Abstract
Agents that target the activity of the mammalian target of rapamycin (mTOR) kinase in humans are associated with proteinuria. However, the mechanisms underlying mTOR activity and signaling within the kidney are poorly understood. In this study, we developed a sensitive immunofluorescence technique for the evaluation of activated pmTOR and its associated signals in situ. While we find that pmTOR is rarely expressed in normal non-renal tissues, we consistently find intense expression in glomeruli within normal mouse and human kidneys. Using double staining, we find that the expression of pmTOR co-localizes with nephrin in podocytes and expression appears minimal within other cell types in the glomerulus. In addition, we found that pmTOR was expressed on occasional renal tubular cells within mouse and human kidney specimens. We also evaluated mTOR signaling in magnetic bead-isolated glomeruli from normal mice and, by Western blot analysis, we confirmed function of the pathway in glomerular cells vs. interstitial cells. Furthermore, we found that the activity of the pathway as well as the expression of VEGF, a target of mTOR-induced signaling, were reduced within glomeruli of mice following treatment with rapamycin. Collectively, these findings demonstrate that the mTOR signaling pathway is constitutively hyperactive within podocytes. We suggest that pmTOR signaling functions to regulate glomerular homeostasis in part via the inducible expression of VEGF.
Keywords: kidney, glomerulus, podocyte, mTOR, signaling
BACKGROUND
The mammalian target of rapamycin (mTOR) exists in two structurally and functionally distinct multiprotein complexes within many human cells types [1; 2; 3]. mTOR complex 1 (mTORC1) mediates the temporal control of cell growth by regulating translation, transcription, ribosome biogenesis and nutrient transport [4]. mTORC2 transmits signals towards the phosphorylation and activation of the AGC family of kinases, including Akt, SGK and PKC, as well as towards actin cytoskeleton rearrangements [5; 6; 7]. The activity of mTORC1/2 is classically mediated by nutrients, growth factors and cytokines, as well as by events mediated via select receptor-inducible signals [4; 8]. Overall, activity of mTOR controls fundamental cellular processes including growth, proliferation, survival and the stabilization of protein translation.
Therapeutics that inhibit the activity of mTOR were initially developed and approved based on their potent immunosuppressive effects and their ability to inhibit effector T cell activation and T cell-dependent immune responses [9; 10]. However, the mTOR kinase and its biological signaling response is important in many other cell types, and it is thus not surprising that mTOR inhibitors have far reaching effects beyond their ability to target the activation of T cells [2; 10; 11; 12; 13]. Indeed, beyond a role in alloimmune T cell activation, mTOR signaling is known to be of functional importance in vascular endothelial cells [14], podocytes [15] and renal tubular epithelial cells [16; 17], and it may function in several renal diseases. For instance, mTOR signaling in renal epithelial cells mediate cyst formation in polycystic kidney disease (PKD) in rat and mouse models [18] as well as in the human autosomal dominant variant [19], and mTOR inhibitor therapy has shown promise to delay the progression of PKD in humans [19; 20; 21]. Also, mTOR inhibitors have been demonstrated to attenuate the progression of HIV nephropathy [22], renal angiomyolipomas and tuberous sclerosis [23]. Nevertheless, little is known about basal activity of the pathway in the normal kidney, and the relative cell-specific activity of mTOR signals has not been previously visualized in situ.
In this study, we developed immunofluorescence techniques to assess cell-specific expression patterns of pmTOR within the kidney. Our findings illustrate prominent hyperactivity of the signaling pathway within the normal glomerulus, notably within podocytes.
METHODS
Specimens
These studies were performed on kidneys harvested from 6-week-old C57BL/6 mice (Jackson Laboratories) either untreated or following treatment with intraperitoneal injections of rapamycin (1.5mg/kg/day of 4mg/kg/day, gifted by Wyeth Pharmaceuticals) or vehicle control. All experiments were performed according to the policies of the Institutional Animal Care and Use Committee of Children's Hospital Boston. Human tissue was obtained as the normal portion of kidneys excised for renal cancer and as biopsy specimens from renal transplant recipients after clinical care and diagnosis was completed. Receipt of clinical material was approved by the Committee on Clinical Investigation at Children's Hospital Boston and at the Beth Israel Deaconess Medical Center, Boston MA.
Light and Electron Microscopy
Kidneys were serially sectioned and fixed in 2.5% Glutaraldehyde, post-fixed with osmium, processed, stained with uranyl acetate and embedded in an araldite-epon mixture. 1-micron sections were cut, stained using both Toluidine Blue and Geimsa and examined by light microscopy. Thin sections of appropriate areas were then cut and studied by electron microscopy.
