Abstract
Approximately 20 percent of all strokes will occur in the Infratentorial brain. This is within the vascular territory of the posterior vascular circulation. Very few clinical specifics are known about the therapeutic needs of this patient sub-population. Most evidence-based practices are founded from research about the treatment of anterior circulatory stroke. As a consequence, little is known about how stroke in the Infratentorial brain region would require a different approach. We characterized the neurovascular features of Infratentorial stroke, pathophysiological responses, and experimental models for further translational study.
Keywords: Experimental models, Stroke, Infratentorial, Posterior circulation
Introduction
The posterior brain circulation is a common vascular region affected by stroke [1–4]; and one fifth of ischemic and hemorrhagic stroke subtypes will occur there [5–9]. The primary Infratentorial vasculature consists of the single basilar and paired vertebral arteries that collectively supply the thalamus (inferior), occipital lobes, midbrain, brainstem, and cerebellum (Figure 1, A-C). The Infratentorial vertebrobasilar circumferential, paramedian, and perforator vessels are terminal vascular branches; they lack collateral flow and are common sources of the ischemic occlusion or brain hemorrhage [10–11]. Several neurological signs are described for posterior vascular injury, and these are summarized in table 1 [12].
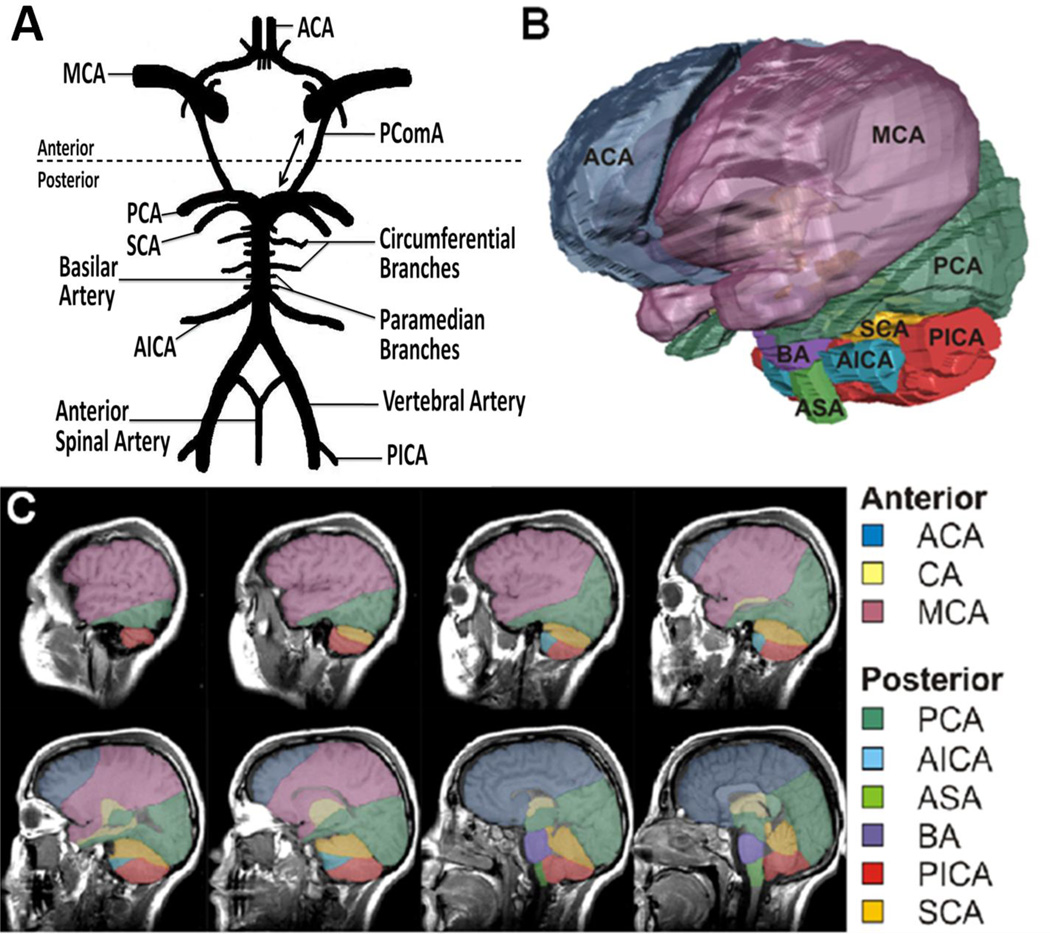
Figure 1.
