Abstract
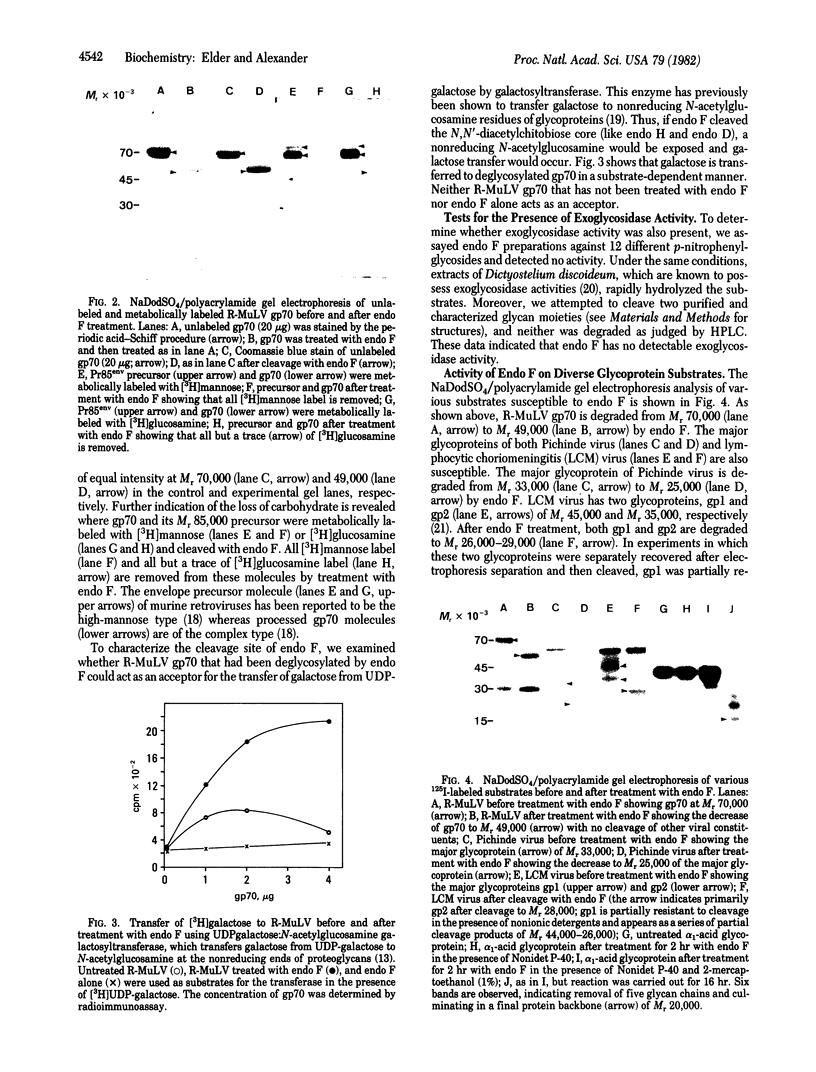
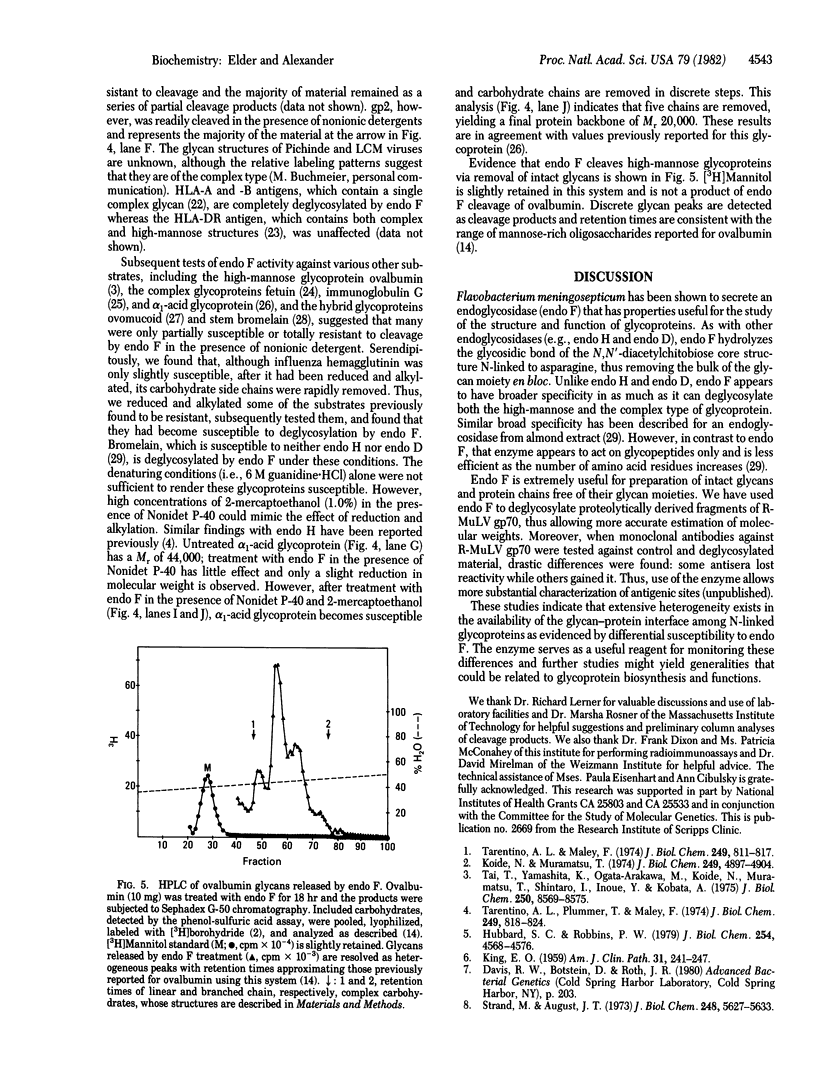
We have detected an endoglycosidase activity produced by Flavobacterium meningosepticum. This enzyme, named endo F, cleaves glycans of both the high-mannose and the complex type linked through asparagine to the protein backbone. The data indicate that cleavage occurs via hydrolysis of the glycosidic bond of the N,N'-diacetylchitobiose core structure adjacent to asparagine, similar to that due to endo H and endo D. Extreme variability was noted in the availability of this cleavage site among N-linked glycoproteins. Glycoproteins of retrovirus, lymphocytic choriomeningitis virus, Pichinde virus, and HLA-A and -B antigens were readily cleaved in the presence of nonionic detergent. Others, such as ovalbumin, fetuin, bromelain, ovomucoid, alpha 1-acid glycoprotein, immunoglobulin G, and influenza virus hemagglutinin became susceptible only after reduction and alkylation or when cleavage was performed in the presence of 1% 2-mercaptoethanol. Endo F should prove useful in the study of glycans and protein backbones as discrete entities and for defining the nature of the glycan-protein interface.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Fiete D. Structure of the complex oligosaccharides of fetuin. J Biol Chem. 1979 Feb 10;254(3):789–795. [PubMed] [Google Scholar]
- Baenziger J., Kornfeld S., Kochwa S. Structure of the carbohydrate units of IgE immunoglobulin. II. Sequence of the sialic acid-containing glycopeptides. J Biol Chem. 1974 Mar 25;249(6):1897–1903. [PubMed] [Google Scholar]
- Buchmeier M. J., Oldstone M. B. Protein structure of lymphocytic choriomeningitis virus: evidence for a cell-associated precursor of the virion glycopeptides. Virology. 1979 Nov;99(1):111–120. doi: 10.1016/0042-6822(79)90042-4. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- Ishihara H., Takahashi N., Oguri S., Tejima S. Complete structure of the carbohydrate moiety of stem bromelain. An application of the almond glycopeptidase for structural studies of glycopeptides. J Biol Chem. 1979 Nov 10;254(21):10715–10719. [PubMed] [Google Scholar]
- KING E. O. Studies on a group of previously unclassified bacteria associated with meningitis in infants. Am J Clin Pathol. 1959 Mar;31(3):241–247. doi: 10.1093/ajcp/31.3.241. [DOI] [PubMed] [Google Scholar]
