Abstract
Establishment of Planar Cell Polarity (PCP) in epithelia, in the plane of an epithelium, is an important feature of the development and homeostasis of most organs. Studies in different model organisms have contributed a wealth of information regarding the mechanisms that govern PCP regulation. Genetic studies in Drosophila have identified two signaling systems, the Fz/PCP and Fat/Dachsous system, which are both required for PCP establishment in many different tissues in a largely non-redundant manner. Recent advances in vertebrate PCP studies have added novel factors of PCP regulation and also new cellular features requiring PCP signaling input, including the positioning and orientation of the primary cilium of many epithelial cells. This review focuses mostly on several recent advances made in the Drosophila and vertebrate PCP field and integrates these within the existing PCP signaling framework.
Keywords: PCP, Frizzled, Dishevelled, Cilia, Convergent extension, Organ patterning
Polarization of epithelia is an important feature for the development, patterning, maintenance and homeostasis of individual organs and whole organisms. All epithelia are defined by their apical-basal polarization, which is critical for its function in vectorial secretion and uptake and also as barrier between a fluid filled space and the organism internal tissues. In addition, many (if not all) epithelia are also polarized within the plane of the epithelium. Polarization within the plane is referred to as Planar Cell Polarity (PCP).
The study of PCP originates from an observation in insects some 40 years ago, then referred to as “tissue polarity”1–3. Emerging from its obscurity, a lot has been uncovered since these early PCP studies and this type of polarity has become a highly studied topic of mainstream research4–8 and links to human disease states9. Drosophila has been a highly valuable organism for the study of PCP, as all of its adult cuticular structures display PCP features and are thus easy to study (Figure 1)4–6. Drosophila PCP establishment has contributed a wealth of information regarding the mechanisms that govern PCP establishment4–6, 10, 11. Studies in Drosophila have uncovered two groups of PCP factors/systems: (1) the Frizzled (Fz)/PCP core group, which includes Fz, Van Gogh/Strabismus (Vang/Stbm), Flamingo (Fmi; a.k.a. Starry Night/Stan), Dishevelled (Dsh), Prickle (Pk) and Diego (Dgo) (see Table 1 for details), and (2) the Fat/Dachsous (Ds) group containing Fat, Ds, Four-jointed (Fj), Dachs, and Approximated5, 12 (Table 1).
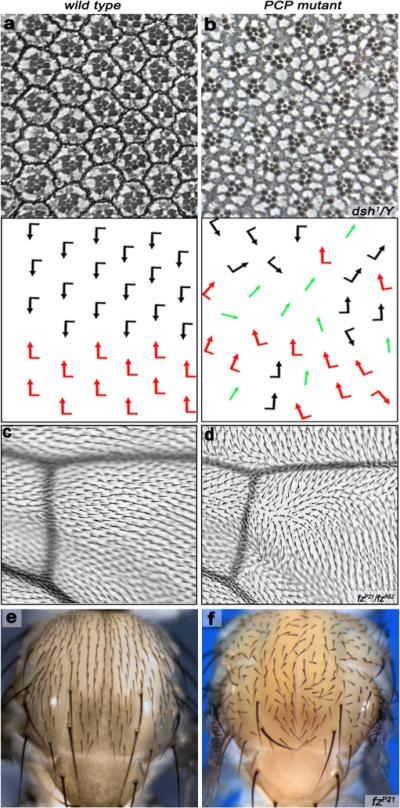
Figure 1. Examples of PCP in adult Drosophila tissues.
(a–b) PCP features in the eye. Anterior is left and dorsal is up. Tangential eye sections showing wild-type adult eye (a) and a dsh1 eye (b) centered on the equator; bottom panels show schematic representations reflecting ommatidial orientation and polarity. Black and red arrows represent the dorsal and ventral chiral forms respectively while green arrows represent R3-R3 symmetrical clusters. In the PCP mutant (dsh1 in b) the arrangement of ommatidia is disorganized. (c–d) PCP aspects of wing patterning. Anterior is up and distal right. Each wing cell gives rise to an actin based hair (trichome) that is pointing distally in wild-type (c). Mutations in PCP genes (a fz example is shown in d) disrupt this near perfect orientation of wing hairs/trichomes, creating swirls and waves.
(e–f) Aspects of PCP establishment on the thorax/notum. Anterior is up. Wild type adult thorax (e) showing mechano-sensory bristles, which are patterned uniformly across the notum and oriented in the anterior-posterior axis of the Drosophila body. In fz mutant (f) this regular pattern is randomized. In addition to the sensory bristles, all body wall cells form actin-rich trichomes (like in the wing), which are also oriented in the Anterior-posterior axis (not visible at this magnification).
Table 1.
Core PCP genes and selected associated regulators in Drosophila and vertebrates
| Drosophila genes | Vertebrate genes | Molecular features | Tissues/processes affected | Refs | |
|---|---|---|---|---|---|
| Drosophila | vertebrates# | ||||
| Core Fz/PCP components | |||||
|
| |||||
| frizzled (fz) | Fz1 (mouse) | Seven-pass transmembrane receptor; can bind Wnt ligands; binds Dsh; recruits Dsh and Dgo to membrane. | All adult tissues | Inner ear, epidermis,neu ral tube closure, CE | 3, 15, 79,166–170 |
| Fz2 (mouse) | |||||
| Fz3 (mouse) | |||||
| Fz6 (mouse) | |||||
| Fz7 (Xenopus) | |||||
| and others | |||||
| dishevelled (dsh) |
mDvll/2/3 (mouse)
XDsh (Xenopus) |
Cytoplasmic protein containing DIX, PDZ, DEP domains; recruited to membrane by Fz; binds Fz, Pk, Stbm and Dgo; undergoes extensive phosphorylation. | All adult tissues | CE, inner ear, | 21, 103, 115, 119, 120, 144, 171–176 |
| strabismus (stbm)/Van Gogh (Vang) | Vangl2 (looptail; mouse), Vangl1 (mouse), trilobite (tri; zebrafish), xStbm (Xenopus) | Novel 4-pass transmembrane protein; binds Pk, Dsh and Dgo; recruits Pk to membrane. | All adult tissues | CE, inner ear,limb elongation, growth cone guidance | 24, 98, 99,177–182 |
| flamingo (fmi)/starry night (stan) | Celsr1/3 (mouse) | Cadherin with seven-pass transmembrane receptor features; capable of homophillic cell adhesion. | All adult tissues | CE, epidermis, inner ear, growth cone guidance | 50, 181, 183–189 |
| prickle (pk) (a.k.a. prickle-spiny legs) | Pk1/2 (mouse, zebrafish) | Cytoplasmic protein with 3 LIM domains and PET domain; recruited to membrane by Stbm; physically interacts with Dsh, Stbm and Dgo; competes with Dgo for Dsh binding. | All adult tissues | CE | 52, 190–194 |
| diego (dgo) | Diversin (ankyrin repeat domain 6)) Inversin (invs) | Cytoplasmic ankyrin repeat protein; recruited to membrane by Fz; binds Dsh, Stbm and Pk; competes with Pk for Dsh binding. | Eye, wing, notum (GOF)* | CE | 56, 64, 142, 195–197 |
|
| |||||
| Regulators of Fz/PCP signaling | |||||
|
| |||||
| Casein Kinase lε/ CK1ε (discs overgrown,dco) | CK1 ε | Serine/Threonine protein kinase; acts positively on Fz/PCP signaling; regulates Dsh localization in the wing. | Eye and wing* | only analyzed in cell culture | 30, 132, 198 |
|
G protein o-α47A
(Goα47A; brokenheart, bkh) |
N.D. | The α subunit of the Go protein; has asymmetric localization in the wing which requires Fz. Also shown to regulate Fz localization in the wing. | Wing* | N.D. | 40, 199 |
| Abelson kinase (dAbl) | Abl1, Abl2 | Member of non receptor tyrosine kinases; binds and phosphorylate Dsh on tyrosine residues | Eye and Wing | only analyzed in cell culture | 31 |
| N.D. |
Glypican 4/6/knypek (kny; zebrafish) |
Heparan sulfate proteoglycan with a GPI-anchor; binds Wnt11, Wnt5 and Fz7; enhances Wnt-fz/PCP signaling. | N.D. | CE | 200, 201 |
| N.D. |
Protein tyrosine kinase 7 (PTK7; mouse)
Xpkt7 (Xenopus) |
A single pass transmembrane protein with tyrosine kinase homology. Binds to dsh via its PDZ domain and is required for Fz7 mediated Dsh localization. | N.D. | Inner ear CE | 106, 107, 202 |
| N.D. | Wnt5/pipetail (ppt; zebrafish) | Secreted cysteine-rich glycoprotein; act as ligands for Fz receptors. | N.D. | CE, limb elongation, growth cone guidance | 98–102, 159, 181 |
| Wnt11/silberbl ick (slb; zebrafish) | CE | ||||
| Wnt9b | Kidney | ||||
| N.D | mRor2(mouse), XRor2 (Xenopus) | Transmembrane protein tyrosine kinase contains extracellular Frizzled-like cysteine-rich domain (CRs) and kringle domain acts as a coreceptor for Wnt5a to mediate non canonical Wnt signaling | N.D. | CE, Inner ear, Limb elongation | 32–34, 99 |
|
| |||||
| N.D | Ryk | Atypical receptor related tyrosine kinase (contains dead tyrosine kinase domain), Binds to Wnt5a | N.D. | CE | 35, 36 |
|
| |||||
| N.D. | Smurf1 and Sm (mouse) | Smurf1 (Smad ubiquitination regulatory factor-1) and Smurf2 are related E3 ubiquitin ligases; targets Prickle1 for ubiquitin-mediated degradation | N.D. | Inner ear CE | 39 |
|
| |||||
| N.D | RACK1 | Receptor for activated C kinase 1; physically interacts with Vangl2 and required for its membrane localization. Also inhibits canonical Wnt signaling. | N.D. | CE, Inner ear | 37, 38 |
| Fat/Dachsous PCP factors | |||||
| fat (ft) | Fat4 | Divergent member of the Cadherin super-family; heterophillic interaction with Ds; binds Atro. | All adult tissues | Kidney | 43, 44, 93, 203–205 |
| dachsous (ds) | N.D. | Divergent member of the Cadherin super-family; heterophillic interaction with Fat. | All adult tissues | N.D. | 44, 93 |
|
| |||||
| four-jointed (fj) | N.D. | Golgi resident kinase that phosphorylate in extracellular cadherin domains of ft and ds. | All adult tissues | N.D. | 206 – 209 |
|
| |||||
| atrophin (atro) | N.D. | Transcriptional co-repressor; binds to Fat. | Eye and wing | N.D. | 210, 211 |
|
| |||||
| dachs (d) | N.D. | Atypical myosin; negatively regulates ft signaling. | Wing | N.D. | 212, 213 |
|
| |||||
| approximated (app) | N.D. | Member of DHHC family, control subcellular localization of Dachs. | Wing | N.D. | 12 |
only tested tissues mentioned, combination of analysis in Xenopus, zebrafish and mouse
other tissues were not tested
CE convergent extension
N.D. not determined
PCP studies in vertebrates revealed that the Fz/PCP core factors are functionally conserved4, 7, 8 (Table 1). Such vertebrates studies have identified several processes that now serve as model systems for PCP analyses (Fig. 2). These include, for example, the polarization of rows of sensory cells in the mammalian inner ear/cochlea13, 14, mouse skin hair orientation15, mucus secreting and ciliated cells in the Xenopus skin16, somitic patterning in the chick17, cell orientation of the lateral line in fish development18, left-right asymmetry generated by ciliary function in the node19–24, and other cilia associated functions throughout development8, 9, 25. In addition to these largely epithelia-associated PCP features, Fz/PCP-signaling is required during the convergent extension (CE) process in gastrulation and neurulation, a cellular process involving mesenchymal cells that require polarization to allow cells to converge and intercalate leading to body axis elongation26–29.