Illustrations are showing vascular distributions within the human brain. (A) The circle of Willis supplies abundant collateral circulation to the forebrain and hindbrain. Dotted line demarcates anterior /posterior circulation separation at posterior communicating artery (PComA). Double-headed arrow indicates potential reversal of flow across PComA. (B) Volumetric 3-dimensional (3D) reconstruction of the human brain: color-coded to display predominant vascular distributions. (C) Serial sagittal sections demonstrating depth dependant distribution of the respective circulations. ACA indicates anterior cerebral artery; CA, carotid artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; AICA, anterior inferior cerebellar artery; ASA, anterior spinal artery; BA, basilar artery; PICA, posterior inferior cerebellar artery; SCA, superior cerebellar artery
Table 1.
| Vessel | Brain Region | Ipsilateral Signs | Contralateral Signs |
|---|---|---|---|
| SCA | Superior and middle cerebellar peduncles |
Cerebellar ataxia, Dysarthria, Nausea, Vomiting |
Horner’s syndrome, Loss of pain and temperature sensation, Nausea, Vomiting |
| AICA | Middle cerebellar peduncle, Pons (lateral-caudal), Caudal Medulla |
Horner’s syndrome, Facial and lateral gaze weakness, Deafness, Tinnitus, Nausea, Vomiting |
Loss of pain and temperature sensation, Nausea, Vomiting |
| PICA | Cerebellum (inferior), Medulla (lateral) |
Horner’s syndrome, Sensory loss, Diplopia, Nystagmus, Hiccups, Nausea, Vomiting |
Loss of pain and temperature sensation, Nausea, Vomiting |
| BA (caudal) | Medulla (medial) | Tongue paralysis (hypoglossal nerve) |
Hemipalagia, but facial structures unaffected |
SCA indicates superior cerebellar artery; AICA, anterior inferior cerebellar artery; BA, basilar artery; PICA, posterior inferior cerebellar artery
Within the evolution from basic principle and concept to clinical trial translations: few studies will account for Infratentorial stroke cases. Many trials will commonly claim to enroll far too few, or even completely excluding, Infratentorial patients [13–17]. Although these strokes are indeed too rare in some population centers to achieve sufficient numbers, others will control for confounding pathophysiological heterogeneities between anterior and posterior circulations [18–19].
These practices have led to evidence-based guidelines that may not sufficiently represent some important spectrums of stroke. For these reasons, experimental animal models would be useful to help address treatment strategies [18, 20]. Therefore, this review will describe: the neurovascular features, experimental findings, and animal models of posterior circulation stroke, for further study of vascular injury to this brain region.
Pathophysiology: Vascular Responses
Similar vascular mechanisms are shared between ischemic and hemorrhagic strokes [21]. In the brain, cerebrovascular autoregulation maintains optimal tissue perfusion by constricting or dilating the arterial system in response to wide variations of systemic pressures (MABP) and local levels of CO2 [22]. Stroke leads to damaged cerebral autoregulation capacity and a greater dependence upon systemic arterial pressure [23–25]. This occurs after both carotid and vertebrobasilar-based ischemic strokes [24, 26]. Impaired autoregulation has been recognized as an important mechanism of secondary brain injury and edema formation in patients after ischemic stroke [27] and intracerebral hemorrhage [28]. For this reason, MABP and respiratory ability are closely controlled at intensive care units.
Compared to the MCA, vertebrobasilar vessels have a greater capacity to mechanically dilate and constrict, which suggests a greater dynamic autoregulatory ability of the posterior circulation [29–31]. This may enable the hindbrain to divert blood flow to the carotid system during cerebrovascular strain, since a drop in CNS perfusion leads to a proportionally greater diminished flow across the BA compared to the MCA [32]. Systemic CO2 and MABP changes superimposed upon permanent PCA occlusion in dogs showed graded autoregulatory decompensation caudally from the supratentorium to the brainstem, while the MCA autoregulation was preserved [33]. Experimental work in rats showed cerebral sparing when systemic hypotension led to progressive declines of cerebellar autoregulatory kinetics while MCA autoregulatory kinetics remain intact [34]. Cerebellar autoregulatory impairment also occurred after bilateral carotid ligation in spontaneously hypertensive rats [35]. In comparison, the addition of hypocapnia to systemic hypotension in cats, led to greater ischemic susceptibility in the MCA-region compared to the cerebellum [36]. Therefore cerebellar autoregulatory kinetics may handle CO2 changes more favorably in the face of hypoperfusion, while a drop in MABP without systemic CO2 changes would affect the cerebellum more severely [34].
Pathophysiology: Neural Consequences
Ischemic interruption of cerebral blood flow leads to hypoxic and anoxic brain injury, increased neuronal excitability, and cell death [37]. Reperfusion following cerebrovascular ischemia augments this injury through free radial production and mitochondrial dysfunction [38–39]. Similar mechanisms are to blame after hemorrhagic stroke as well (discussed elsewhere) [21].
The cells comprising the CA1 hippocampal region are well known for vulnerability to ischemia; however even these cells may be more resistant to hypoxic-ischemic events than several areas of the hindbrain [40–41]. Notably, electrophysiological studies after hypoxic injury have shown greater neuronal excitability in the hypoglossal (CNXII) and dorsal vagal motor (DVMN) cranial nuclei of the brainstem compared to hippocampal CA1 regions [41]. After anoxia, the hypoglossal nucleus has shown both greater initial injury, and also impaired recovery as compared to temporal lobe neurons [42]. In-vitro simulation of ischemic reperfusion injury, using cell cultures of oxygenglucose deprivation followed by re- oxygenation (OGD-R) showed greater free-radical injury (lipid peroxidation) and mitochondrial impairment in cerebellar cells compared to cerebral cortical cell culture [43].
