Abstract
Two important features of amphibian metamorphosis are the sequential response of tissues to different concentrations of thyroid hormone (TH) and the development of the negative feedback loop between the pituitary and the thyroid gland that regulates TH synthesis by the thyroid gland. At the climax of metamorphosis in Xenopus laevis (when the TH level is highest), the ratio of the circulating precursor thyroxine (T4) to the active form 3,5,3′-triiodothyronine (T3) in the blood is many times higher than it is in tissues. This difference is because of the conversion of T4 to T3 in target cells of the tadpole catalyzed by the enzyme type II iodothyronine deiodinase (D2) and the local effect (cell autonomy) of this activity. Limb buds and tails express D2 early and late in metamorphosis, respectively, correlating with the time that these organs undergo TH-induced change. T3 is required to complete metamorphosis because the peak concentration of T4 that is reached at metamorphic climax cannot induce the final morphological changes. At the climax of metamorphosis, D2 expression is activated specifically in the anterior pituitary cells that express the genes for thyroid-stimulating hormone but not in the cells that express proopiomelanocortin. Physiological concentrations of T3 but not T4 can suppress thyrotropin subunit β gene expression. The timing and the remarkable specificity of D2 expression in the thyrotrophs of the anterior pituitary coupled with the requirement for locally synthesized T3 strongly support a role for D2 in the onset of the negative feedback loop at the climax of metamorphosis.
The metamorphosis of anurans is controlled by a steadily increasing concentration of thyroid hormone (TH) in tadpoles. TH reaches a peak at the climax of metamorphosis and then falls as the final change, tail resorption, occurs (1, 2). This gradual increase in TH concentration is essential for the sequential development of frog tissues and organs (3, 4). The growth of the hind limbs is the earliest TH-induced morphological modification. In Xenopus laevis, the hind-limb buds form but cannot develop beyond Nieukoop Faber (NF) stage 53 (5) in the absence of TH. TH-induced growth and development of limbs occur when the endogenous TH concentration is low and is completed before the climax of metamorphosis (when TH concentration is the highest). Many metamorphic changes occur in rapid succession at climax. In about 4 days, the intestine remodels and the gills resorb followed by tail resorption, which takes another 3–4 days.
A negative feedback loop between the thyroid gland, the pituitary, and the hypothalamus maintains the endogenous TH at a constant level in adults of higher vertebrates (6). The rising TH concentration in X. laevis tadpoles, which is so important for their sequential development, occurs paradoxically as the pituitary content of thyroid-stimulating hormone (TSH) mRNA is increasing also (7). Etkin (8) hypothesized that the rise in TH during the early part of tadpole development was caused by a positive feedback loop between the pituitary, the hypothalamus, and the thyroid glands. At climax, this control changes to a negative feedback. An alternative theory (3) states that the rise of TH occurs until the receptors are saturated, and then the negative feedback loop is initiated. The level of TSH mRNA does drop at climax (7) followed by a decrease of TH at the end of climax (1), signaling the establishment of the negative feedback loop. However, components of the negative feedback loop have been demonstrated clearly in tadpoles at earlier stages when both TH and TSH are still rising. Surgical removal of a tadpole's pituitary causes complete cessation of thyroid function within a few days, presumably because of an absolute requirement for TSH (3). Inhibition of thyroid gland function with goitrogens at any time before climax arrests tadpole development and leads to an increase of TSH mRNA in the pituitary (ref. 7; see Fig. 4) and ultimately to thyroid gland enlargement (3). Another hypothesis is simply that the pituitary gland, like so many other tadpole organs, differentiates during tadpole life and at climax develops competence to respond to the elevated TH concentration. In this paper, we address possible explanations for this competence.
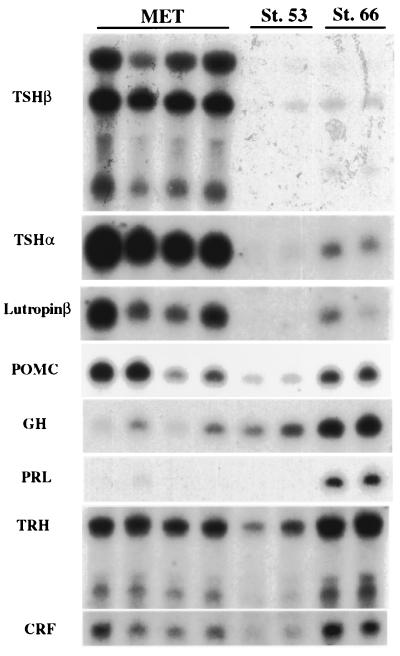
Figure 4.
Northern blot of various pituitary and hypothalamic hormones. Total RNA was purified from pituitary and part of the brain (from midbrain to cerebellum) of individual animals, and the entire sample was loaded in a lane. The tadpoles treated with methimazole (MET) for several months were arrested at stage 53, judging from the limb criteria. Two normal stage-53 tadpoles and 2 stage-66 froglets were used as controls. The same blot was hybridized successively with the radioactive probes.