Figure 2. Examples of PCP features in vertebrates.
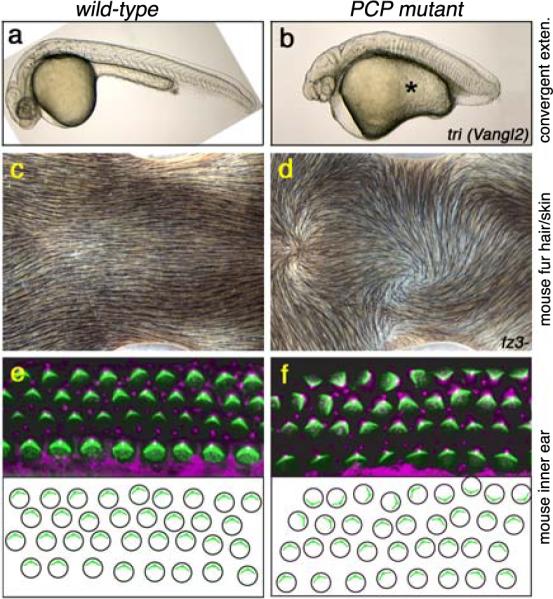
PCP features of convergent extension gastrulation movements in the zebrafish (a–b), the mouse skin (c–d) and the mouse inner ear (e–f). Anterior is right in all panels. Wild-type is on the left and PCP mutants in the right column.
(a–b) Zebrafish embryos: the mutant PCP genotype is a maternal-zygotic mutant of trilobite/Vangl2. Note short and wide (fat) body axis in PCP mutant. The original pictures were provided by Brian Ciruna.
(c–d) Dorsal view of mouse neck displaying the orientation of fur hair (and underlying skin) in wild-type (c) and mfz3 mutants (d). Note random waves and whorls in the fz3- genotype, compare to the normal anterior-posterior orientation in wild-type (c).
(e–f) Orientation of sensory hair cells of the mouse choclea (inner ear). Each cell contains polarized bundles of actin-based stereocilia (green; labeled with phalloidin) and a tubulin based kinocilium (labeled with anti-acetylated tubulin; magenta). In PCP mutants these bundles still form but their orientation becomes randomized (f; Looptail/Vangl2 mutant). The lower panels show schematic representation of the cellular (actin bundle) orientation.. The original pictures of c–f were kindly provided by Jeremy Nathans.
Apart from the core PCP factors, regulatory proteins have been identified that specifically modulate core Fz/PCP group members. These include among others Casein kinase 1ε30 and Abelson kinases 31 with a documented PCP function in several organisms or more specifically in vertebrates, Ror232–34, Ryk35, 36, RACK137, 38, and Smurf1/239. Additionally, other factors have been proposed to act in PCP like Gαo and Widerborst (a regulatory subunit of PP2A) in Drosophila40, 41, or PAR 1 in Xenopus42 just to mention a few (see also Table 1). These factors are thought to modulate the core components of the Fz/Dsh-PCP signaling pathway.
In this review we highlight the latest developments in the PCP field in Drosophila and integrate these findings with the previous data. Likewise we compare these studies to recent advances in vertebrate PCP research. We will focus primarily on Fz/PCP signaling, but will also briefly discuss its relationship to the Fat/Ds-system.
HOW MANY PCP-SIGNALING SYSTEMS REGULATE PCP ESTABLISHMENT?
The Fz/PCP signaling cassette regulates PCP establishment in all adult external Drosophila tissues and in many if not all vertebrate contexts. The general features are conserved, with the vertebrate systems having a larger set of regulatory components as compared to Drosophila (see below and Table 1). The Fat/Ds-system is also required in all PCP contexts in Drosophila and likely also in many PCP models in vertebrates5, 43.
The relationship between local Fz/PCP-signaling and the long-range regulation of PCP across a field of cells remains a topic of great interest. In the past it was proposed that both Fz/PCP and the Ft/Ds-systems function in a single pathway to establish PCP, with the Fat/Ds-group asymmetrically regulating the activity of Fz/PCP-signaling44. This hypothesis was based on observations that components of the Fat/Ds system (notably Ds itself and Four-jointed) are expressed in a gradient, whereas the core factors of the Fz/PCP pathway are expressed uniformly across all tissues analyzed45, 46. As such, they would lend themselves as an asymmetric input to Fz/PCP signaling, as to date an upstream regulatory input to the Fz/PCP pathway remains unknown in Drosophila (see below). However, the single-pathway hypothesis has been questioned and refuted by a comprehensive set of experiments in the Drosophila abdomen model of PCP47. This study elegantly demonstrated that the Fz/PCP and Fat/Ds-systems act independently of one another and thus are two parallel pathways. Both fz and fat/ds display non-autonomous effects (in both gain and loss-of-function scenarios) on neighboring wild-type cells in the absence of the other system. For example, fz and/or fmi mutant backgrounds do not affect the non-autonomous effects of the clonal expression of Ft or Ds, and similarly the inverse holds true as well47. The proper interpretation of these data must be that the Ft/Ds system does not require the presence of Fz/PCP signaling for its polarity information and vice versa5. The global mechanism(s) by which PCP is regulated across a field of cells still remain unclear (see below).
PCP ESTABLISHMENT IN DROSOPHILA
In Drosophila, PCP features are the most evident and thus preferentially studied in the eye, wing, thorax and abdomen4–6 (also Fig. 1). Much of the present day understanding of PCP establishment comes from numerous studies in these Drosophila tissues. Strikingly, several of the Drosophila tissues, while relying on a conserved signaling cassette, show variations in PCP establishment: in the eye Fz/PCP signaling sets up cell fate determination through gene expression; in the thorax/notum, PCP mediates spindle orientation in the sensory organ precursor cells; and PCP in the abdomen and wing largely results from localized asymmetric actin polymerization giving rise to asymmetrically positioned cellular hairs. Each of the Drosophila tissues shares unique advantages and features and thus studying PCP in multiple tissues is very helpful to understand the underlying conserved general features and also the differences between tissues.
In the following sub-chapters, we will discuss the specifics of PCP establishment in these Drosophila tissues.
PCP in the Drosophila wing
Historically this is one of the most studied “model organs” in animal development, serving as a paradigm for many patterning events including the action of morphogens. One of the last steps in wing development is the precise orientation of wing cells in the proximal-distal axis mediated by PCP signaling.
In adult wings, cells are uniformly packed in a hexagonal manner with each cell producing a single actin-rich hair that originates from the distal vertex and points distally (Fig. 1c–d and 3a). PCP generation in the wing follows earlier patterning processes that define the wing regions and cell fates. PCP in the wing is initiated during late 3rd instar larval and early pupal stages48, 49. Although it was thought until recently that the first apparent signs of polarity become evident in mid-pupal stages50–53, recent work has firmly established that PCP in the wing is already visible in the prepupa and early pupal stages48, 49. Strikingly at these early stages, PCP orientation is aligned in a radial manner perpendicular to the wing margin, rather than in the proximal-distal axis evident in the adult48. The late proximal-distal arrangement is generated from the early radial pattern through a series of cellular rearrangements, including cell flow and rotation induced by anisotropic mechanical stress, originating from the contraction of the hinge region during wing development in pupal stages48. Hinge contraction causes wing cells (besides their proximal streaming) to rotate in opposing directions in anterior and posterior compartments and these cell movements eventually reorient PCP to the proximal-distal axis48, 54.