Comparing cerebellar to brainstem injury after vertebral arterial occlusion, in gerbils (experimental models are summarized in table 2), showed the greatest amount of cell death near areas of coordination and balance (cerebellar interpositus and lateral vestibular nuclei), while brainstem cardio-respiratory areas remained relatively intact [40]. Due to the scattered nature of brainstem nuclei, it is unlikely this finding simply represents re-distribution of blood flow. This is therefore more likely a brain region dependant phenomenon.
Table 2.
Experimental animal models of posterior circulation stroke
| Study | Stroke Type | Species | Experimental Method | Injury Region |
|---|---|---|---|---|
| Chung et al, 1993 | ICH | Cat | Autologous blood injection | Brain Stem |
| Cossu et al, 1994 | ICH | Rat | Autologous blood injection | Cerebellum |
| Guo et al, 1995 | Ischemic | Dog | Embolized PComA and SCA, then clamped VA and ventral spinal artery |
Brainstem |
| Hata et al, 1994 | Ischemic | Cat | Extra-cranial VA occlusion | Brainstem Cerebellum |
| Henninger et al, 2006 | Ischemic | Rat | Injected Autologous clots into VA |
Brainstem Cerebellum |
| Kuwabara et al, 1988 | Ischemic | Dog | Occluded perforators of PCA | Brainstem |
| Nakahara et al, 1991 | Ischemic | Cat | Radiographic embolization of VA |
Brainstem Cerebellum |
| Qureshi et al, 2004 | Ischemic | Dog | Radiographic embolization BA | Brainstem |
| Sekiguchi et al, 2005 | Ischemic | Rat | Microspheres into right CCA | Cerebellum Forebrain |
| Shiroyama et al, 1991 | Ischemic | Rat | Endoluminal suture into BA | Brainstem |
| Wojak et al, 1991 | Ischemic | Rat | Coagulated BA | Brain Stem |
| Yamada et al, 1984 | Ischemic | Gerbil | Vascular-clip to BA | Brainstem Cerebellum |
| Yao et al, 1990 | Ischemic | Rat | Cauterized VA and decreased MAP |
Brainstem Cerebellum |
ICH indicates intracerebral hemorrhage; VA, vertebral artery; BA, basilar artery; CCA, common carotid artery; MAP, mean arterial pressure; PCA, posterior cerebral artery; PComA, posterior communicating artery; SCA, superior cerebellar artery
In support of this notion, magnetic resonance imaging (MRI) perfusion and diffusion studies in humans have determined white matter to have an infarction threshold of 20ml/100g/minute, while gray matter can sustain flow down to infarctions starting at 12ml/100g/minute [44–47]. The cerebellum and brainstem have an abundance of white matter tracts, and this implicates a greater vulnerability to ischemic injury. Therefore, the viability of brainstem cardiorespiratory centers during periods of stress, such as severe systemic hypotension, global cerebral ischemia, and cardiac arrest will require further investigation- as this could yield many lasting clinical implications.
Experimental Studies: Ischemic Stroke
Animal models of posterior circulation stroke (see table 2) have revealed several mechanisms of injury as targets for future study. In progressive hypotension in rats the autoregulatory kinetics remained intact at the cerebrum, while there was a progressive loss of autoregulatory efficacy in the cerebellum [34]. However, a manipulation of both mean arterial blood pressure (MABP) and CO2 levels (in cats) and measuring blood flow (hydrogen clearance method) in the cerebrum, cerebellum and spinal cord, found a greater susceptibility to pressure dependant ischemia in the cerebrum and spinal cord than the cerebellum, which was relatively resistant [36].
De Bray et al [48] used transcranial Doppler to compare blood flow in the supratentorial and infratentorial compartments under increasing intracranial pressure (in rabbits). The maximum amplitude of vasomotor activity occurred 30 seconds later in the basilar artery compared to the carotid siphon. This indicates a delayed effect of intracranial pressure on hindbrain microvascular tone. Matsumoto et al [33] caused permanent occlusion of posterior cerebral artery perforators (canine model). They monitored cerebral blood flow (autoregulation) and carbon dioxide reactivity in response to induced hypotension or hypertension during the occlusion. The cerebral cortex maintained autoregulation and carbon dioxide reactivity, while thalamic autoregulation was maintained during hypotension, but not hypertension. On the other hand, the midbrain had markedly impaired autoregulation and carbon dioxide reactivity. This suggests a differential vulnerability to permanent vascular occlusion, and the brainstem may decompensate compared to the forebrain areas, in spite of abundant posterior collateral circulation.
Using a model of bilateral carotid ligation (in spontaneously hypertensive rats), impaired autoregulation was demonstrated in the cerebrum [49]. However, the addition of stepwise drop in blood pressure caused impairment of cerebellar autoregulation as well. This suggests a vulnerability to hypotension in a distant area from the original stroke location, an effect possibly modulated by the alpha-adrenoceptor system (vasoconstrictive), secondary to cerebral hypertensive stimuli or other transtentorial signals [35, 49–50]. The chronic collateral vascular response may be age dependant, since bilateral carotid occlusion led to a greater dependence on basilar flow in adult rats, compared to extra-cerebral midline collaterals in the younger animals [51–53].