Thyroxine (T4) is the main product of the thyroid gland in all vertebrates tested to date (9), including anuran tadpoles (1), and it is converted to the more active hormone 3,5,3′-triiodothyronine (T3) in peripheral tissues (10). Two kinds of iodothyronine deiodinases that metabolize TH in target tissues of Rana catesbeiana tadpoles have been identified (11). Type II iodothyronine deiodinase (D2) synthesizes T3 from T4, whereas type III iodothyronine deiodinase (D3) inactivates the hormone by removing an iodine molecule from the inner ring of the hormone. An important issue for the control of TH-induced changes is the extent to which a high local activity of deiodinase in one tissue can influence the T3 concentration of another tissue. If the action of D2 generates T3 from T4 for local use only, a phenomenon referred to as tissue or cell autonomy, then the enzyme could play an important role in the sequential timing of metamorphic change. We will present data supporting this idea.
In this study we have investigated the role of D2 in the development and metamorphosis of X. laevis. Whereas the D3 expression pattern correlates with protection against TH-induced change (12–15), the expression of D2 correlates with susceptibility to metamorphic change. In agreement with the findings of Becker et al. (11), constitutive expression of D2 in X. laevis limb buds results in the local conversion of T4 to T3 at a time when D2 expression is absent or low elsewhere. D2 activity appears at late climax in the tail just before tail resorption begins. Expression of D2 is activated specifically in the TSH-producing cells of the anterior pituitary at the climax of metamorphosis. We propose that activation of D2 in the anterior pituitary at the climax of metamorphosis establishes the negative feedback loop by producing a high enough local concentration of T3 that can suppress TSH synthesis.
Materials and Methods
Growth of Tadpoles on Inhibitors and Assays for Radioactive and Nonradioactive T3 and T4.
Wild-type X. laevis tadpoles were purchased at various stages from Xenopus I (Dexter, MI). Ten NF-stage 52 tadpoles were grown and fed in 8 liters of dechlorinated tap water containing 10 μM iopanoic acid (Sigma) and/or 1 mM methimazole (Sigma). The water was changed weekly.
Blood was collected by cardiac puncture of anesthetized tadpoles that had been injected i.p. 20 min earlier with 30 μg of heparin. The whole blood was expelled directly into cold methanol. Radioactive [125I]T3, -T4, and -I− were purchased from NEN and either added to the rearing water or injected i.p. into X. laevis tadpoles. Whole tadpoles or isolated organs were cut into small pieces with a razor blade, weighed, suspended in 6 volumes of cold methanol, and homogenized with a Tekmar (Cincinnati) Tissuemizer. The use of methanol to extract T3 and T4 was described by Tagawa and Hirano (16). For RIA measurements, [125I]T4 or -T3 was added to the methanol homogenates at a concentration of 5–10 pM, the precipitate was removed by centrifugation, and the methanol evaporated by using a vacuum centrifuge. Samples were diluted to 1 ml with 0.1 M sodium barbital (pH 8.6) for T4 or 0.02 M sodium phosphate (pH 7.5), containing 0.02 M EDTA and 0.75% sodium salicylate, for T3. The solutions were transferred to Ab-coated tubes (ICN). After incubation for 2 h at 37°C, total radioactivity was measured. Then the tubes were washed twice with water and the radioactivity bound to the tubes was determined again. A standard dilution series was performed by adding known amounts of T3 or T4 (Sigma) to identical premetamorphic tadpole extracts. About 50% of the [125I]TH was bound in control experiments. Tadpole extracts that contained very high concentrations of TH, like the iopanoic acid-treated tadpoles, were mixed in varying proportions with premetamorphic tadpole extracts.
Identification of [125I]T3 and -T4 was performed by chromatography on Whatman LK5D silica gel 150A TLC plates. The solvent was 2-methylbutanol/t-butyl alcohol/25% NH3/acetone, 7:14:14:56, vol/vol (17). Samples were prepared by evaporating methanol extracts to dryness and then dissolving the dried radioactivity with 20 mM NaOH. About 20% of the original methanol extract of one tadpole can be chromatographed in one lane. Known radioactive T3 and T4 markers were added to methanol extracts and dried for chromatography in the same way as the samples.
Cloning X. laevis D2 cDNA and the Source of Pituitary Hormone cDNAs.
Degenerate primers (5′-GAYGCCTAYAARCARGTNAAR-3′ and 5′-GGRTGAGCYTCRTCDATRTAN-3′) corresponding to a conserved region of known D2 sequences were used to amplify a 250-bp fragment by reverse transcription–PCR (RT-PCR), using stage 61 tadpole brain and tadpole tail total RNA as the template. Then the fragment was cloned and identified by its sequence. This cDNA fragment was used as a probe to screen a randomly primed cDNA library derived from X. laevis tail mRNA. A 2,291-bp fragment was obtained that contained almost all of the ORF and a portion of the 3′ untranslated region (GenBank accession no. AF354707). Full-length X. laevis D2 mRNA is estimated to be about 7 kb. The amino acid sequence is 82% identical to the R. catesbeiana D2 sequence (18).