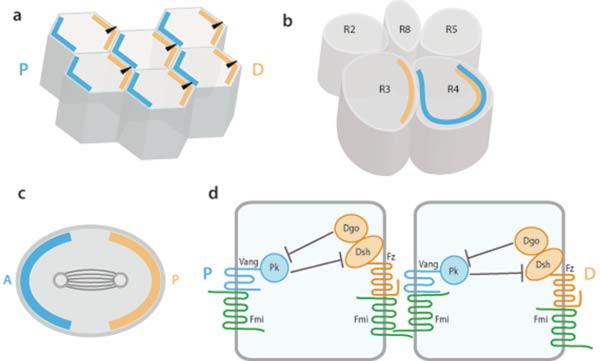
Figure 3. Schematic Presentation of Asymmetric Core Fz/PCP Protein Localization.
(a) Schematic of wing cells highlighting the epithelial nature of these cells. These hexagonal cells display PCP orientation in the proximal-distal axis). Single actin rich hairs (black arrowheads in each cell) project from the distal vertex of each cell, where the Fz-Dsh complex (orange) gets localized. The asymmetric localization of the core protein complexes at the end of PCP establishment (shown in blue and orange) serves as molecular markers for cell orientation. The blue complex contains Vang/Stbm, Pk and Fmi proteins, while the orange complex contains Fz, Dsh, Dgo, and Fmi (see panel d for molecular interactions). (b) Asymmetric distribution of core PCP proteins is also observed during eye patterning in the precursors to the R3 and R4 photoreceptors, which is a prerequisite for proper fate specification of R3 and R4, at the time of the 5-cell precluster posterior to the furrow (individual R-cells are numbered according to their final fate) (c) Example of a dividing sensory organ precursor (SOP) cell, showing polarized orientation of the mitotic spindle. The orientation of the spindle depends on the asymmetric localization of the core PCP proteins (shown in blue and orange as above).
(d) Schematic presentation of asymmetric localization and molecular interactions of core PCP proteins across two wing epithelial cells. Fz–Dsh–Dgo–Fmi is enriched in a form of complex at the distal edges of cells, while the Vang–Pk–Fmi complex is concentrated to proximal edges of cells. Fz (orange) binds to Vang (blue) primarily via its CRD and this interaction is stabilized by homophilic interactions mediated by the atypical cadherin Fmi (green). Fz forms an intracellular complex with Dsh and Dgo, while Vang interacts intracellularly with Pk. Dsh and Dgo physically interact with each to promote Fz-Dsh signaling and can antagonize Pk. Diego competes with Prickle for binding to Dsh and thus antagonizes the inhibitory effect of Pk on Dsh.
The first sign of PCP-type polarization is evident in the asymmetric localization of the proteins of the Fz/PCP core group (Fig. 3). How is this established? Initially and prior to the stage when PCP is visible, all core PCP factors are uniformly localized in a subapical ring, partially overlapping with adherens junctions55. This is best understood and studied in the Drosophila wing, but largely applies to all Drosophila tissues and probably also to vertebrate PCP establishment. First, the subapical localization of Fz/PCP components is a prerequisite for the interactions that will define polarization in the plane55. Through subsequent interactions among the core Fz/PCP factors, two stable complexes form on opposing sides of each cell: a Vang/Stbm-Pk complex and a Fz-Dsh-Dgo complex (Fig. 3; asymmetric localization of the core PCP factors is the first visible sign of planar polarization/PCP). Fmi becomes enriched at both ends of the apical membrane, helping to stabilize both complexes (see below). The two complexes are formed through a series of molecular interactions: (1) negative (inhibitory) intracellular interactions mostly between Pk and Dsh and modulated by Dgo52, 56, (2) clustering of “like” complexes (e.g. Pk clusters Vang/Stbm intracellularly and Dsh causes Fz clustering through their multimerization features56, 57), (3) intercellular stabilization across cell membranes mediated by Fz-Fmi complexes binding to Vang-Fmi complexes53, 58–61, likely through a direct physical intertaction between Fz and Vang60, 61, and (4) differential trafficking and stability of such complexes49, 62, 63. Within this molecular network Fz recruits Dsh to the membrane, while Vang/Stbm recruits Pk to the membrane. Dgo binds to Dsh and protects it from antagonistic binding by Pk and thus Dgo competes with Pk for Dsh binding (see earlier reviews4, 6, 10 and also Fig. 3d). Of note, apical membrane localization of the core PCP proteins depends on Fmi, as well as on Fz and Vang/Stbm, as it is lost or strongly reduced in mutant clones of either fz, Vang/stbm and fmi64, 65. Besides these molecular interactions and interdependence of the core PCP factors, non-centrosomal microtubules (MT), which are aligned in the proximal-distal axis of wing epithelia66, 67, contribute to the asymmetric localization. MTs appear to be polarized and direct the movement of Fz-Fmi complexes along them62, 63. Intriguingly, Ds and Ft have been shown to control both proximal-distal alignment and asymmetric polarization of MT, possibly through involving the Ser/Thr kinase PAR-1, the subcellular localization of which was altered in ft mutant and over expression clones63.
Although these events are best understood in the Drosophila wing due to its relatively simple structure, many of these mechanisms are also key in establishing PCP in other Drosophila tissues and also likely in vertebrate PCP contexts (see below).
Once the PCP factor asymmetry is set, downstream effectors then set-up the initiation point for the formation of the actin hair, the so-called trichome. Mutations in core PCP genes not only disrupt cellular and trichome orientation, but can also result in cells with multiple wing hairs/trichomes. These downstream effectors which include inturned (in), fritz (frtz), multiple wing hair (mwh) and fuzzy (fy) are also proposed to localize asymmetrically and act downstream of core PCP genes68–72. mwh among these downstream effectors seems to be different in a way that it is genetically downstream of both core PCP members as well as the other downstream effectors like in, frtz and fy. Further, mwh is not required for the asymmetric localization of core PCP factors or the other downstream effectors, though proximal localization of Mwh itself depends on the other downstream effectors 73.
PCP in the fly eye
In the Drosophila eye, PCP is evident in the regular arrangement of ommatidia in the retina with respect to both the dorsal-ventral and anterior-posterior axes10, 74, 75. The Drosophila eye is a compound eye made up of ~800 ommatidia, each consisting of eight photoreceptor cells (R1–R8) and twelve non-neuronal accessory cells. Fz/PCP signaling sets up the asymmetric specification of the photoreceptor pair, R3 and R4, via nuclear signaling within each ommatidial precluster, giving rise to the two chiral forms of ommatidia across the dorso-ventral midline, the equator (Fig. 1a). This is then followed by a 90° rotation of ommatidial preclusters in opposing directions around the equator, resulting in a mirror-symmetric arrangement of ommatidia in the adult eye across the equator. Mutations in the core PCP genes disrupt R3/R4 specification, resulting in stochastic cell fate specification and random ommatidial chirality and rotation (Fig. 1b).
PCP in the eye is established in the third instar larval eye imaginal disc, where the R3/R4 precursor pair is part of the 5-cell precluster. In the 5-cell precluster (emerging from the morphogenetic furrow via an “arc”-configuration;76), the R3 precursor within the R3/R4 pair is closer to the equator than R4 and hence is thought to receive higher levels of Fz/PCP-signaling and adopts an R3 fate77–79. Fz activity in R3 then upregulates Delta (Dl) and neuralized (neur) transcription77, 80, 81, which in turn activates Notch-signaling in the adjacent cell and specifies it as R477, 78, 80. This two-tiered mechanism allows the robust amplification of small signaling differences at the Fz/PCP level to large and robust difference in Notch signaling. Once Notch is active, the R4 fate is determined and, as such, indiscriminate activation of Notch in both cells of the R3/R4 pair results in symmetrical R4/R4-type ommatidia77, 78, 80. The initial Fz/PCP factor interactions in the R3/R4 cells are mediated by a very similar molecular circuitry among the Fz/PCP core set as in the wing, leading to their asymmetric localization (and activity) with the R3/R4 pair (Fig. 3b).
Following the cell fate specification of Fz/PCP signaling, ommatidial preclusters rotate 90° towards the equator in opposing direction in each half of the eye. Mutations in the core PCP factors usually not only result in random R3/R4 specification and associated chirality, but also in random ommatidial rotation. Generally, the rotation process is much less understood than the R3/R4-cell fate specification. As such rotation could be considered to be at a comparable effector level as trichome/wing hair formation. Accordingly, mutations in some of the Fz-Dsh effectors like Rho-associated kinase (dRok) display rotation defects when mutated in addition to a “multiple wing hair”-phenotype82. Besides the general downstream effectors of Fz/PCP-signaling such as dROK, Myosin Regulatory Light Chain (MRLC, Sqh in Drosophila) and Myosin II (Zipper) have been proposed to control ommatidial rotation82, 83. In addition, the cell adhesion factors E-cadherin/Shotgun (shg) and Drosophila N-cadherins (cadN1 and cadN2) and their associated β-catenin (arm in Drosophila) protein have been implicated in rotation84. Few rotation specific genes are known, and of those Nemo (Nmo, the founding member of the Nlk subfamily of MAPKs), is the best characterized. Originally it was shown that mutations in nmo caused ommatidia to rotate only partially85. It was later shown that nmo is required for the entire rotation process86. Recent work has demonstrated that nmo serves as a link between the core PCP factors and the cell adhesion E-cad/β-catenin machinery, providing input to promote rotation and thus Nmo regulates the rate of rotation by affecting the activity of E-cad/β-catenin complexes87. Strikingly though, it is the Vang-Pk complex that interacts physically and genetically with Nmo (not the Fz-Dsh complex) and regulates Nmo activity by affecting its localization to the adherens junctions where it can then phosphorylate components of the E-cad/β-catenin complex87. Although the core PCP factors clearly feed into the rotation process, its regulation is more complex as it is also affected by Notch-signaling and EGF-receptor signaling input88–90. Thus although the specification of the R3/R4 pair is quite well understood, the integration of different signaling pathways91 and the downstream events of ommatidial rotation remains less defined.