Many animal studies of anterior circulation ischemic stroke have demonstrated impaired autoregulation after ischemic stroke. The extent of which would depend on occlusion duration and extent of reperfusion hyperemia [54–56]. This physiological response would be expected to contribute to injury in the posterior brain region, and the effects of global brain ischemia after cardiac arrest needs further study as well.
Experimental Studies: Hemorrhagic Stroke
One-fifth of the approximate 2 million worldwide intracerebral hemorrhages (ICHs) each year will occur in the infratentorium [7–9]. Brainstem hemorrhages have an approximate 65% mortality rate and around 40% after cerebellar hemorrhage [57–59]. Prolonged endovascular cerebrovascular damage from uncontrolled hypertension leads to arteriosclerotic and amyloid angiopathic changes, vessel fragility and rupture at the deep cerebellar vessels or brainstem basilar (paramedian) branches [8, 60]. Less common causes of occurrence are: cancer, coagulopathy, or vascular anomalies (arterial-venous malformations, aneurysms, cavernomas and dural arteriovenous fistulas) [8, 60]. For most patients, supportive care is the best treatment rendered, since surgery is only available for one-quarter of hospitalized cerebellar hemorrhage patients, and the brainstem is not surgically accessible [61–64].
Mechanisms of infratentorial hemorrhage have not been studied. Due to the small size of the hindbrain region, previous attempts using autologous blood injection could not reproduce consistent hematomas, and consequently have received no further study [65–66]. Therefore our preliminary studies developed experimental models of infratentorial intracerebral hemorrhage (Figure 2) using clostridial collagenase to induce a hematoma in the cerebellum or brainstem [67–68]. These animal models successfully mimic the clinical hemorrhage at the infratentorial region (Figure 3). In clinical agreement, these animals were highly ataxic, with motor-sensory, cognitive, and cranial nerve deficits. Most animals survived past 30 days, so long as gustatory, cardiovascular and reticular-activating systems remained intact. These approaches produced consistent bleeding inside the tissue borders of these small brain regions, with reproducible neurological and morphological features which can be intervened with neuroprotective treatments in future studies.
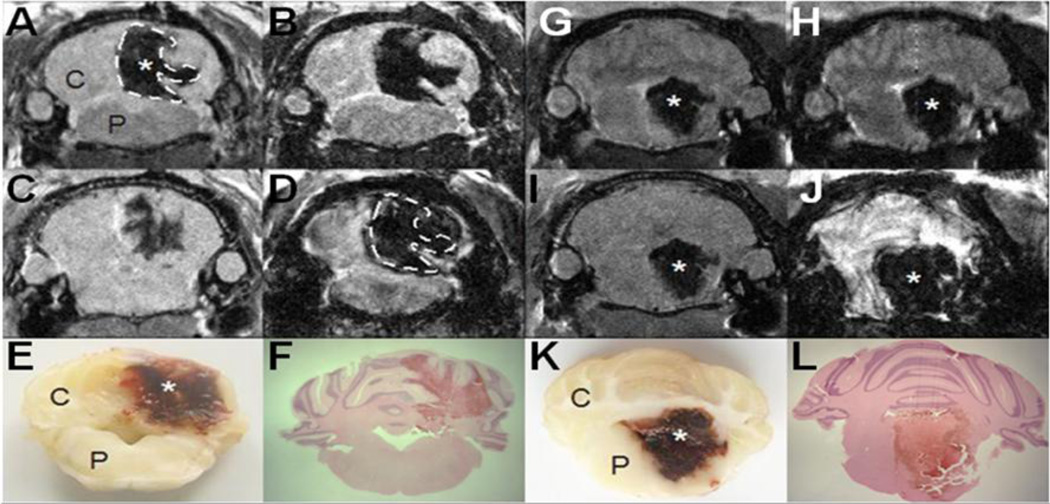
Figure 2.
Photomicrographs demonstrate how multiple MRI contrasts can characterize infratentorial hemorrhage in the rodent brain (*). T2 weighted imaging (T2WI; A and G) can easily identify the location of the ICH injury based on loss of signal within the hemorrhage and it can also provide information on peri-lesional edema. Diffusion-weighted imaging (DWI; B and H) can also delineate the ICH, but is more useful to evaluate ongoing cellular changes such that there is an increased signal around the ICH lesion consistent with cellular swelling. T1 weighted imaging (T1WI; C and I) can readily evaluate the blood-brain barrier if an exogenous contrast agent such as Gadolinium is administered. More recently, susceptibility weighted imaging (SWI; D and J) has been shown to be extraordinarily sensitive to extravascular blood, as shown with the dotted line, SWI identifies a larger region of hemorrhage than the T2W, and is particularly useful for small hemorrhages not be visible on standard imaging modalities. All data can be readily correlated with gross (E and K) and histological (F and L) specimens. C=cerebellum and P=pons.