The cDNAs for thyrotropin subunit α (TSHα), thyrotropin subunit β (TSHβ), growth hormone (GH), prolactin (PRL; ref. 7), and D3 (19) have been described. X. laevis lutropin β (GenBank accession number AF360397) was cloned from the same X. laevis pituitary cDNA library. The amino acid sequence is 72% identical to R. catesbeiana lutropin β (20). The X. laevis cDNA encoding thyrotropin-releasing hormone (TRH) was a gift of Klaus Richter (Salzburg, Austria; ref. 21). Corticotropin-releasing factor (CRF) cDNA was amplified from genomic DNA with primers derived from the published sequence (22). The cDNAs encoding thyroid receptor (TR)-α and TRβ have been described (23). Northern blotting (24) and in situ hybridization (13) methods have been described.
Results
Local Expression of D2 Can Contribute to the Timing of Metamorphosis Because its Action is Cell Autonomous.
The thyroid gland synthesizes mainly T4 and releases it into the circulation (1, 9). In peripheral tissues, T4 is converted to T3, which has a 10–15 times higher affinity to the TRs than T4. Deiodinases are intracellular enzymes (25, 26). If D2 acts in a truly cell-autonomous manner, then locally generated T3 will not be returned to the circulation where it could influence the development of a cell type that has no D2 activity. To test the cell autonomy of D2, we have compared the ratio of T4/T3 in the circulating blood with that of the carcass at metamorphic climax by RIA measurements. Whole blood collected from tadpoles at climax by heart puncture was assayed for T4 and T3 as were carcasses from the same animals. The T4/T3 ratio in the blood at climax is 5.5-fold higher than that of the carcass (Table 1). This result is in contrast to the reported T4/T3 ratio of about 1.0 in plasma (1).
Table 1.
The T4/T3 ratio in the carcass and the blood of climax tadpoles
| T4/T3 ratio | T4, nM | T3, nM | |
|---|---|---|---|
| Carcass | 1.2 ± 0.5 | 6.0 ± 2.3 | 5.0 ± 1.0 |
| Blood | 5.5 ± 2.9 | — | — |
Six NF-stage 62/63 tadpoles were bled individually by cardiac puncture. The collected blood was divided in half for T4 and T3 RIA measurements. Their carcasses were assayed for the same hormones. Values are mean ± standard deviation. The differences between the ratios is significant (P < 0.004) by Student's t test.
We have observed that the addition of exogenous T4 to premetamorphic tadpoles can duplicate closely the normal progression of early development to stage 59, the beginning of climax (Fig. 1A). This change is characterized by the induction of limb development. In contrast, exogenous T3 added in amounts as low as 0.3 nM induces many changes simultaneously that normally do not occur until climax in addition to limb development. The most obvious of these changes is gill resorption (Fig. 1A). There are two possible explanations for this observation. First, T4 might be the functional hormone that induces early limb development. The original analysis of T4 and T3 content in plasma of X. laevis during the period of limb growth (NF stages 54–57) detected only T4 (1). Our RIA measurements of tadpole carcasses were not sensitive enough to detect either hormone before NF-stage 57. The second possibility is that there is a local conversion of T4 to T3 in limbs that is not detected by measurements of plasma or whole-tadpole homogenates. Local generation of T3 from T4 could provide limbs with a high enough concentration of the more active form of the hormone. In fact, D2 is expressed in early stages of limb development in the bull frog (11). We cloned part of the X. laevis cDNA encoding D2 to test its expression in various tissues during development and have confirmed that D2 is expressed early in limbs at stages when expression is not detectable in the tail, gills, or intestine (Fig. 2). Furthermore, limb buds of premetamorphic tadpoles can convert efficiently the precursor T4 to T3 at a stage when tails cannot (Fig. 3A). We agree with the conclusion (11) that the ability of limbs to grow and develop early when the endogenous TH is low is aided by the expression of D2 that enables the limb buds to convert circulating T4 to T3. Expression of D2 cannot be detected in the tail until climax of X. laevis (Fig. 2), just as has been described for R. catesbeiana (11). Gills are very sensitive to low concentrations of added T3 and insensitive to T4 throughout tadpole development, and they never express detectable D2 mRNA (data not shown).
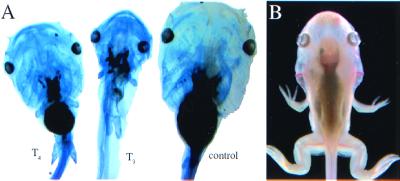
Figure 1.
(A) Sibling tadpoles grown in 1 mM methimazole for several weeks. Control tadpole (Right) at stage 53 treated for 8 days with 3 nM T3 (Center) and 3 nM T4 (Left). They were stained for cartilage with Alcian blue. (B) Tadpole grown for 2 months in 1 mM methimazole/10 μM iopanoic acid/5 nM T4. In 1 month, the tadpole developed to stage 59 but then did not change further during the final month of treatment.