PCP on the Drosophila thorax
Similar to the wing, most of the fly cuticle is covered by cellular hairs (trichomes) aligned in the antero-posterior axis. Thus, common mechanistic interactions have been proposed to direct cellular orientation in other Drosophila tissues, including the abdomen and thorax (notum)5, 47, 92, 93. In addition, many cuticular structures like the notum display polarized orientation of a large number of sensory bristles (Fig. 1e and f). The development of these sensory bristles involves a primary cell (sensory organ precursor/SOP), which divides asymmetrically giving rise to two daughter cells (pIIa and pIIb). The asymmetric division per se is not affected in PCP mutants, but the orientation of the division axis/mitotic spindle is randomized in fz- and other core PCP mutant backgrounds94. The molecular interactions among the core Fz/PCP factors are largely the same, but in the case of the SOPs they result in an alignment of the spindle with the body axis. Although the mechanisms by which the PCP pathway regulates spindle orientation are not clear (and some redundancy might exist between the Fz/Dsh and Vang-Pk complexes94, 95), it has been shown recently that the DEP domain of Dsh is sufficient to orient spindle polarity in Drosophila S2 cells96. The Dsh DEP binds to C-terminal domain of the protein Mud (mushroom body defective, the Drosophila nuclear mitotic apparatus [NuMA] orthologue), and Mud is required for Dsh mediated spindle orientation. Mud is localized to the posterior cortex of the SOP, requiring Dsh for this localization, which itself is localized to the posterior cortex of SOP cells as part of the Fz/Dsh complex. Similarly, during zebrafish gastrulation, reducing levels of NuMA disrupts spindle orientation suggesting a conserved mechanism by which the spindle is orientated in a proper axis96. Thus, although all three tissues/contexts use the same molecular system or cassette to establish PCP, the downstream effectors and interactions are context specific, ranging from actin polymerization in wing and body wall cells, nuclear signaling in photoreceptors, to spindle orientation in asymmetric cell divisions.
Intriguingly, the notum recently added a new cell behavior to the PCP puzzle: mechanical stress and cell flexibility can also affect PCP orientation97. Most of the cuticle/body wall cells on the notum are actually tendon cells where the muscles are attached (although externally they do not look different from other trichome bearing body wall cells). As such the tendon cells need to buffer the mechanical stress of muscle contraction as they establish PCP orientation. Filamin (jitterbug in flies) or the associated factor chascon (chas), expressed in tendon cells, are important for the maintenance of cellular PCP orientation on the notum by balancing mechanical stress generated by the attachment of the indirect flight muscles (IFMs) to the exoskeleton/cuticle. This mechanism is independent of and acts in parallel to Fz/PCP signaling97. It is likely that in other tissues, including vertebrate examples, a similar balance between mechanical pull and Fz/PCP signaling is required for proper cellular orientation.
REGULATION OF FZ/PCP CORE FACTOR SIGNALING
Upstream input to Fz/PCP signaling
Most studies of Fz/PCP signaling have addressed intracellular interactions and how these lead to the asymmetric PCP core complex localizations (Fig. 3). However, a big remaining question is how the initial directional bias is established in the first place. Although it was suggested that the Fat/Ds-system could be acting upstream of Fz/PCP signaling in Drosophila (see above), it has been quite convincingly demonstrated that two systems act in parallel5, 47. Fz/PCP and canonical Wnt/Fz-signaling share several membrane associated components, most notably Fz itself and Dsh. Canonical Wnt/Fz-signaling is activated by Wnt family members in vertebrates and Drosophila. In vertebrates, Fz/PCP signaling is linked to regulatory input from Wnts98–102, but the precise mechanistic function of Wnts in vertebrate PCP regulation remains unclear. Do they activate Fz/PCP signaling or do they regulate other core PCP factors, e.g. as suggested for Ror and Vangl99. Alternatively they might regulate the asymmetric localization of any of the core Fz/PCP factors without directly regulating Fz/PCP signaling “activity” as asymmetric localization is more important in this context than cellular activation (see also below). Moreover, in Drosophila the involvement of Wnts (equivalent to mammalian non-canonical Wnts) and/or other “global” upstream regulatory input to Fz/PCP signaling remains a big question mark53.
In vertebrates, Wnt5 and Wnt11 appear dedicated to Fz/PCP signaling. In particular, in zebrafish Wnt5/pipetail and Wnt11/silberblick have been shown genetically to regulate CE during gastrulation100, 103. Similarly, these Wnts act in PCP signaling in other vertebrate contexts as mainly shown through mammalian cell culture and mouse genetics (reviewed in6, 29). Interestingly, the non-canonical (PCP) Wnts do not appear to bind to the LRP5/6-Fz co-receptor complex, but bind only to Fz family members104, 105. The identification of Wnt-binding domains in the Ror2 and Ryk receptor tyrosine kinases (RTKs) and the fact these RTKs are also involved in PCP signaling31, 99, 106–108 has suggested a complex co-receptor scenario for Wnt-Fz/PCP signaling. In particular, it has been demonstrated that Wnt5 can bind to a Ror2-Fz co-receptor complex32, 34, 109, 110. Interestingly, Wnt5a mediated activation of Ror2 also requires Dsh (and Fz) causing Ror2 phosphorylation of Ser864 (by GSK3), suggesting an analogous mechanism to Fz/Dsh-dependent canonical Wnt3a induced phosphorylation of LRP6110. Whether the equivalent Ror2 and Ryk homologues play any role in Drosophila PCP remains to be explored.
Are these PCP Wnts providing instructive information to orient PCP? The genetic data from zebrafish would suggest that Wnt5 and Wnt11 act in a permissive manner as the respective mutants can be rescued by RNA injections into the one or two-cell stage embryos100, 101. In contrast, recent papers suggest that non-canonical Wnts provide directional information to PCP establishment. Both Wnt11 (during chicken somite patterning) and Wnt5a (in mouse limb patterning) have been shown to provide directional cues to PCP signaling and cellular orientation17, 99. It is thus likely that the non-canonical Wnts do indeed serve an instructive PCP function and provide global orientation cues. However, the mechanism(s) by which non-canonical Wnts activate PCP signaling in vertebrates and whether Wnt5a or Wnt11 act through similar or different mechanisms remain unclear. It will be very interesting to dissect the mechanism(s) of their regulatory inputs; for example, it is not clear whether Wnt5a and Wnt11 act through the same or different Fz (and/or co-receptor) members. Moreover, the issue needs to be resolved also in Drosophila as well, where in tissues such as the wing (see above), dissection of a precise mechanism should be possible.
Dsh regulation and Fz/PCP pathway selection
At the level of the membrane, the core Fz/PCP factors interact among each other to resolve their initial homogeneous localization into two polarized complexes. These interactions are discussed in some detail above (see subchapter “PCP in the Drosophila wing” and Fig. 3d) and thus not repeated here. However, one member of the Fz core group Dsh (Dvl in mammals) deserves special attention. Dsh/Dvl proteins are quite central to the intracellular aspects of Fz/PCP establishment. Moreover, Dsh/Dvl also act in canonical Wnt-Fz signaling, required for beta-catenin stabilization and thus are at the branch point of canonical Wnt-Fz and PCP signaling pathways. As such, Dsh is central to signaling specificity regulation111–113. Second, it is both an integral component of the intracellular interactions between the core PCP complexes (see above; Fig. 3) and a critical link of the core factors to the downstream Fz/PCP-effector pathways, as it is thought to bind and localize downstream effectors (see for example reviews4, 7, 114 for more detail on downstream Fz-Dsh signaling events). All Dsh/Dvl proteins contain three highly conserved domains, the DIX, PDZ and DEP domains, but have no catalytic activity111–113. There is also a conserved proline-rich region with a SH3 protein-binding motif downstream of the PDZ domain, which might act as binding substrate for SH3-domain containing proteins.
Whereas, there is a single Dsh in Drosophila, there are three Dvls (Dvl1, Dvl2 and Dvl3) in mammals. Genetic studies in mice have defined functional redundancy among all three Dvls as a result of their conserved structure and overlapping expression patterns during development115. Individual dvl knock out mice display very weak PCP defects or no defects115. Single homozygous mutants combined with trans-heterozygotes for another dvl display stronger phenotypes116–118. dvl1−/−; dvl2−/− double knock out mice display neural tube closure defects, cochlear defects, and cardiovascular outflow tract defects116. These dvl1−/−; dvl2−/− double knock phenotypes can be rescued by exogenous expression of Dvl3, consistent with functional redundancy among all Dvls118.