Glomerular Isolation
Glomerular isolation was performed as previously described [24]. After ligation of the iliac artery, 8 × 107 M-450 tosylactivated Dynabeads (Invitrogen Dynal, Oslo, Norway) diluted in 40mls of Hanks’ balanced salt solution (HBSS) (Lonza, Walkersville, MD) were perfused through the mice via cardiac puncture. After perfusion, the kidneys were removed, were minced into 1mm2 cubes and were digested in 1mg/ml collagenase A (Worthington, Lakewood, NJ) and 100U/ml deoxyribonuclease (Invitrogen, Oslo, Norway) for 30 minutes at 37°C. The tissue was gently passed through a 100μm cell strainer (Fisher Scientific, Morris Plains, NJ) and washed with 15mls of HBSS. After passage through a second cell strainer, the tissue was washed and centrifuged for 5 minutes. The supernatant was discarded and the remaining cells were resuspended in 5 ml HBSS. Glomeruli were separated from the surrounding tissue using a magnet and washed twice in HBSS. The glomerular and non-glomerular isolates were stored at minus 20°C until use.
Immunofluorescence Studies
Kidneys were snap frozen in OCT (Sakura Finetek, Torrance, CA) and 3 μm cryosections were used for immunofluorescence. The tissue was fixed in 3% paraformaldehyde (PFA) for 10 minutes. Endogenous peroxidase was blocked with 3% hydrogen peroxide and non-specific binding was blocked with a proprietary blocking reagent (Perkin Elmer, Boston, MA). The tissue was incubated in primary antibody at 4°C overnight, washed with 3 changes of PBS and then incubated with the appropriate secondary antibody for 30 minutes. After washing in PBS, the tissue was incubated for 5 minutes with a Cy3-conjugated tyramide solution (1:200, Perkin Elmer, Boston, MA) and the slides were mounted using DAPI mounting medium (Vector, Burlingame, CA). Primary antibodies were used at optimal dilutions and included anti-phospho-mTOR (Ser2448) (Cell Signaling, Danvers, MA), anti-mouse CD31 (BD Biosciences, San Jose, CA) and anti-nephrin (Progen, Heidelberg, Germany). Secondary antibodies were alexafluor 488 goat anti-rat (Invitrogen, Eugene, OR), FITC-conjugated goat anti-guinea-pig (Jackson Immunoresearch, West Grove, PA) and HRP-conjugated goat anti-rabbit (Jackson Immunoresearch, West Grove, PA) also used at optimal dilutions. The slides were visualized with a Nikon Eclipse 80i microscope with a Qimaging Retiga 200R Fast 1394 camera using NIS-Elements BR 3.00 software (Micro Video Instruments, Avon, MA).
Western Blot Analysis
Glomerular and non-glomerular kidney isolates were lysed in RIPA buffer containing protease inhibitors (Boston BioProducts, Worcester, MA). Equal amounts of protein lysate were resolved by 4-15% Tris-HCL polyacrylamide gel electrophoresis (BioRad Laboratories, Hercules, CA). Primary antibodies used for immunoblotting were anti-phospho mTOR (Ser2448) (Cell Signaling, Danvers, MA), anti-phospho S6 kinase (Thr389) (Epitomics, Burlingame, CA), anti-VEGFA (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-GAPDH (Sigma Life Sciences, St Louis, MO). Secondary antibody was anti-rabbit HRP (Santa Cruz Biotechnologies, Santa Cruz, CA), and bands were resolved by chemoluminescence (Thermo Scientific, Rockford, IL).
In situ hybridization
Kidneys were dissected and washed in RNAase-free phosphate-buffered saline (PBS) and fixed overnight in DEPC-treated 4% PFA. Subsequently, they were immersed in 30% sucrose for 4 hours, embedded in OCT (Sakura Finetek, Torrance, CA) and snap frozen in liquid nitrogen. Sense and anti-sense digoxigenin-labeled VEGF-A riboprobes were prepared using standard techniques. In situ was performed on 10μm cryosections, initially fixed in 4% PFA for 10 minutes and subsequently rinsed in PBS, treated with 15ug/ml Proteinase K (Roche) in PBS for 20 minutes, washed and fixed 4% PFA for 5 minutes. The tissue was next immersed in 1.3X SSC hybridization buffer (50% formamide, 5x sodium chloride-sodium citrate (SSC) pH 4.5, 5mM EDTA, 50ug/ml yeast tRNA, 0.2% Tween-20, 0.5% CHAPS (Fisher Scientific, Fair Lawn, NJ), 100ug/ml heparin) and hybridization of the tissue with the riboprobe was performed overnight at 68° in a 50% formamide-humidified chamber. After hybridization, the slides were washed in 0.2X SSC at 72°C and blocked with 2% blocking reagent (Roche Applied Sciences, Indianapolis, IN) with 5% heat inactivated sheep serum in NTT (0.15M NaCl, 0.1M Tris pH 7.5, 0.1% Tween-20). The sections were finally incubated overnight with an anti-Digoxigenin antibody (Roche Applied Sciences, Indianapolis, IN) diluted to optimal concentrations in NTT. After washing in NTT x3 for 30 minutes, the slides were immersed overnight in BM purple AP substrate coupled to alkaline phosphatase (Roche Applied Sciences, Indianapolis, IN). Once the slides had developed, they were rinsed in PBS, fixed in 4% PFA in PBS for 2 hours and mounted with an aqueous mounting medium.