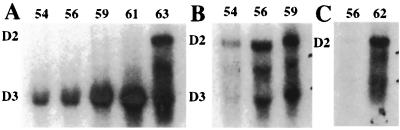
Figure 2.
Developmental Northern blots with simultaneous hybridization with D2 and D3 mRNAs. (A) Total tail RNA. (B) Hind-limb total RNA. (C) Pituitary total RNA. This blot was hybridized only with the D2 probe. The numbers on the tops of the lanes are tadpole stages.
Figure 3.
(A) Conversion of T4 to T3 in tadpole limb buds but not tail tissue. Stage-54 tadpoles were reared in methimazole with or without iopanoic acid for 24 h and then incubated with [125I]T4 for 24 h followed by nonradioactive medium for another 24 h. Limbs and tails were extracted in methanol for radioactive TH, and the concentrated extracts were chromatographed. A lane contains extract from four hind limbs or one tail. Hind limb (lane 1), tail (lane 2), and hind limb with iopanoic acid (lane 3). (B) Stage-59 tadpoles were incubated in either iopanoic acid (IOP) or methimazole (MET) for 1 day and then were incubated for a day in 0.5 μCi/ml radioactive NaI. TH was extracted from whole tadpoles and separated by TLC. Duplicate samples were chromatographed, and each lane represents about one-third of a tadpole. CON, control.
The Control of Gene Expression in the Tadpole Pituitary.
The goitrogen methimazole is an effective and nontoxic inhibitor of TH synthesis by the thyroid gland in X. laevis. Uptake of 125I− by the thyroid gland is inhibited completely by methimazole, and these animals do not synthesize T3 or T4 (Fig. 3B). Tadpoles were placed in 1 mM methimazole 1 week after fertilization when they had begun to feed, but before their thyroid glands were functional. We have kept these tadpoles for more than 1 year in the presence of the inhibitor. They grow to several times the size of control tadpoles, but their hind limbs never develop beyond NF-stage 54 in the absence of TH (data not shown). The larval skull and vertebrae ossify, a developmental change that normally begins at NF-stage 56. After several months in methimazole, primary gonads are formed, and males and females can be distinguished. All of these tadpoles have enlarged thyroid glands, and about 20% develop goiters after several months in the inhibitor as is expected for interference with the negative feedback loop between the thyroid and the pituitary glands.
We screened the pituitaries and brains of control and methimazole-treated tadpoles for the expression of genes that encode pituitary hormones and the hypothalamic-releasing factors that have been implicated in the control of TH synthesis (Fig. 4). Over time, the pituitaries of these inhibited tadpoles accumulate high levels of mRNA encoding the α and β subunits of TSH (7) and lutropin β. GH is expressed at a lower level by the pituitaries of the methimazole-inhibited tadpoles. Prolactin synthesis is inhibited completely by methimazole (7). Expression of CRF and TRH mRNAs, the two hormones that are candidates for hypothalamic involvement in the negative feedback loop with the thyroid, is active even in the brains of young tadpoles and does not change much during normal tadpole development or growth in methimazole. These releasing hormones are expressed widely in the brain, thus any local hypothalamic change in expression could have been missed in these Northern blots. Addition of 10 nM T3 to the rearing water suppresses the synthesis of TSHβ mRNA in stage-58 tadpoles within about 2 days, but it does not affect proopiomelanocortin (POMC) mRNA (Fig. 5). If CRF is a participant in the negative feedback loop that regulates TSH, then excess TH would be expected to suppress CRF synthesis, which in turn would shut off POMC mRNA. Tadpoles that are grown in iopanoic acid accumulate about 5 times the control levels of T4 but do not shut off synthesis of TSH mRNA at climax (data not shown). These tadpoles have reduced but detectable levels of T3. We conclude that amounts of T4 that greatly exceed the highest normal concentrations at climax do not suppress TSH mRNA synthesis.