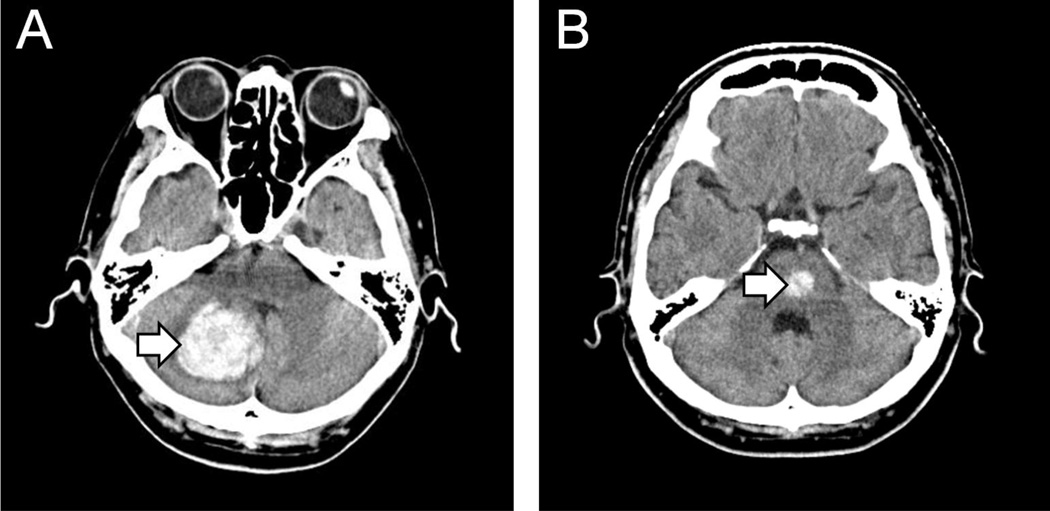
Figure 3.
Images showing computed tomography of the head. The lesion foci (white arrows) represent Infratentorial hemorrhage in the Human cerebellum (A), and the pons (B).
Conclusion
The hindbrain has many neural tracts and nuclei that are critical and involved with orchestrating, processing and transmitting information between the cerebral cortexes and spine. Cerebrovascular injuries to the Infratentorium can therefore be particularly devastating. In support of this notion, several animal studies and clinical reports together indicate that the Infratentorial brain region may have less innate neurovascular protective mechanisms, and greater amounts of cell death and injury, in comparison to supratentorial brain regions, after stroke.
Though a very limited, yet significant, amount of experimental study has been done for ischemic posterior circulation stroke, hemorrhage into the infratentorium has received almost no study to date. In spite of shared mechanisms between ischemic and hemorrhagic strokes, there is an urgent need to study ICH in the hindbrain. Future studies can use these experimental models of ICH, and an array of other ischemic models, to test interventions for reversing the mechanisms of injury in this brain region.
Acknowledgments
This review was partially supported by a grant from NIH NS53407 to John H. Zhang. The neuroimaging support was provided in part by a NASA cooperative agreement (NCCQ-XX) to Loma Linda University. The authors wish to thank Pete Hayes for assistance with animal imaging.
References
- 1.Caplan L. Posterior circulation ischemia: then, now, and tomorrow. The Thomas Willis Lecture-2000. Stroke. 2000;31(8):2011–2023. doi: 10.1161/01.str.31.8.2011. [DOI] [PubMed] [Google Scholar]
- 2.Belden JR, Caplan LR, Pessin MS, Kwan E. Mechanisms and clinical features of posterior border-zone infarcts. Neurology. 1999;53(6):1312–1318. doi: 10.1212/wnl.53.6.1312. [DOI] [PubMed] [Google Scholar]
- 3.Bogousslavsky J, Regli F, Maeder P, Meuli R, Nader J. The etiology of posterior circulation infarcts: a prospective study using magnetic resonance imaging and magnetic resonance angiography. Neurology. 1993;43(8):1528–1533. doi: 10.1212/wnl.43.8.1528. [DOI] [PubMed] [Google Scholar]
- 4.Bogousslavsky J. Posterior circulation strokes. In: Fisher M, editor. Stroke Part II: Clinical Manifestations and Pathogenesis. Elsevier B.V.; 2009. pp. 537–558. [Google Scholar]
- 5.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 6.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19(9):1083–1092. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty ML, Woo D, Haverbusch M, Sekar P, Khoury J, Sauerbeck L, et al. Racial variations in location and risk of intracerebral hemorrhage. Stroke. 2005;36(5):934–937. doi: 10.1161/01.STR.0000160756.72109.95. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland GR, Auer RN. Primary intracerebral hemorrhage. J Clin Neurosci. 2006;13(5):511–517. doi: 10.1016/j.jocn.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Oliveira E, Tedeschi H, Rhoton A, Peace D. Microsurgical anatomy of the posterior circulation: vertebral and basilar arteries. In: Carter L, Spetzler R, Hamilton M, editors. Neurovascular Surgery. New York: McGraw-Hill Inc.; 1995. pp. 25–34. [Google Scholar]
- 11.Duvemoy H. Human Brain Stem Vessels. Berlin: Springer; 1999. [Google Scholar]
- 12.Bogousslavsky J. Stroke Syndromes. Second ed. Cambridge University Press; 2001. p. 770. [Google Scholar]
- 13.Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. 1988;19(5):547–554. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- 14.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282(21):2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 15.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274(13):1017–1025. [PubMed] [Google Scholar]
- 16.Mendelow AD, Unterberg A. Surgical treatment of intracerebral haemorrhage. Curr Opin Crit Care. 2007;13(2):169–174. doi: 10.1097/MCC.0b013e3280a9e5c2. [DOI] [PubMed] [Google Scholar]