Due to the above mentioned complexities of Dvl redundancy, functional studies have been more informative in Drosophila. However, it is still largely unclear how Dsh becomes “activated” and how it is differentially regulated between canonical Wnt-Fz and Fz/PCP signaling. One possible mechanism for specific Dsh regulation could be differential binding, where binding partners may interact with distinct domains of Dsh. Accordingly, distinct domains of Dsh are required in the different signaling pathways. Whereas the DIX domain functions exclusively in canonical Wnt-Fz signaling, the PDZ domain is required for both pathways, and the DEP domain acts in Fz/PCP signaling111, 112, 119, 120. Each of these domains has a defined set of binding partners. The PDZ domain, in particular, is quite promiscuous and can be bound by many factors acting in either pathway. Moreover, the PDZ domain binds the C-tail of Fz receptors in either pathway context. For example, CK1 family members, GSK3, GBP/FRAT, Frodo, Dapper, Naked cuticle (Nkd), PP2A, IDAX (Inhibitor of Dsh and Axin) and Daple among others have been shown to associate with the PDZ domain and function in canonical signaling. DAAM1, Vang/Stbm, Pk, Dgo, and PAR1 can bind the PDZ domain in the context of PCP signaling112. The DEP domain acting specifically in PCP signaling also binds to a host of factors including potential downstream effectors and, importantly, it also plays a key role in the stable PCP specific membrane association of Dsh112, 113, 121, 122 (see below). Although the mechanistic differences of Dsh membrane association for either canonical or Fz/PCP pathway signaling still remain largely elusive, it is a likely mechanism for differential activation of Dsh. The PCP specific membrane recruitment requires the DEP domain, while the DEP domain is dispensable for canonical signaling. In particular, a PCP-specific mode of regulation at the level of Dsh membrane recruitment has recently been documented122. It involves a basic surface within the DEP domain and its binding to an acidified intracellular plasma-membrane, making the Fz/PCP-specific Dsh-membrane recruitment pH dependent. This is generated by dNhe2, a Na+/H+ exchange pump, which co-localizes with Fz at the plasma membrane122. Intriguingly, other studies have documented that Prorenin receptor (PRR) and vacuolar H+-ATPase mediated acidification are important in both the canonical and Fz/PCP pathways. PRR, as the name suggests binds to Renin, is a single pass membrane protein consisting of a large extracellular domain. PRR forms a complex with Fz and LRP6 thus acting as an adaptor between the Wnt receptors and vacuolar H+-ATPase (V-ATPase)123. This was independently confirmed by two reports, demonstrating that Drosophila PRR binds Fz to regulate both canonical and Fz/PCP signaling pathways124, 125.
Subcellular membrane localization is likely to provide signaling specificity cues even at the level of the Fz receptors. In Drosophila, distinct localization of Fz and Fz2 (dedicated to canonical signaling) is important for signaling. Fz is mainly localized subapically at adherens junctions, whereas Fz2 is distributed throughout the entire plasma membrane including the baso-lateral side55. The difference in subcellular localization is critical for Fz/PCP signaling and thus contributes to the signaling outcome: apical localization of Fz favors Fz/PCP signaling and is less effective for canonical signaling. Thus a (over)recruitment of Dsh to the subapical Fz site, depletes it from the canonical signaling pool55. The role of membrane association and/or subcellular localization of various Dvls in vertebrates remain poorly understood. For example, In Xenopus animal cap explants and in mammalian cells canonical Wnt signaling activation results in the membrane localization of Dsh/Dvls, while Wnt stimulation in embryonic mouse kidney cells results in the accumulation of Dsh/Dvl in and around the nucleus126, 127.
A third potential regulatory input to Dsh is phosphorylation, which has been thought to provide pathway specificity. Dsh is hyperphosphorylated upon activation of either pathway, but the phosphorylation pattern is thought to vary between pathways. Due to the presence of many Ser/Thr residues (over 100 out of ~600 residues in Dsh/Dvls are Ser and Thr) identification of physiological pathway specific phosphorylation events remains challenging. A host of serine/threonine kinases has been identified which can phosphorylate Dsh, including many members of the Casein Kinase 1 superfamily, as well as Casein Kinase 2 family members, Par-1 and PKC family members30, 42, 128–132. The physiological relevance of these phosphorylation events remains unclear. Recently, Tyrosine (Tyr) phosphorylation has emerged as a likely contributor to Dsh/Dvl activity and signaling pathway selection. Dsh/Dvls are phosphorylated by Abelson family kinases on Tyr residues within the DEP domain and C-term31. Abl phosphorylation of Dsh on Tyr473 appears essential for its function in Fz/PCP signaling, while it is dispensable for canonical signaling. This appears to be conserved, as Abl1−/−, Abl2−/− double-knockout MEFs (mouse embryonic fibroblasts; removing all Abl function) display changes in Dvl2/3 phosphorylation patterns and subcellular localization, but display no change in nuclear β-catenin levels and signaling31. The mechanistic insight of how Dsh/Dvl phosphorylation could provide pathway specificity however still remains obscure. One possibility is that different phosphorylation events result in changes in Dsh conformation, which results in differential protein-protein interactions and activation of a specific pathway.
Downstream effectors of Fz/PCP signaling
The downstream effectors of the Fz/PCP pathway differ depending on the tissue in which the pathway is being activated. This can range from activation of nuclear signaling, asymmetric organization of the cytoskeleton, or orientation of the mitotic spindle in epithelial cells. In the eye, Fz/Dsh signaling leads to transcriptional activation of for example the Notch ligand Delta, while in the wing epithelium actin cytoskeletal rearrangements lead to proper hair orientation.
Genetic and biochemical studies have shown that Fz/PCP signaling downstream of Dsh consists of small GTPases of the Rho subfamily (Rho, Rac and cdc42), the STE20 like kinase Misshapen (Msn), the Rho associated kinase (dROK), and the JNK MAPK cascades66, 82, 120, 133. Downstream of dROK, the role of Sqh (Myosin Regulatory Light Chain, MRLC) and Myosin II (Zipper) has been proposed to control ommatidial rotation in the eye and to restrict the formation of a single actin rich hair in developing wing cells82. Further studies in Xenopus have shown a similar requirement for RhoA during CE, suggesting a conserved mechanism of regulation downstream of Dsh134. This study has also identified a Formin homology domain protein, Daam1, which binds to both Dsh and Rho GTPase134. The N-terminus fragment of Daam1 has been shown to bind RhoA, while its C-terminal binds PDZ and DEP domains of Dsh thus linking Dsh and its downstream effector RhoA134. More recently in Xenopus, the role of Septins have been postulated downstream of the PCP effector Frtz to control collective cell migration and ciliogenesis, thus highlighting the diversity of effector proteins that are involved downstream of Fz/PCP signaling pathway135.
The effectors of the Fz/PCP pathway downstream of Dsh have distinct functions and their requirement depends on the context in which they are activated. Although cytoskeletal organization is the main response in wing PCP and probably in CE during gastrulation, a transcriptional response is also important in the Drosophila eye. In the eye, genetic interactions suggest that JNK/p38 signaling acts downstream of Dsh. In biochemical assays, Drosophila Dsh acts as potent activators of JNK suggesting JNK downstream of Dsh120. Intriguingly, loss of JNK in the eye does not show a phenotype, possibly because of genetic redundancy in the JNK/p38 kinases in Fz/PCP signaling. Nevertheless JNK signaling has also been implicated in the context of CE in vertebrates, suggesting a general requirement of JNK in PCP establishment136. Downstream of JNK, the AP-1 transcription factor (consisting of Jun and Fos) has been proposed as one of the necessary nuclear factors for R3/R4 cell fate specification during eye development137.
Recent work has also identified effectors of the Vang/Stbm-Pk complex71, 72, 87, suggesting that this complex has signaling roles independent of its function to restrict the Fz/Dsh complex to one side of the cell. As such the regulator of ommatidial rotation, Nemo, is linked to its function in cell adhesion regulation by binding to the Vang/Stbm-Pk complex87. Similarly, the asymmetric localization of several of the factors that inhibit the formation of too many actin hairs (trichomes) being formed in individual cuticle/wing cells, is mediated by the Vang-Pk complex71, 72. These observations suggest that there is more effector signaling to be expected from the Vang/Stbm-Pk side of PCP signaling.
PCP, CILIA AND DISEASE
The cilium, a microtubule-based organelle present on the surface of many cells plays a critical role in many aspects of developmental patterning, signaling, and disease. The role of cilia in vertebrate Hh-signaling has been studied extensively138, while its role in other signaling pathways including canonical Wnt and PCP signaling remains unclear. The first study highlighting a connection between Wnt-signaling and ciliary function showed that Inversin (Inv, one of the two mammalian homologues of the core Fz/PCP factor Dgo), mutated in Nephronophthisis (NPHP, an autosomal recessive cystic kidney disease), was localized to cilia139. Inversin was originally identified due to its left-right polarity defect in mouse (hence its name), which provided a further link to PCP signaling (PCP signaling is thought to regulate at least some aspects of left-right specification8, 140, 141). Inversin was later shown to co-localize with Dvl1 in MDCK cells and to inhibit canonical Wnt-signaling by targeting Dvl for degradation, while also being required for CE in Xenopus embryos142.
The primary cilium is anchored to the cell surface by a microtubule-based structure, the basal body, which serves as a nucleation site for the growth of the axoneme as well as plays a role in cell division. Directional beating of motile cilia has been shown to require PCP signaling in several tissues, primarily by regulating the orientation of the basal body143–145. Mutations in components of the basal body such as bbs1, bbs4, bbs6, mkks, ift88 and kif3a not only show PCP associated defects (disorganized stereocilia) in the cochlea and CE but these mutants also interact with core PCP pathway components (e.g.146–149). Conversely, components of ciliary and centrosomal machinery such as the ciliary kinesin Kif3A, the Bardet-Biedl (BBS) proteins, the nephronophthisis protein 3 (NPHP3), and the oro-facial-digital syndrome protein (OFD1), have been implicated in Wnt signaling pathways147, 148, 150. Mutations in these ciliary proteins have been linked to rare diseases referred to as ciliopathies, including Bardet-Biedl syndrome (BBS), Autosomal-dominant polycystic kidney disease (ADPKD), Oro-facio-digital syndrome (OFD), Meckel-Gruber syndrome (MKS), and Nephronophthisis (NPHP) and are caused by defects in cilia formation and function151, 152. As such, defects in primary cilia underlie the pathogenesis of Bardet-Biedl syndrome (BBS), a genetic disorder whose symptoms include among others obesity, retinal degeneration, and nephropathy153. The depletion of BBS proteins also impair CE movements in zebrafish and genetically interacts with the mouse Vangl2 allele looptail in the context of neural tube closure and stereociliary bundle orientation in the cochlea146, 147. Furthermore PCP proteins have also been implicated in neural tube closure defect (NTD). This was first shown in patients with familial and sporadic NTDs, where mutations in Vangl1 were reported154, 155.