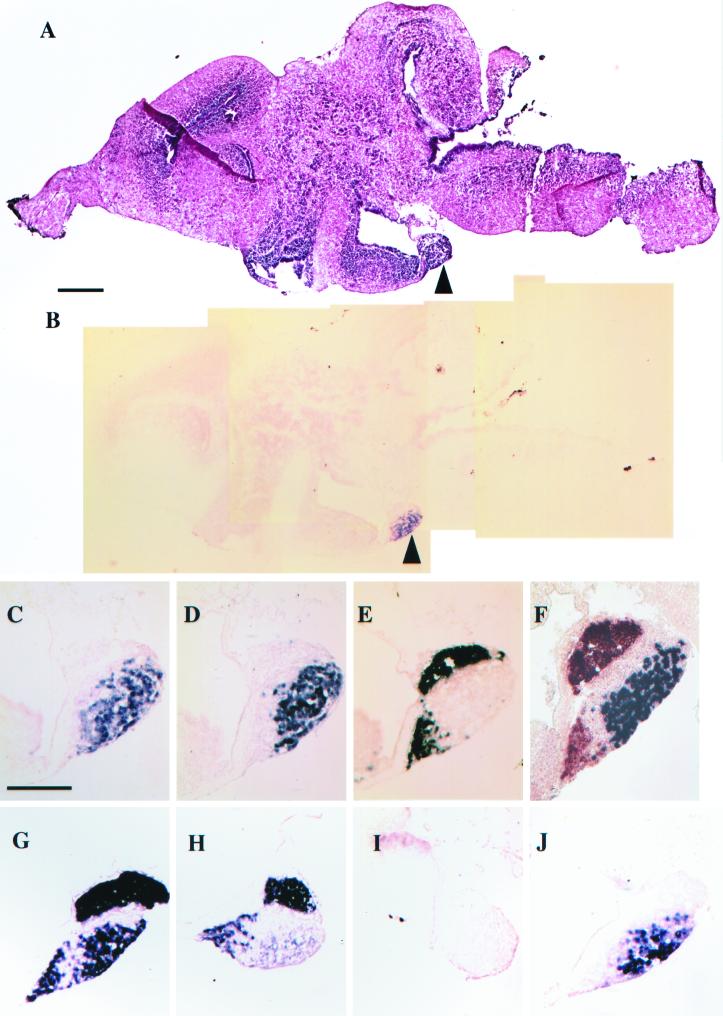
Figure 5.
In situ hybridization of sagittal sections of the pituitary (arrowhead) of stage-62 brains. (A) Hematoxylin and eosin stain. (B–J) In situ hybridization. (B) D2 in situ in a section adjacent to A. (C) Higher magnification of D2 in the pituitary in B. (D) TSHβ. (E) POMC. (F) Simultaneous in situ with POMC (orange) and TSHβ (purple). (G–J) Stage-58 tadpoles treated for 4 days with methimazole and iopanoic acid. (G and H) Simultaneous POMC and TSHβ in situ, but both probes were labeled with digoxigenin. (G) Stage-58 tadpoles treated for another 4 days with 10 nM T4. (H) Same as G, but treated for another 4 days with 10 nM T3. (I) D2 in situ of doubly inhibited stage-58 tadpole. (J) Same as I except treated for 4 days with 10 nM T4. (Bar for A and B = 200 μm.) (Bar for C–J = 100 μm.) In all figures, anterior is left and dorsal is up.
Because T3 but not T4 can shut down the pituitary TSH, we investigated a possible role of D2 in establishing the negative feedback loop. A Northern blot shows that the D2 mRNA levels are increased at climax in the pituitary (Fig. 2C). In situ hybridization reveals D2 gene expression specifically in the cells that express TSHβ, but not in those that express POMC (Fig. 5). In fact, the only detectable D2 mRNA in these sagittal sections of the brain at climax is in the thyrotrophs of the anterior pituitary (Fig. 5B). Tadpoles that were inhibited with both methimazole and iopanoic acid were incubated with either 10 nM T4 or 10 nM T3. The use of the two inhibitors allowed us to assess the influence of climax levels of the two hormones independently on TSH gene expression. In situ hybridization reveals the suppression of TSH synthesis by T3 (Fig. 5H) but not by T4 (Fig. 5I). Although added 10 nM T4 does not suppress TSH gene expression (Fig. 5G), it up-regulates D2 mRNA in the anterior pituitary (Fig. 5J). The addition of 10 nM T3 also up-regulates D2 (data not shown).
Discussion
How Limbs Develop Early and Tails Resorb Late in Metamorphosis.
Limb development is extraordinarily sensitive to TH concentration. Some of the same genes are up-regulated by TH in the limb and tail, but this occurs at different stages when the endogenous concentration of TH is widely different (27). The most comprehensive RIA analysis of TH during X. laevis tadpole development did not detect any plasma T3 up to NF-stage 57 (1). Yet by this stage, limbs have completed their TH-controlled development. Two features of limb buds correlate with their early development. Limb buds have a highly constitutive level of TRα (27) that decreases as the limbs develop. Hind-limb buds have a highly constitutive level of D2 (11) that efficiently converts T4 to T3 (Fig. 3A). This local generation of T3 must enhance the early induction of limb development. In contrast, the tail has low levels of receptor early and no detectable D2 (Fig. 2). As metamorphosis progresses, the levels of TR rise in the tail (28). TRβ is induced by the rising concentration of TH (23) and becomes the most abundant form of the receptor in the tail at climax. D2 gene expression is activated just before tail resorption (Fig. 2A). When a tadpole is treated with methimazole to inhibit TH synthesis by the thyroid gland and iopanoic acid to inhibit D2 activity, the influence of added T4 per se can be assessed. The addition of 5 nM T4, a concentration that mimics the endogenous level, can induce a doubly inhibited tadpole to develop to the beginning of climax (Fig. 1B). However, the final events of metamorphosis do not occur in these tadpoles. Remarkably, only low levels of T3 are needed to induce gill resorption at any time during metamorphosis, whereas limb growth responds to low levels of either T3 or T4. The sensitivity to T3 and resistance to T4 of gills is explained in part by the absence of D2 in gills at any stage (data not shown). Therefore, gill resorption at metamorphic climax must depend on the low but measurable T3 that is synthesized by the thyroid gland and released into the circulation. On the other hand, hind-limb buds express D2 and can convert even trace amounts of T4 to T3 (Fig. 3A). The tail and the anterior pituitary do not express D2 until the climax of metamorphosis when there is a dramatic up-regulation of D2 mRNA.