- 17.Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g) : results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke. 2000;31(4):811–816. doi: 10.1161/01.str.31.4.811. [DOI] [PubMed] [Google Scholar]
- 18.Macleod M. Current issues in the treatment of acute posterior circulation stroke. CNS Drugs. 2006;20(8):611–621. doi: 10.2165/00023210-200620080-00001. [DOI] [PubMed] [Google Scholar]
- 19.Macleod MR, Davis SM, Mitchell PJ, Gerraty RP, Fitt G, Hankey GJ, et al. Results of a multicentre, randomised controlled trial of intra-arterial urokinase in the treatment of acute posterior circulation ischaemic stroke. Cerebrovasc Dis. 2005;20(1):12–17. doi: 10.1159/000086121. [DOI] [PubMed] [Google Scholar]
- 20.Lekic T, Zhang JH. Posterior circulation stroke and animal models. Front Biosci. 2008;13:1827–1844. doi: 10.2741/2803. [DOI] [PubMed] [Google Scholar]
- 21.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5(1):53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 22.Kety SS, Schmidt CF. The Effects of Altered Arterial Tensions of Carbon Dioxide and Oxygen on Cerebral Blood Flow and Cerebral Oxygen Consumption of Normal Young Men. J Clin Invest. 1948;27(4):484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eames PJ, Blake MJ, Dawson SL, Panerai RB, Potter JF. Dynamic cerebral autoregulation and beat to beat blood pressure control are impaired in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2002;72(4):467–472. doi: 10.1136/jnnp.72.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson SL, Panerai RB, Potter JF. Serial changes in static and dynamic cerebral autoregulation after acute ischaemic stroke. Cerebrovasc Dis. 2003;16(1):69–75. doi: 10.1159/000070118. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of body position on intracranial pressure and cerebral perfusion in patients with large hemispheric stroke. Stroke. 2002;33(2):497–501. doi: 10.1161/hs0202.102376. [DOI] [PubMed] [Google Scholar]
- 26.Dawson SL, Blake MJ, Panerai RB, Potter JF. Dynamic but not static cerebral autoregulation is impaired in acute ischaemic stroke. Cerebrovasc Dis. 2000;10(2):126–132. doi: 10.1159/000016041. [DOI] [PubMed] [Google Scholar]
- 27.Dohmen C, Bosche B, Graf R, Reithmeier T, Ernestus RI, Brinker G, et al. Identification and clinical impact of impaired cerebrovascular autoregulation in patients with malignant middle cerebral artery infarction. Stroke. 2007;38(1):56–61. doi: 10.1161/01.STR.0000251642.18522.b6. [DOI] [PubMed] [Google Scholar]
- 28.Diedler J, Sykora M, Rupp A, Poli S, Karpel-Massler G, Sakowitz O, et al. Impaired cerebral vasomotor activity in spontaneous intracerebral hemorrhage. Stroke. 2009;40(3):815–819. doi: 10.1161/STROKEAHA.108.531020. [DOI] [PubMed] [Google Scholar]
- 29.Ito H, Yokoyama I, Iida H, Kinoshita T, Hatazawa J, Shimosegawa E, et al. Regional differences in cerebral vascular response to PaCO2 changes in humans measured by positron emission tomography. J Cereb Blood Flow Metab. 2000;20(8):1264–1270. doi: 10.1097/00004647-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Hida W, Kikuchi Y, Okabe S, Miki H, Kurosawa H, Shirato K. CO2 response for the brain stem artery blood flow velocity in man. Respir Physiol. 1996;104(1):71–75. doi: 10.1016/0034-5687(96)00011-4. [DOI] [PubMed] [Google Scholar]
- 31.Reinhard M, Waldkircher Z, Timmer J, Weiller C, Hetzel A. Cerebellar autoregulation dynamics in humans. J Cereb Blood Flow Metab. 2008;28(9):1605–1612. doi: 10.1038/jcbfm.2008.48. [DOI] [PubMed] [Google Scholar]
- 32.Garbin L, Habetswallner F, Clivati A. Vascular reactivity in middle cerebral artery and basilar artery by transcranial Doppler in normals subjects during hypoxia. Ital J Neurol Sci. 1997;18(3):135–137. doi: 10.1007/BF02048480. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto S, Kuwabara S, Moritake K. Effects of cerebrovascular autoregulation and CO2 reactivity in experimental localized brainstem infarction. Neurol Res. 2000;22(2):197–203. doi: 10.1080/01616412.2000.11741061. [DOI] [PubMed] [Google Scholar]
- 34.Merzeau S, Preckel MP, Fromy B, Leftheriotis G, Saumet JL. Differences between cerebral and cerebellar autoregulation during progressive hypotension in rats. Neurosci Lett. 2000;280(2):103–106. doi: 10.1016/s0304-3940(00)00763-1. [DOI] [PubMed] [Google Scholar]