In kidney, defective PCP signaling has been linked to polycystic kidney disease (PKD), probably via proper orientation of the cilia/basal body apparatus. There is strong genetic co-relation showing that the defect in cilia is one of the contributing factors to the PKD pathogenesis thus providing an indirect link between PCP and PKD156, 157. ADPKD is caused by mutations in Pkd1, which encodes a large transmembrane protein polycystin-1 (PC1) or by mutations in Pkd2 encoding a TRP cation channel polycystin-2 (PC2) that regulates calcium entry inside a cell. These proteins localize to the ciliary axoneme of kidney tubule epithelial cells158. Direct evidence of PCP protein involvement in cystic kidney disease comes from studies of mouse fat4 loss-of-function, which resulted in disruption of tubule formation during kidney development43. fat4 mutant kidneys showed randomization in spindle orientation, resulting in cysts containing dilated tubules43. Recently, Wnt9b was also shown to play a role in kidney morphogenesis by disrupting the planar polarization of oriented cell division during development159. This lead to tubules with significantly increased diameter, affecting normal kidney development and leading to cyst formation. These studies highlight the importance of oriented cell division for convergent extension processes, which regulate kidney tubule diameter in vertebrates. For additional discussion of PCP associated diseases see respective review9.
Earlier studies postulated that PCP is important for cilia formation as well as for planar polarization of basal bodies144, 160–162. Loss of Dsh in bronchial epithelial cells disrupts cilia formation as a result of defective apical docking of basal bodies144. In Xenopus embryos, the PCP effector genes inturned and fuzzy control the assembly of an apical actin network that is essential for the normal orientation of ciliary microtubules and thus regulate ciliogenesis160. However, recent studies showed that zebrafish embryos, which were devoid of trilobite/vangl2, surprisingly showed no defects in cilia formation. Instead, these cells showed abnormal localization of the cilia19. This was further confirmed in mouse, where in the absence of Vangl1 and Vangl2, cilia are positioned randomly around the centre of the posterior notochord (PNC) cells, leading to turbulent nodal flow that results in disrupted left-right asymmetry24. This would suggest that the relationship between PCP proteins and cilia formation/localization is not that simple and may depend upon the specific protein and the context in which it is studied. Thus the molecular mechanism of interaction between cellular polarization, PCP signaling, and cilia still remains elusive.
CONCLUSIONS
PCP studies in Drosophila have provided a wealth of information and have laid a framework for the better understanding of PCP in vertebrates. Still there are many open areas in the field that lack genetic and molecular understanding. Moreover, there are also additional molecular pathways that can regulate polarity within the epithelial plane that appear (at least for now) unrelated to either the Fz/PCP or Fat/Ds systems. For example, in Drosophila embryos planar polarity is established by the enrichment of Bazooka/Par3 and Myosin II at the borders between dorsal and ventral cellular interfaces leading to the formation of polarized structures, consisting of actin-myosin cables along adherens junctions11, 163–165.
A critical issue that is not yet resolved among the Fz/PCP and Fat/Ds systems is how they are linked (if at all) and how they might converge on cellular effector pathways. Moreover, the long-range global regulation of PCP orientation via the Fz/PCP core genes remains unresolved, as it is certainly lacking some players in Drosophila53 and mechanistic insight in vertebrates. Thus, the coordination of cellular polarization across whole tissues and organs remains mysterious.
In vertebrates, PCP regulation is more complex than in Drosophila as it plays very diverse roles during development (including in mesenchymal cells during CE) and disease in different tissues and thus it is likely that some unexpected turns will be made before we have a more complete picture. This includes the molecular relationship between cilia and PCP proteins/signaling. Genetic studies in Drosophila and other model organisms will be needed to continue to provide new insights and enhance our understanding the molecular mechanisms associated with PCP establishment in development and disease.
Acknowledgements
We are grateful to William Gault, Lindsay Kelly and Carlo Iomini for carefully reading and thoughtful comments on the manuscript, we thank Patricio Olguin and Jun Wu for providing pictures of the fz mutant thorax and wing, and Susanna Franks for help with drawings presented in Figure 3. We are grateful to Jeremy Nathans, Brian Ciruna, and Mireille Montcouquiol for pictures shown in Figure 2. We thank all members of the Mlodzik lab for their input, comments, and support. We apologize to investigators whose work could not be cited due to space limitations. Our work in the Mlodzik lab is supported by grants from the NIH/NIGMS and the NIH/NEI.
References
- 1.Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- 2.Lawrence PA, Shelton PM. The determination of polarity in the developing insect retina. J Embryol Exp Morphol. 1975;33:471–486. [PubMed] [Google Scholar]
- 3.Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- 4.Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 7.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 9.Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenny A. Planar cell polarity signaling in the Drosophila eye. Curr Top Dev Biol. 2010;93:189–227. doi: 10.1016/B978-0-12-385044-7.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 12.Matakatsu H, Blair SS. The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Curr Biol. 2008;18:1390–1395. doi: 10.1016/j.cub.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 14.Dabdoub A, Kelley MW. Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. J Neurobiol. 2005;64:446–457. doi: 10.1002/neu.20171. [DOI] [PubMed] [Google Scholar]
- 15.Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. Proc Natl Acad Sci U S A. 2004;101:9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 2009;19:924–929. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gros J, Serralbo O, Marcelle C. WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature. 2009;457:589–593. doi: 10.1038/nature07564. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Schier H, Hudspeth AJ. A two-step mechanism underlies the planar polarization of regenerating sensory hair cells. Proc Natl Acad Sci U S A. 2006;103:18615–18620. doi: 10.1073/pnas.0608536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12:407–412. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- 20.Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, et al. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol. 2010;12:170–176. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- 22.Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol. 2005;3:e268. doi: 10.1371/journal.pbio.0030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada Y, Takeda S, Tanaka Y, Belmonte JC, Hirokawa N. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell. 2005;121:633–644. doi: 10.1016/j.cell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanto M, McNeill H. Planar polarity from flies to vertebrates. J Cell Sci. 2004;117:527–533. doi: 10.1242/jcs.00973. [DOI] [PubMed] [Google Scholar]
- 26.Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 27.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 28.Myers DC, Sepich DS, Solnica-Krezel L. Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genetics. 2002;18:447–455. doi: 10.1016/s0168-9525(02)02725-7. [DOI] [PubMed] [Google Scholar]
- 29.Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol. 2009;20:986–997. doi: 10.1016/j.semcdb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein TJ, Jenny A, Djiane A, Mlodzik M. CKIepsilon/discs overgrown promotes both Wnt-Fz/beta-catenin and Fz/PCP signaling in Drosophila. Curr Biol. 2006;16:1337–1343. doi: 10.1016/j.cub.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 31.Singh J, Yanfeng WA, Grumolato L, Aaronson SA, Mlodzik M. Abelson family kinases regulate Frizzled planar cell polarity signaling via Dsh phosphorylation. Genes Dev. 2010;24:2157–2168. doi: 10.1101/gad.1961010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green JL, Kuntz SG, Sternberg PW. Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol. 2008;18:536–544. doi: 10.1016/j.tcb.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hikasa H, Shibata M, Hiratani I, Taira M. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development. 2002;129:5227–5239. doi: 10.1242/dev.129.22.5227. [DOI] [PubMed] [Google Scholar]
- 34.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim GH, Her JH, Han JK. Ryk cooperates with Frizzled 7 to promote Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent extension movements. J Cell Biol. 2008;182:1073–1082. doi: 10.1083/jcb.200710188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin S, Baye LM, Westfall TA, Slusarski DC. Wnt5b-Ryk pathway provides directional signals to regulate gastrulation movement. J Cell Biol. 2010;190:263–278. doi: 10.1083/jcb.200912128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S, Esterberg R, Lachance V, Ren D, Radde-Gallwitz K, Chi F, Parent JL, Fritz A, Chen P. Rack1 is required for Vangl2 membrane localization and planar cell polarity signaling while attenuating canonical Wnt activity. Proc Natl Acad Sci U S A. 2011;108:2264–2269. doi: 10.1073/pnas.1013170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wehner P, Shnitsar I, Urlaub H, Borchers A. RACK1 is a novel interaction partner of PTK7 that is required for neural tube closure. Development. 2011;138:1321–1327. doi: 10.1242/dev.056291. [DOI] [PubMed] [Google Scholar]
- 39.Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, Sakuma R, Luga V, Roncari L, Attisano L, et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Hannus M, Feiguin F, Heisenberg CP, Eaton S. Planar cell polarization requires Widerborst, a B' regulatory subunit of protein phosphatase 2A. Development. 2002;129:3493–3503. doi: 10.1242/dev.129.14.3493. [DOI] [PubMed] [Google Scholar]
- 42.Ossipova O, Dhawan S, Sokol S, Green JB. Distinct PAR-1 proteins function in different branches of Wnt signaling during vertebrate development. Dev Cell. 2005;8:829–841. doi: 10.1016/j.devcel.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 44.Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 45.Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. Bioessays. 2005;27:1218–1227. doi: 10.1002/bies.20318. [DOI] [PubMed] [Google Scholar]