In X. laevis, D3 is a direct-response gene of TH (27). Yet its constitutive expression in certain tissues accounts for some of its influence on metamorphosis (15). The elevated expression of D2 in limb buds is another example of constitutive expression. D2 is up-regulated at climax in the tail and in the pituitary and therefore is responding to the elevated TH levels. However, unlike D3, D2 is not a direct-response gene of TH in X. laevis. D2 requires several days of TH treatment to up-regulate its expression (data not shown). D2 gene expression is down-regulated by TH in the mammalian pituitary (29, 30), yet it has been implicated still in the feedback loop in mammals.
Control of the Negative Feedback Loop.
The part of the negative feedback loop that must develop at the climax of metamorphosis is the ability of the rising TH to inhibit the pituitary's synthesis of TSH. The sensitivity of the pituitary to TH in turn determines the set point of TH production. The major hormone produced and released into the circulation is T4. When the concentration of TH is high enough to ensure the completion of metamorphosis at climax, D2 is activated in the thyrotrophs of the anterior pituitary (Fig. 5), and TSH synthesis is reduced (7). Although the concentration of T4 at climax can up-regulate D2 in the pituitary (Fig. 5J), it cannot repress TSH gene expression (Fig. 5G). Only physiological concentrations of T3 shut off TSH synthesis (Fig. 5H). In mammals, D2 is shown to be distributed throughout the central nervous system and pituitary (29, 30). Larsen et al. (31) have shown that T4 is converted to T3 by D2 in the rat pituitary, and that T3 is the physiological hormone that shuts down TSH mRNA production in the anterior pituitary. In the mouse, TRβ is important for the pituitary's response to TH as judged by the TRβ knockouts (32). We have carried out TRα and TRβ in situ hybridization in the tadpole pituitary at climax. The expression of both genes is detected but neither gene's activity is unusually high or specific within the thyrotrophs (data not shown). We have tested the expression of a variety of pituitary-specific genes in tadpoles inhibited with methimazole, a well known goitrogen (Fig. 4), and after subsequent addition of T3 or T4. The expression of GH and POMC is not influenced by TH. Cells synthesizing GH are known to give rise to PRL-producing cells (33). Because methimazole-inhibited tadpoles never activate PRL synthesis (ref. 7; Fig. 4), this conversion requires TH.
The Role of the Hypothalamus.
The role of the hypothalamus in the negative feedback loop in tadpoles is not yet settled. In mammals, TSH gene expression in the pituitary and TRH expression by the hypothalamus are repressed by TH by way of their thyroid receptors. The early experiments that implicated the hypothalamus in amphibian metamorphosis were surgical lesions that isolated the pituitary from its connections in the brain. These tadpoles were reported to be inhibited in their metamorphosis (3). Even the surgical removal of the hypothalamus has given controversial results (34). Several investigators have injected the TRH tripeptide into amphibian tadpoles and found no effect on TH synthesis (3). Denver (35) and others (36) have reported that CRF but not TRH stimulates TH synthesis by the thyroid gland, presumably by stimulating TSH release. If hypothalamic CRF was part of the negative feedback loop, then excess TH should shut down POMC mRNA synthesis along with TSH. POMC gene expression is detected in embryogenesis long before the thyroid gland is formed (37), and POMC mRNA is expressed in an entirely different set of cells in the pituitary than those that synthesize TSH (Fig. 5). POMC gene expression is not affected by TH concentration (Figs. 4 and 5). Likewise, TH does not regulate TRH or CRF gene expression generally in the brain (Fig. 4). In situ hybridization through the region of the hypothalamus with either TRH or CRF cDNA probes did not reveal any specific change when TH synthesis is inhibited, or as a result of exogenous T3 at levels that shut off TSH mRNA synthesis (data not shown). D2 mRNA was not detected in the hypothalamus (Fig. 5B). Furthermore, the i.p. injection of ovine CRF or bovine TRH into stage-56 tadpoles did not alter the amount of TSHβ mRNA by Northern blot or the timing of metamorphosis (data not shown). Therefore, we have not been able to demonstrate a role for either TRH or CRF in the negative feedback loop of the X. laevis tadpole.
The Importance of Deiodinases in Metamorphosis Is Predicated on the Local Effect (Cell Autonomy) of Their Expression.
The local expression of D2 results in a lower tissue T4/T3 ratio than the value in blood, confirming that the two are not in equilibrium. This finding emphasizes the cell autonomy of deiodinase action, which is crucial if D2 is to have a role in the timing of metamorphosis. The same large difference in the T4/T3 ratio between plasma and tissues has been reported in fish (38) and rats (39).