- 35.Shiokawa O, Sadoshima S, Fujii K, Yao H, Fujishima M. Impairment of cerebellar blood flow autoregulation during cerebral ischemia in spontaneously hypertensive rats. Stroke. 1988;19(5):615–622. doi: 10.1161/01.str.19.5.615. [DOI] [PubMed] [Google Scholar]
- 36.Sato M, Pawlik G, Heiss WD. Comparative studies of regional CNS blood flow autoregulation and responses to CO2 in the cat. Effects of altering arterial blood pressure and PaCO2 on rCBF of cerebrum, cerebellum, and spinal cord. Stroke. 1984;15(1):91–97. doi: 10.1161/01.str.15.1.91. [DOI] [PubMed] [Google Scholar]
- 37.Fujiwara N, Higashi H, Shimoji K, Yoshimura M. Effects of hypoxia on rat hippocampal neurones in vitro. J Physiol. 1987;384:131–151. doi: 10.1113/jphysiol.1987.sp016447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Back T. Pathophysiology of the ischemic penumbra--revision of a concept. Cell Mol Neurobiol. 1998;18(6):621–638. doi: 10.1023/a:1020629818207. [DOI] [PubMed] [Google Scholar]
- 39.Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 1998;18(6):667–682. doi: 10.1023/a:1020685903186. [DOI] [PubMed] [Google Scholar]
- 40.Hata R, Matsumoto M, Hatakeyama T, Ohtsuki T, Handa N, Niinobe M, et al. Differential vulnerability in the hindbrain neurons and local cerebral blood flow during bilateral vertebral occlusion in gerbils. Neuroscience. 1993;56(2):423–439. doi: 10.1016/0306-4522(93)90343-e. [DOI] [PubMed] [Google Scholar]
- 41.Donnelly DF, Jiang C, Haddad GG. Comparative responses of brain stem and hippocampal neurons to O2 deprivation: in vitro intracellular studies. Am J Physiol. 1992;262(5 Pt 1):L549–L554. doi: 10.1152/ajplung.1992.262.5.L549. [DOI] [PubMed] [Google Scholar]
- 42.O'Reilly JP, Jiang C, Haddad GG. Major differences in response to graded hypoxia between hypoglossal and neocortical neurons. Brain Res. 1995;683(2):179–186. doi: 10.1016/0006-8993(95)00373-x. [DOI] [PubMed] [Google Scholar]
- 43.Scorziello A, Pellegrini C, Forte L, Tortiglione A, Gioielli A, Iossa S, et al. Differential vulnerability of cortical and cerebellar neurons in primary culture to oxygen glucose deprivation followed by reoxygenation. J Neurosci Res. 2001;63(1):20–26. doi: 10.1002/1097-4547(20010101)63:1<20::AID-JNR3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 44.Bristow MS, Simon JE, Brown RA, Eliasziw M, Hill MD, Coutts SB, et al. MR perfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J Cereb Blood Flow Metab. 2005;25(10):1280–1287. doi: 10.1038/sj.jcbfm.9600135. [DOI] [PubMed] [Google Scholar]
- 45.Arakawa S, Wright PM, Koga M, Phan TG, Reutens DC, Lim I, et al. Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke. 2006;37(5):1211–1216. doi: 10.1161/01.STR.0000217258.63925.6b. [DOI] [PubMed] [Google Scholar]
- 46.Liu S, Levine SR, Winn HR. Targeting ischemic penumbra: part I - from pathophysiology to therapeutic strategy. J Exp Stroke Transl Med. 2010;3(1):47–55. doi: 10.6030/1939-067x-3.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moustafa RR, Baron JC. Pathophysiology of ischaemic stroke: insights from imaging, and implications for therapy and drug discovery. Br J Pharmacol. 2008;153(Suppl 1):S44–S54. doi: 10.1038/sj.bjp.0707530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Bray JM, Tranquart F, Saumet JL, Berson M, Pourcelot L. Cerebral vasodilation capacity: acute intracranial hypertension and supra- and infra-tentorial artery velocity recording. Clin Physiol. 1994;14(5):501–512. doi: 10.1111/j.1475-097x.1994.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 49.Shiokawa O, Sadoshima S, Kusuda K, Nishimura Y, Ibayashi S, Fujishima M. Cerebral and cerebellar blood flow autoregulations in acutely induced cerebral ischemia in spontaneously hypertensive rats--transtentorial remote effect. Stroke. 1986;17(6):1309–1313. doi: 10.1161/01.str.17.6.1309. [DOI] [PubMed] [Google Scholar]
- 50.Shiokawa O, Sadoshima S, Okada Y, Nagao T, Fujishima M. Alpha- and beta-adrenergic receptors of noradrenergic innervation modulate the lower limits of cerebral and cerebellar blood flow autoregulation in spontaneously hypertensive rats. Gerontology. 1989;35(2–3):106–112. doi: 10.1159/000213007. [DOI] [PubMed] [Google Scholar]
