J. Neurosci. 1996;16:7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sohn JW, et al. Serotonin 2C receptor activates a dinstinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71:488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]