RESULTS
We initially visualized the expression of pmTOR in situ in different organs harvested from C57BL/6 mice using immunofluorescence. Although pmTOR can be visualized in endothelial cells in skin in association with inflammation [25], we found minimal expression of pmTOR and its downstream substrates in most normal uninflamed tissues (data not shown). However, consistently, we found intense basal constitutive levels of expression of pmTOR within the normal kidney and expression was most notable and prominent within glomeruli (Figure 1A-B), even at low power magnification (Figure 1A). In addition, pmTOR was visible along the brush border of occasional proximal tubules, but relative to the glomeruli, the level of expression in tubular epithelial cells was low intensity. To determine the physiological relevance of these observations, we also evaluated pmTOR expression in native human kidneys and in biopsy material obtained from human renal allografts. As illustrated in Figures 1C-D, we found that the expression of pmTOR localized to glomerular capillary loops in human tissues, in an identical pattern as that observed in the mouse. To validate these observations further, we isolated whole glomeruli from healthy mice using an established magnetic bead technique [24], and expression patterns were evaluated by Western blot analysis. Similar to our findings using immunofluorescence, we found high levels of expression of pmTOR and its downstream substrate pS6k within these glomerular isolates (Figure 1E). These findings indicate that the mTOR signaling pathway is constitutively hyperactive within normal renal glomeruli.
Figure 1. pmTOR is expressed at high levels in normal mouse and human glomeruli.
Kidneys were harvested from C57BL/6 mice, and immunofluorescence was performed on 3μm cryostat sections. Illustrated are low power (Panel A) and high power (Panel B) micrographs of the renal cortex showing intense expression of pmTOR within glomeruli and minimal expression on occasional tubules in extra-glomerular tissues. Panels C and D illustrate a similar pattern of expression of pmTOR in a human kidney transplant biopsy (Panel C) and in normal human kidney tissue (Panel D). In Panel E, glomeruli were isolated from C57BL/6 mice using magnetic beads (as described in Methods), and the expression of pmTOR, pS6 kinase and GAPDH was evaluated by Western blot analysis. Illustrated is the expression of pmTOR and pS6 kinase in the glomerular isolates (Glom) and in non-glomerular (Non-G) renal tissue.
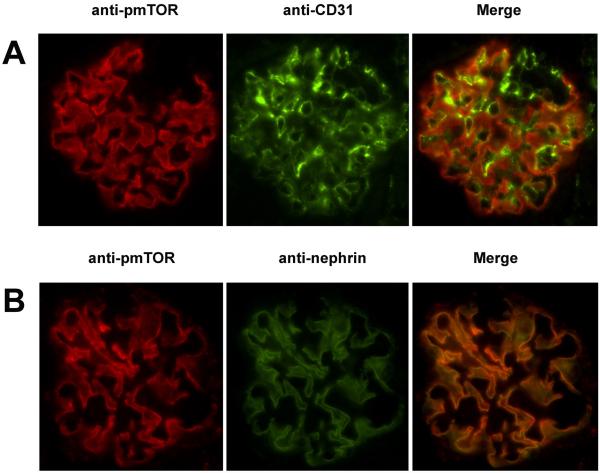
We hypothesized that mTOR-induced signaling is functional in both endothelial cells (EC) and podocytes within glomeruli. To test this hypothesis, we performed double immunofluorescence staining of kidneys using anti-pmTOR and either anti-CD31 (an EC-specific marker) or anti-nephrin (a podocyte-specific marker). As illustrated in Figure 2A-B, we found that few CD31-expressing glomerular EC co-express pmTOR. Rather, we found significant co-expression of pmTOR with nephrin in all glomerular capillary loops (Figure 2), indicating that expression is reflective of high basal activity of the signaling pathway within normal podocytes in situ.
Figure 2. pmTOR is primarily expressed by the podocyte.
Double immunofluorescence staining of normal mouse kidneys was performed using anti-pmTOR and either anti-CD31 or anti-nephrin. Panel A, immunofluorescence staining for pmTOR (left panel, red), CD31 (mid panel, green) and the merged image (right panel) showing a lack of co-localization (yellow) of pmTOR with CD31. Panel B, immunofluorescence staining for pmTOR (left panel, red), nephrin (mid panel, green) and the merged image (right) showing co-localization (yellow) of pmTOR with podocyte-specific nephrin in the glomerulus.