- 46.Eaton S. Cell biology of planar polarity transmission in the Drosophila wing. Mech Dev. 2003;120:1257–1264. doi: 10.1016/j.mod.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 49.Strutt H, Warrington SJ, Strutt D. Dynamics of core planar polarity protein turnover and stable assembly into discrete membrane subdomains. Dev Cell. 2011;20:511–525. doi: 10.1016/j.devcel.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 51.Strutt DI. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Molecular Cell. 2001;7:367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- 52.Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olguin P, Mlodzik M. A new spin on planar cell polarity. Cell. 2010;142:674–676. doi: 10.1016/j.cell.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J, Klein TJ, Mlodzik M. Subcellular localization of frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol. 2004;2:E158. doi: 10.1371/journal.pbio.0020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- 57.Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. Embo J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- 59.Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, Nusse R, Axelrod JD. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–1105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–1564. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu J, Jenny A, Mirkovic I, Mlodzik M. Frizzled-Dishevelled signaling specificity outcome can be modulated by Diego in Drosophila. Mech Dev. 2008;125:30–42. doi: 10.1016/j.mod.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 63.Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, Uemura T. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19:389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- 65.Strutt D, Strutt H. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev Biol. 2007;302:181–194. doi: 10.1016/j.ydbio.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eaton S, Wepf R, Simons K. Roles for Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila. J Cell Biol. 1996;135:1277–1289. doi: 10.1083/jcb.135.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turner CM, Adler PN. Distinct roles for the actin and microtubule cytoskeletons in the morphogenesis of epidermal hairs during wing development in Drosophila. Mech Dev. 1998;70:181–192. doi: 10.1016/s0925-4773(97)00194-9. [DOI] [PubMed] [Google Scholar]
- 68.Park WJ, Liu J, Sharp EJ, Adler PN. The Drosophila tissue polarity gene inturned acts cell autonomously and encodes a novel protein. Development. 1996;122:961–969. doi: 10.1242/dev.122.3.961. [DOI] [PubMed] [Google Scholar]
- 69.Collier S, Gubb D. Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development. 1997;124:4029–4037. doi: 10.1242/dev.124.20.4029. [DOI] [PubMed] [Google Scholar]
- 70.Collier S, Lee H, Burgess R, Adler P. The WD40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the Drosophila epidermis. Genetics. 2005;169:2035–2045. doi: 10.1534/genetics.104.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adler PN, Zhu C, Stone D. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr Biol. 2004;14:2046–2051. doi: 10.1016/j.cub.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 72.Strutt D, Warrington SJ. Planar polarity genes in the Drosophila wing regulate the localisation of the FH3-domain protein Multiple Wing Hairs to control the site of hair production. Development. 2008;135:3103–3111. doi: 10.1242/dev.025205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu Q, Yan J, Adler PN. The Drosophila planar polarity proteins inturned and multiple wing hairs interact physically and function together. Genetics. 2010;185:549–558. doi: 10.1534/genetics.110.114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mlodzik M. Planar polarity in the Drosophila eye: a multifaceted view of signaling specificity and cross-talk. EMBO J. 1999;18:6873–6879. doi: 10.1093/emboj/18.24.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strutt H, Strutt D. Polarity determination in the Drosophila eye. Curr. Opin. Genet. Dev. 1999;9:442–446. doi: 10.1016/S0959-437X(99)80067-7. [DOI] [PubMed] [Google Scholar]
- 76.Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991;113:841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- 77.Fanto M, Mlodzik M. Asymmetric Notch activation specifies photoreceptors R3 and R4 and planar polarity in the Drosophila eye. Nature. 1999;397:523–526. doi: 10.1038/17389. [DOI] [PubMed] [Google Scholar]
- 78.Tomlinson A, Struhl G. Decoding vectorial information from a gradient: sequential roles of the receptors Frizzled and Notch in establishing planar polarity in the Drosophila eye. Development. 1999;126:5725–5738. doi: 10.1242/dev.126.24.5725. [DOI] [PubMed] [Google Scholar]
- 79.Zheng L, Zhang J, Carthew RW. frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development. 1995;121:3045–3055. doi: 10.1242/dev.121.9.3045. [DOI] [PubMed] [Google Scholar]
- 80.Cooper MT, Bray SJ. Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature. 1999;397:526–530. doi: 10.1038/17395. [DOI] [PubMed] [Google Scholar]
- 81.del Alamo D, Mlodzik M. Frizzled/PCP-dependent asymmetric neuralized expression determines R3/R4 fates in the Drosophila eye. Dev Cell. 2006;11:887–894. doi: 10.1016/j.devcel.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 82.Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 83.Fiehler RW, Wolff T. Drosophila Myosin II, Zipper, is essential for ommatidial rotation. Dev Biol. 2007;310:348–362. doi: 10.1016/j.ydbio.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mirkovic I, Mlodzik M. Cooperative activities of drosophila DE-cadherin and DN-cadherin regulate the cell motility process of ommatidial rotation. Development. 2006;133:3283–3293. doi: 10.1242/dev.02468. [DOI] [PubMed] [Google Scholar]
- 85.Choi KW, Benzer S. Rotation of photoreceptor clusters in the developing Drosophila eye requires the nemo gene. Cell. 1994;78:125–136. doi: 10.1016/0092-8674(94)90579-7. [DOI] [PubMed] [Google Scholar]
- 86.Fiehler RW, Wolff T. Nemo is required in a subset of photoreceptors to regulate the speed of ommatidial rotation. Dev Biol. 2008;313:533–544. doi: 10.1016/j.ydbio.2007.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mirkovic I, Gault WJ, Rahnama M, Jenny A, Gaengel K, Bessette D, Gottardi CJ, Verheyen EM, Mlodzik M. Nemo kinase phosphorylates beta-catenin to promote ommatidial rotation and connects core PCP factors to E-cadherin-beta-catenin. Nat Struct Mol Biol. 2011 doi: 10.1038/nsmb.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strutt H, Strutt D. EGF signaling and ommatidial rotation in the Drosophila eye. Curr Biol. 2003;13:1451–1457. doi: 10.1016/s0960-9822(03)00545-1. [DOI] [PubMed] [Google Scholar]
- 89.Gaengel K, Mlodzik M. Egfr signaling regulates ommatidial rotation and cell motility in the Drosophila eye via MAPK/Pnt signaling and the Ras effector Canoe/AF6. Development. 2003;130:5413–5423. doi: 10.1242/dev.00759. [DOI] [PubMed] [Google Scholar]
- 90.Brown KE, Freeman M. Egfr signalling defines a protective function for ommatidial orientation in the Drosophila eye. Development. 2003;130:5401–5412. doi: 10.1242/dev.00773. [DOI] [PubMed] [Google Scholar]
- 91.Weber U, Pataki C, Mihaly J, Mlodzik M. Combinatorial signaling by the Frizzled/PCP and Egfr pathways during planar cell polarity establishment in the Drosophila eye. Dev Biol. 2008;316:110–123. doi: 10.1016/j.ydbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adler PN, Taylor J. Asymmetric cell division: plane but not simple. Curr Biol. 2001;11:R233–236. doi: 10.1016/s0960-9822(01)00113-0. [DOI] [PubMed] [Google Scholar]
- 93.Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131:4651–4664. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]
- 94.Bellaiche Y, Gho M, Kaltschmidt JA, Brand AH, Schweisguth F. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat Cell Biol. 2001;3:50–57. doi: 10.1038/35050558. [DOI] [PubMed] [Google Scholar]
- 95.Bellaiche Y, Beaudoin-Massiani O, Stuttem I, Schweisguth F. The planar cell polarity protein Strabismus promotes Pins anterior localization during asymmetric division of sensory organ precursor cells in Drosophila. Development. 2004;131:469–478. doi: 10.1242/dev.00928. [DOI] [PubMed] [Google Scholar]
- 96.Segalen M, Johnston CA, Martin CA, Dumortier JG, Prehoda KE, David NB, Doe CQ, Bellaiche Y. The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Dev Cell. 2010;19:740–752. doi: 10.1016/j.devcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olguin P, Glavic A, Mlodzik M. Intertissue mechanical stress affects Frizzled-mediated planar cell polarity in the Drosophila notum epidermis. Curr Biol. 2011;21:236–242. doi: 10.1016/j.cub.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, Hseih JC, Rubin JS, Salinas PC, Kelley MW. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–2384. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- 99.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 101.Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 102.Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 104.Liu G, Bafico A, Harris VK, Aaronson SA. A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol Cell Biol. 2003;23:5825–5835. doi: 10.1128/MCB.23.16.5825-5835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu G, Bafico A, Aaronson SA. The mechanism of endogenous receptor activation functionally distinguishes prototype canonical and noncanonical Wnts. Mol Cell Biol. 2005;25:3475–3482. doi: 10.1128/MCB.25.9.3475-3482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shnitsar I, Borchers A. PTK7 recruits dsh to regulate neural crest migration. Development. 2008;135:4015–4024. doi: 10.1242/dev.023556. [DOI] [PubMed] [Google Scholar]
- 107.Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A. PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development. 2009;136:2039–2048. doi: 10.1242/dev.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, Tarui H, Sasaki H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 109.Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 110.Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 112.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 113.Xu W, He X. DEEP Insights through the DEP Domain. Structure. 2010;18:1223–1225. doi: 10.1016/j.str.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–571. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- 115.Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 116.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 117.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 121.Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, Lee HJ, Wu AL, Fang Y, Satlin LM, et al. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol. 2009;11:286–294. doi: 10.1038/ncb1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 124.Hermle T, Saltukoglu D, Grunewald J, Walz G, Simons M. Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol. 2010;20:1269–1276. doi: 10.1016/j.cub.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 125.Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, Spirohn K, Niehrs C, Boutros M. Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol. 2010;20:1263–1268. doi: 10.1016/j.cub.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 126.Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- 127.Torres MA, Nelson WJ. Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J Cell Biol. 2000;149:1433–1442. doi: 10.1083/jcb.149.7.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bernatik O, Ganji RS, Dijksterhuis JP, Konik P, Cervenka I, Polonio T, Krejci P, Schulte G, Bryja V. Sequential activation and inactivation of Dishevelled in the Wnt/beta-catenin pathway by casein kinases. J Biol Chem. 2011;286:10396–10410. doi: 10.1074/jbc.M110.169870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- 131.Willert K, Brink M, Wodarz A, Varmus H, Nusse R. Casein kinase 2 associates with and phosphorylates dishevelled. EMBO J. 1997;16:3089–3096. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Strutt H, Price MA, Strutt D. Planar polarity is positively regulated by casein kinase Iepsilon in Drosophila. Curr Biol. 2006;16:1329–1336. doi: 10.1016/j.cub.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 133.Paricio N, Feiguin F, Boutros M, Eaton S, Mlodzik M. The Drosophila STE20-like kinase misshapen is required downstream of the Frizzled receptor in planar polarity signaling. EMBO J. 1999;18:4669–4678. doi: 10.1093/emboj/18.17.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 135.Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Weber U, Paricio N, Mlodzik M. Jun mediates Frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development. 2000;127:3619–3629. doi: 10.1242/dev.127.16.3619. [DOI] [PubMed] [Google Scholar]