The significance of the two kinds of deiodinases in metamorphosis was emphasized first in R. catesbeiana (11). A close correlation was found between the sequence of TH-induced change in tissues and the expression of D2 and D3. In X. laevis, D3 modulates certain local metamorphic changes. Wherever we have found high expression of D3, that tissue is resistant to TH-induced metamorphic change (13, 14). The level of D3 in X. laevis limb buds is low, but there is a constitutive level of D3 in tail early in premetamorphosis that rises during prometamorphosis and then drops just before tail resorption (27). Constitutive expression of D3 in the dorsal retina accounts for the asymmetric growth of the ventral retina during tadpole growth and also the ipsilateral projections from the retina to the optic tecta that occur late in prometamorphosis (15). Clearly, D3 functions in the retina in a cell-autonomous manner. Overexpression of D3 by transgenesis interferes with induced and spontaneous metamorphosis by reducing the effective level of TH (12).
D2 expression marks the time in the developmental program when a subset of tadpole tissues or organs will be induced to change by TH. The early-limb and late-tail expressions of D2 correlate with their times of change. This relationship is supported by the demonstration that T3 is required to complete the final events at climax (Fig. 1B). These observations are entirely in agreement with the original findings for D2 expression profiles in R. catesbeiana (11). We also have shown here that expression of D2 is up-regulated specifically in those cells of the anterior pituitary that express the genes for TSH (Fig. 5). This up-regulation happens at climax, when the pituitary responds to the high endogenous TH levels that orchestrate the final changes of metamorphosis. Then the feedback loop is completed, as the locally synthesized T3 reduces TSH production by the pituitary marking the end of metamorphosis.
Acknowledgments
We thank Heather Henry for technical support. We are grateful to Drs. V. Galton and P. R. Larsen and our colleagues for critical reading of this manuscript. This research was supported by grants to D.D.B. from the National Institutes of Health and the G. Harold and Leila Y. Mathers Charitable Trust.
Abbreviations
- TH
thyroid hormone
- NF
Nieukoop Faber stages
- TSHα and TSHβ
thyrotropin subunits alpha and beta
- TR
thyroid receptor
- TRH
thyrotropin-releasing hormone
- CRF
corticotropin-releasing factor
- GH
GH
- PRL
prolactin
- POMC
proopiomelanocortin
- D2
type II iodothyronine deiodinase
- D3
type III iodothyronine deiodinase
- T3
3,5,3′-triiodothyronine
- T4
thyroxine or 3,5,3′,5′-tetraiodothyronine
Footnotes
References
- 1.Buscaglia M, Leloup J, deLuze A. In: Metamorphosis. Balls M, Bownes M, editors. New York: Clarendon; 1985. pp. 273–293. [Google Scholar]
- 2.Regard E, Taurog A, Nakashima T. Endocrinology. 1978;102:674–684. doi: 10.1210/endo-102-3-674. [DOI] [PubMed] [Google Scholar]
- 3.Dodd M H I, Dodd J M. In: Physiology of the Amphibia. Lofts B, editor. III. New York: Academic; 1976. pp. 467–599. [Google Scholar]
- 4.Kollros J J. Am Zool. 1961;1:107–114. [Google Scholar]
- 5.Nieuwkoop P D, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: Elsevier Biomedical; 1956. [Google Scholar]
- 6.Larsen P R. Adv Exp Med Biol. 1989;261:11–26. doi: 10.1007/978-1-4757-2058-7_3. [DOI] [PubMed] [Google Scholar]
- 7.Buckbinder L, Brown D D. Proc Natl Acad Sci USA. 1993;90:3820–3824. doi: 10.1073/pnas.90.9.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etkin W. In: Metamorphosis: A Problem in Developmental Biology. Etkin W, Gilbert L I, editors. New York: Appleton-Century-Croft; 1968. pp. 313–339. [Google Scholar]
- 9.Diamond E J. In: Endocrine Methods. Thomas J A, editor. San Diego: Academic; 1996. pp. 157–186. [Google Scholar]
- 10.Frieden E, Yoshizato K. Endocrinology. 1974;95:188–194. doi: 10.1210/endo-95-1-188. [DOI] [PubMed] [Google Scholar]