- 51.Vavilala MS, Kincaid MS, Muangman SL, Suz P, Rozet I, Lam AM. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr Res. 2005;58(3):574–578. doi: 10.1203/01.PDR.0000179405.30737.0F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choy M, Ganesan V, Thomas DL, Thornton JS, Proctor E, King MD, et al. The chronic vascular and haemodynamic response after permanent bilateral common carotid occlusion in newborn and adult rats. J Cereb Blood Flow Metab. 2006;26(8):1066–1075. doi: 10.1038/sj.jcbfm.9600259. [DOI] [PubMed] [Google Scholar]
- 53.Tontisirin N, Muangman SL, Suz P, Pihoker C, Fisk D, Moore A, et al. Early childhood gender differences in anterior and posterior cerebral blood flow velocity and autoregulation. Pediatrics. 2007;119(3):e610–e615. doi: 10.1542/peds.2006-2110. [DOI] [PubMed] [Google Scholar]
- 54.Drummond JC, Oh YS, Cole DJ, Shapiro HM. Phenylephrine-induced hypertension reduces ischemia following middle cerebral artery occlusion in rats. Stroke. 1989;20(11):1538–1544. doi: 10.1161/01.str.20.11.1538. [DOI] [PubMed] [Google Scholar]
- 55.Cipolla MJ, McCall AL, Lessov N, Porter JM. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997;28(1):176–180. doi: 10.1161/01.str.28.1.176. [DOI] [PubMed] [Google Scholar]
- 56.Olah L, Franke C, Schwindt W, Hoehn M. CO(2) reactivity measured by perfusion MRI during transient focal cerebral ischemia in rats. Stroke. 2000;31(9):2236–2244. doi: 10.1161/01.str.31.9.2236. [DOI] [PubMed] [Google Scholar]
- 57.Flaherty ML, Haverbusch M, Sekar P, Kissela B, Kleindorfer D, Moomaw CJ, et al. Long-term mortality after intracerebral hemorrhage. Neurology. 2006;66(8):1182–1186. doi: 10.1212/01.wnl.0000208400.08722.7c. [DOI] [PubMed] [Google Scholar]
- 58.Balci K, Asil T, Kerimoglu M, Celik Y, Utku U. Clinical and neuroradiological predictors of mortality in patients with primary pontine hemorrhage. Clin Neurol Neurosurg. 2005;108(1):36–39. doi: 10.1016/j.clineuro.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31(1):123–127. doi: 10.1161/01.str.31.1.123. [DOI] [PubMed] [Google Scholar]
- 60.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 61.Tuhrim S. Intracerebral hemorrhage--improving outcome by reducing volume? N Engl J Med. 2008;358(20):2174–2176. doi: 10.1056/NEJMe0801856. [DOI] [PubMed] [Google Scholar]
- 62.Adeoye O, Woo D, Haverbusch M, Sekar P, Moomaw CJ, Broderick J, et al. Surgical management and case-fatality rates of intracerebral hemorrhage in 1988 and 2005. Neurosurgery. 2008;63(6):1113–1117. doi: 10.1227/01.NEU.0000330414.56390.DE. discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morioka J, Fujii M, Kato S, Fujisawa H, Akimura T, Suzuki M, et al. Surgery for spontaneous intracerebral hemorrhage has greater remedial value than conservative therapy. Surg Neurol. 2006;65(1):67–72. doi: 10.1016/j.surneu.2005.03.023. discussion-3. [DOI] [PubMed] [Google Scholar]
- 64.Fewel ME, Thompson BG, Jr., Hoff JT. Spontaneous intracerebral hemorrhage: a review. Neurosurg Focus. 2003;15(4):E1. [PubMed] [Google Scholar]
- 65.Chung Y, Haines SJ. Experimental brain stem surgery. Neurosurg Clin N Am. 1993;4(3):405–414. [PubMed] [Google Scholar]
- 66.Cossu M, Pau A, Siccardi D, Viale GL. Infratentorial ischaemia following experimental cerebellar haemorrhage in the rat. Acta Neurochir (Wien) 1994;131(1–2):146–150. doi: 10.1007/BF01401465. [DOI] [PubMed] [Google Scholar]
- 67.Lekic T, Tang J, Zhang JH. A rat model of pontine hemorrhage. Acta Neurochir Suppl. 2008;105:135–137. doi: 10.1007/978-3-211-09469-3_28. [DOI] [PubMed] [Google Scholar]
- 68.Lekic T, Tang J, Zhang JH. Rat model of intracerebellar hemorrhage. Acta Neurochir Suppl. 2008;105:131–134. doi: 10.1007/978-3-211-09469-3_27. [DOI] [PubMed] [Google Scholar]
- 69.Goetz C. Textbook of Clinical Neurology. 2nd ed. Philadelphia: Saunders; 2003. Vertebrobasilar Stroke Syndromes; pp. 415–416. [Google Scholar]
- 70.Worthley LI, Holt AW. Acute ischaemic stroke: part II. The vertebrobasilar circulation. Crit Care Resusc. 2000;2(2):140–145. [PubMed] [Google Scholar]