- 138.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aw S, Levin M. Is left-right asymmetry a form of planar cell polarity? Development. 2009;136:355–366. doi: 10.1242/dev.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Y, Levin M. Left-right asymmetry in the chick embryo requires core planar cell polarity protein Vangl2. Genesis. 2009;47:719–728. doi: 10.1002/dvg.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tissir F, Qu Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, Fujimori T, Labeau J, Tyteca D, Courtoy P, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- 146.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 147.Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 148.Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- 149.Jones C, Chen P. Primary cilia in planar cell polarity regulation of the inner ear. Curr Top Dev Biol. 2008;85:197–224. doi: 10.1016/S0070-2153(08)00808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kranzlin B, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet. 2005;6:928–940. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 152.Marshall WF. The cell biological basis of ciliary disease. J Cell Biol. 2008;180:17–21. doi: 10.1083/jcb.200710085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kibar Z, Bosoi CM, Kooistra M, Salem S, Finnell RH, De Marco P, Merello E, Bassuk AG, Capra V, Gros P. Novel mutations in VANGL1 in neural tube defects. Hum Mutat. 2009;30:E706–715. doi: 10.1002/humu.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, Mathieu M, Kirillova I, De Marco P, Merello E, Hayes JM, et al. Mutations in VANGL1 associated with neural-tube defects. N Engl J Med. 2007;356:1432–1437. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- 156.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 159.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 161.Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat Genet. 2006;38:1316–1322. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- 162.Gray RS, Abitua PB, Wlodarczyk BJ, Szabo-Rogers HL, Blanchard O, Lee I, Weiss GS, Liu KJ, Marcotte EM, Wallingford JB, et al. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol. 2009;11:1225–1232. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bertet C, Lecuit T. Planar polarity and short-range polarization in Drosophila embryos. Semin Cell Dev Biol. 2009;20:1006–1013. doi: 10.1016/j.semcdb.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 164.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 165.Rauzi M, Lenne PF, Lecuit T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 2010;468:1110–1114. doi: 10.1038/nature09566. [DOI] [PubMed] [Google Scholar]
- 166.Djiane A, Riou J, Umbhauer M, Boucaut J, Shi D. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2000;127:3091–3100. doi: 10.1242/dev.127.14.3091. [DOI] [PubMed] [Google Scholar]
- 167.Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- 168.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang Y, Thekdi N, Smallwood PM, Macke JP, Nathans J. Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J Neurosci. 2002;22:8563–8573. doi: 10.1523/JNEUROSCI.22-19-08563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yu H, Smallwood PM, Wang Y, Vidaltamayo R, Reed R, Nathans J. Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development. 2010;137:3707–3717. doi: 10.1242/dev.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- 172.Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:3487. doi: 10.1242/dev.121.10.3487. [DOI] [PubMed] [Google Scholar]
- 173.Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- 174.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 175.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Almuedo-Castillo M, Salo E, Adell T. Dishevelled is essential for neural connectivity and planar cell polarity in planarians. Proc Natl Acad Sci U S A. 2011;108:2813–2818. doi: 10.1073/pnas.1012090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- 178.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wolff T, Rubin GM. strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- 181.Shafer B, Onishi K, Lo C, Colakoglu G, Zou Y. Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev Cell. 2011;20:177–191. doi: 10.1016/j.devcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang B, Sinha T, Jiao K, Serra R, Wang J. Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type B. Hum Mol Genet. 2011;20:271–285. doi: 10.1093/hmg/ddq462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 184.Das G, Reynolds-Kenneally J, Mlodzik M. The Atypical Cadherin Flamingo Links Frizzled and Notch Signaling in Planar Polarity Establishment in the Drosophila Eye. Dev. Cell. 2002 doi: 10.1016/s1534-5807(02)00147-8. in press. [DOI] [PubMed] [Google Scholar]
- 185.Carreira-Barbosa F, Kajita M, Morel V, Wada H, Okamoto H, Martinez Arias A, Fujita Y, Wilson SW, Tada M. Flamingo regulates epiboly and convergence/extension movements through cell cohesive and signalling functions during zebrafish gastrulation. Development. 2009;136:383–392. doi: 10.1242/dev.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Qu Y, Glasco DM, Zhou L, Sawant A, Ravni A, Fritzsch B, Damrau C, Murdoch JN, Evans S, Pfaff SL, et al. Atypical cadherins Celsr1–3 differentially regulate migration of facial branchiomotor neurons in mice. J Neurosci. 2010;30:9392–9401. doi: 10.1523/JNEUROSCI.0124-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhou L, Bar I, Achouri Y, Campbell K, De Backer O, Hebert JM, Jones K, Kessaris N, de Rouvroit CL, O'Leary D, et al. Early forebrain wiring: genetic dissection using conditional Celsr3 mutant mice. Science. 2008;320:946–949. doi: 10.1126/science.1155244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A, Zou Y, Pasterkamp RJ. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2010;30:16053–16064. doi: 10.1523/JNEUROSCI.4508-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- 191.Gubb D, Green C, Huen D, Coulson D, Johnson G, Tree D, Collier S, Roote J. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 1999;13:2315–2327. doi: 10.1101/gad.13.17.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, Ueno N. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr Biol. 2003;13:674–679. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 193.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 194.Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS One. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Feiguin F, Hannus M, Mlodzik M, Eaton S. The Ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Developmental Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 196.Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, Huelsken J, Behrens J, Hammerschmidt M, Birchmeier W. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 2002;16:2073–2084. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Itoh K, Jenny A, Mlodzik M, Sokol SY. Centrosomal localization of Diversin and its relevance to Wnt signaling. J Cell Sci. 2009;122:3791–3798. doi: 10.1242/jcs.057067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cong F, Schweizer L, Varmus H. Casein kinase Iepsilon modulates the signaling specificities of dishevelled. Mol Cell Biol. 2004;24:2000–2011. doi: 10.1128/MCB.24.5.2000-2011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Purvanov V, Koval A, Katanaev VL. A direct and functional interaction between Go and Rab5 during G protein-coupled receptor signaling. Sci Signal. 2010;3:ra65. doi: 10.1126/scisignal.2000877. [DOI] [PubMed] [Google Scholar]
- 200.Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- 201.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 202.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 203.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 204.Tepass U. Genetic analysis of cadherin function in animal morphogenesis. Curr Opin Cell Biol. 1999;11:540–548. doi: 10.1016/s0955-0674(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 205.Viktorinova I, Konig T, Schlichting K, Dahmann C. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development. 2009;136:4123–4132. doi: 10.1242/dev.039099. [DOI] [PubMed] [Google Scholar]
- 206.Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Strutt H, Mundy J, Hofstra K, Strutt D. Cleavage and secretion is not required for Four-jointed function in Drosophila patterning. Development. 2004;131:881–890. doi: 10.1242/dev.00996. [DOI] [PubMed] [Google Scholar]
- 208.Zeidler MP, Perrimon N, Strutt DI. Polarity determination in the Drosophila eye: a novel role for unpaired and JAK/STAT signaling. Genes & Dev. 1999;13:1342–1353. doi: 10.1101/gad.13.10.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev. Biol. 2000;228:181–196. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- 210.Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, Kerridge S, McNeill H. The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development. 2003;130:763–774. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- 211.Zhang S, Xu L, Lee J, Xu T. Drosophila atrophin homolog functions as a transcriptional corepressor in multiple developmental processes. Cell. 2002;108:45–56. doi: 10.1016/s0092-8674(01)00630-4. [DOI] [PubMed] [Google Scholar]
- 212.Mao Y, Rauskolb C, Cho E, Hu WL, Hayter H, Minihan G, Katz FN, Irvine KD. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development. 2006;133:2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 213.Mao Y, Tournier AL, Bates PA, Gale JE, Tapon N, Thompson BJ. Planar polarization of the atypical myosin Dachs orients cell divisions in Drosophila. Genes Dev. 2011;25:131–136. doi: 10.1101/gad.610511. [DOI] [PMC free article] [PubMed] [Google Scholar]