- 11.Becker K B, Stephens K C, Davey J C, Schneider M J, Galton V A. Endocrinology. 1997;138:2989–2997. doi: 10.1210/endo.138.7.5272. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Marsh-Armstrong N, Brown D D. Proc Natl Acad Sci USA. 1999;96:962–967. doi: 10.1073/pnas.96.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry D L, Schwartzman R A, Brown D D. Dev Biol. 1998;203:12–23. doi: 10.1006/dbio.1998.8974. [DOI] [PubMed] [Google Scholar]
- 14.Berry D L, Rose C S, Remo B F, Brown D D. Dev Biol. 1998;203:24–35. doi: 10.1006/dbio.1998.8975. [DOI] [PubMed] [Google Scholar]
- 15.Marsh-Armstrong N, Huang H, Remo B F, Liu T T, Brown D D. Neuron. 1999;24:871–878. doi: 10.1016/s0896-6273(00)81034-x. [DOI] [PubMed] [Google Scholar]
- 16.Tagawa M, Hirano T. Gen Comp Endocrinol. 1987;68:129–135. doi: 10.1016/0016-6480(87)90068-2. [DOI] [PubMed] [Google Scholar]
- 17.Koopdonk-Kool J M, van Lopik-Peterse M C, Veenboer G J, Visser T J, Schoenmakers C H, de Vijlder J J. Anal Biochem. 1993;214:329–331. doi: 10.1006/abio.1993.1497. [DOI] [PubMed] [Google Scholar]
- 18.Davey J C, Becker K B, Schneider M J, St Germain D L, Galton V A. J Biol Chem. 1995;270:26786–26789. doi: 10.1074/jbc.270.45.26786. [DOI] [PubMed] [Google Scholar]
- 19.St. Germain D L, Schwartzman R A, Croteau W, Kanamori A, Wang Z, Brown D D, Galton V A. Proc Natl Acad Sci USA. 1994;91:7767–7771. doi: 10.1073/pnas.91.16.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi H, Hayashi T, Hanaoka Y. Eur J Biochem. 1992;205:105–110. doi: 10.1111/j.1432-1033.1992.tb16756.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuchler K, Richter K, Trnovsky J, Egger R, Kreil G. J Biol Chem. 1990;265:11731–11733. [PubMed] [Google Scholar]
- 22.Stenzel-Poore M P, Heldwein K A, Stenzel P, Lee S, Vale W W. Mol Endocrinol. 1992;6:1716–1724. doi: 10.1210/mend.6.10.1448118. [DOI] [PubMed] [Google Scholar]
- 23.Yaoita Y, Brown D D. Genes Dev. 1990;4:1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Brown D D. Proc Natl Acad Sci USA. 2000;97:190–194. doi: 10.1073/pnas.97.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auf dem Brinke D, Kohrle J, Kodding R, Hesch R D. J Endocrinol Invest. 1980;3:73–76. doi: 10.1007/BF03348222. [DOI] [PubMed] [Google Scholar]
- 26.Fekkes D, van Overmeeren-Kaptein E, Docter R, Hennemann G, Visser T J. Biochim Biophys Acta. 1979;587:12–19. doi: 10.1016/0304-4165(79)90215-0. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Brown D D. J Biol Chem. 1993;268:16270–16278. [PubMed] [Google Scholar]
- 28.Eliceiri B P, Brown D D. J Biol Chem. 1994;269:24459–24465. [PubMed] [Google Scholar]
- 29.Croteau W, Davey J C, Galton V A, St. Germain D L. J Clin Invest. 1996;98:405–417. doi: 10.1172/JCI118806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tu H M, Kim S W, Salvatore D, Bartha T, Legradi G, Larsen P R, Lechan R M. Endocrinology. 1997;138:3359–3368. doi: 10.1210/endo.138.8.5318. [DOI] [PubMed] [Google Scholar]
- 31.Larsen P R, Silva J E, Kaplan M M. Endocr Rev. 1981;2:87–102. doi: 10.1210/edrv-2-1-87. [DOI] [PubMed] [Google Scholar]
- 32.Weiss R E, Forrest D, Pohlenz J, Cua K, Curran T, Refetoff S. Endocrinology. 1997;138:3624–3629. doi: 10.1210/endo.138.9.5412. [DOI] [PubMed] [Google Scholar]
- 33.Borrelli E, Heyman R A, Arias C, Sawchenko P E, Evans R M. Nature (London) 1989;339:538–541. doi: 10.1038/339538a0. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto M, Watanabe Y G. Gen Comp Endocrinol. 2000;119:37–42. doi: 10.1006/gcen.2000.7489. [DOI] [PubMed] [Google Scholar]
- 35.Denver R J. Gen Comp Endocrinol. 1993;91:38–51. doi: 10.1006/gcen.1993.1102. [DOI] [PubMed] [Google Scholar]
- 36.Gancedo B, Corpas I, Alonso-Gomez A L, Delgado M J, Morreale de Escobar G, Alonso-Bedate M. Gen Comp Endocrinol. 1992;87:6–13. doi: 10.1016/0016-6480(92)90143-8. [DOI] [PubMed] [Google Scholar]
- 37.Hayes W P, Loh Y P. Development (Cambridge, UK) 1990;110:747–757. doi: 10.1242/dev.110.3.747. [DOI] [PubMed] [Google Scholar]
- 38.Specker J L, Brown C L, Bern HA. Gen Comp Endo. 1992;88:397–405. doi: 10.1016/0016-6480(92)90234-b. [DOI] [PubMed] [Google Scholar]
- 39.Morreale de Escobar G, Calvo R, Escobar del Ray F, Obregon M J. Endocrinology. 1994;134:2410–2415. doi: 10.1210/endo.134.6.8194467. [DOI] [PubMed] [Google Scholar]