- Koide N., Muramatsu T. Endo-beta-N-acetylglucosaminidase acting on carbohydrate moieties of glycoproteins. Purification and properties of the enzyme from Diplococcus pneumoniae. J Biol Chem. 1974 Aug 10;249(15):4897–4904. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Separation of neutral oligosaccharides by high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):276–280. doi: 10.1016/0003-2697(81)90480-2. [DOI] [PubMed] [Google Scholar]
- Parham P., Alpert B. N., Orr H. T., Strominger J. L. Carbohydrate moiety of HLA antigens. Antigenic properties and amino acid sequences around the site of glycosylation. J Biol Chem. 1977 Nov 10;252(21):7555–7567. [PubMed] [Google Scholar]
- Prieels J. P., Dolmans M., Schindler M., Sharon N. The binding of glycoconjugates to human-milk D-galactosyltransferase. Eur J Biochem. 1976 Jul 15;66(3):579–582. doi: 10.1111/j.1432-1033.1976.tb10584.x. [DOI] [PubMed] [Google Scholar]
- Rosner M. R., Grinna L. S., Robbins P. W. Differences in glycosylation patterns of closely related murine leukemia viruses. Proc Natl Acad Sci U S A. 1980 Jan;77(1):67–71. doi: 10.1073/pnas.77.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner M. R., Tung J. S., Hopkins N., Robbins P. W. Relationship of GIX antigen expression to the glycosylation of murine leukemia virus glycoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6420–6424. doi: 10.1073/pnas.77.11.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATAKE M., OKUYAMA T., ISHIHARA K., SCHMID K. THE CARBOHYDRATE-POLYPEPTIDE LINKAGES, THE AMINO ACID SEQUENCES OF THE PEPTIDES ADJACENT TO SOME OF THESE BONDS, AND THE COMPOSITION AND SIZE OF THE CARBOHYDRATE UNITS OF ALPHA-1-ACID GLYCOPROTEIN. Biochem J. 1965 Jun;95:749–757. doi: 10.1042/bj0950749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Mirelman D., Schwarz U. Quantitative determination of N-acetylglucosamine residues at the non-reducing ends of peptidoglycan chains by enzymic attachment of [14C]-D-galactose. Eur J Biochem. 1976 Dec;71(1):131–134. doi: 10.1111/j.1432-1033.1976.tb11098.x. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Lampson L. A., Strominger J. L. Analysis of HLA-DR antigens by using monoclonal antibodies: recognition of conformational differences in biosynthetic intermediates. J Immunol. 1981 Oct;127(4):1403–1410. [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Takahashi N., Nishibe H. Some characteristics of a new glycopeptidase acting on aspartylglycosylamine linkages. J Biochem. 1978 Dec;84(6):1467–1473. doi: 10.1093/oxfordjournals.jbchem.a132270. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Wylie D. E., Klinman N. R. The murine B cell repertoire responsive to an influenza-infected syngeneic cell line. J Immunol. 1981 Jul;127(1):194–198. [PubMed] [Google Scholar]