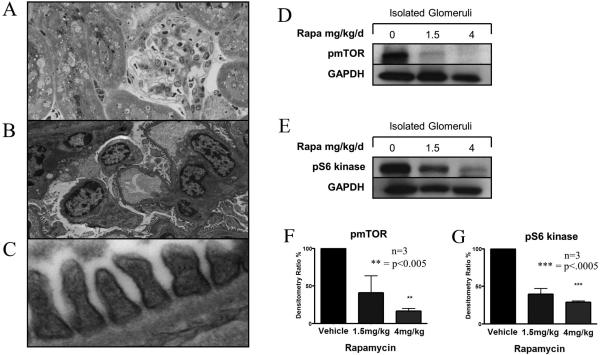
We next treated mice with the pharmacological mTOR inhibitor rapamycin (1.5mg/kg/day or 4mg/kg/day i.p.), and we assessed its effect on pmTOR activity in podocytes in vivo. After 4 days of therapy, the kidneys were harvested and processed for light microscopy, electron microscopy and Western blot analysis. As illustrated in Figure 3A , by light microscopy we found no difference in the morphology of kidneys harvested from vehicle-treated and rapamycin-treated mice. Also, as illustrated in Figure 3B-C, by electron microscopy, we failed to find any effect of rapamycin on EC or podocytes within glomeruli; notably foot processes, basement membrane and capillary loops were normal. However, by Western blot analysis, we found that rapamycin treatment resulted in the inhibition of pmTOR (Figure 3D, F), as well as its downstream substrate pS6K (Figure 3E-G) within lysates generated from glomeruli. This observation suggests that rapamycin targets mTOR signaling within podocytes in vivo. Since it short-term treatment with rapamycin predominant targets of mTORC1 [11]; (vs. mTORC2 [26]) it is expected that there will be only a limited effect on mTORC2-dependent cell survival.
Figure 3. The treatment of mice with rapamycin inhibits glomerular expression of pmTOR and pS6 kinase.
C57BL/6 mice were treated with 1.5mg/kg/day or 4mg/kg/day of rapamycin i.p. or vehicle as a control. After 4 days, the kidneys were harvested and processed for light microscopy, electron microscopy and Western blot analysis. Panel A illustrates the histological appearance by light microscopy, and Panels B and C show the typical electron microscopy appearance of the renal cortex following treatment with rapamycin (4mg/kg). No histological changes are evident. Panels D-G show expression of pmTOR (Panels D, F), and pS6 kinase (Panels E, G) as evaluated by Western blot analysis in magnetic bead isolated glomeruli from control vehicle-treated and rapamycin-treated mice. Representative blots are illustrated in Panels D-E, and the mean densitometric analysis (+/- 1SD) from three different experiments are shown in Panels F and G. As illustrated, we found a dose-dependent reduction of glomerular expression of pmTOR and pS6 kinase in treated mice (vs. controls).
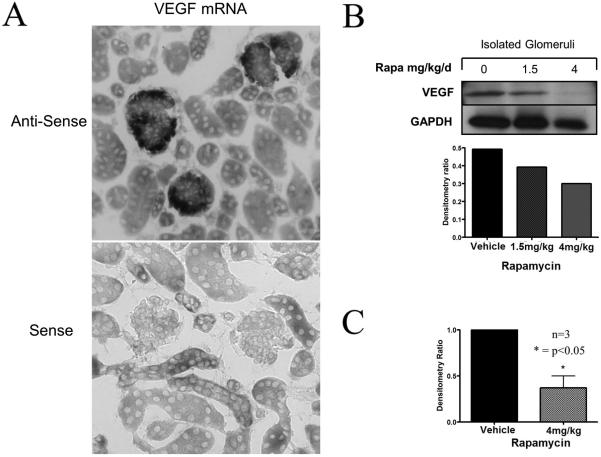
In a final series of experiments, we wished to evaluate the effect of mTOR targeting on the expression of VEGF by podocytes within the glomerulus. The activity of mTORC1 and mTORC2 results in the inducible expression of vascular endothelial growth factor (VEGF) [27; 28; 29; 30], and VEGF is thought to be a key mediator of mTOR-associated glomerular function [31; 32; 33]. However, it is not known whether podocyte-specific activity of mTOR regulates VEGF expression. We used in situ hybridization and Western blot analysis to identify expression of VEGF mRNA and protein in control mice and in mice that were treated with rapamycin. As illustrated in Figure 4A, as expected, we found that VEGF mRNA was primarily expressed within glomeruli in a podocyte specific pattern. Furthermore, rapamycin had no quantitative effect on mRNA patterning as assessed by in situ hybridization, but it inhibited VEGF protein expression in glomerular isolates as determined by Western blot analysis.
Figure 4. The effect of rapamycin on glomerular expression of VEGF.
Kidneys were harvested from C57BL/6 mice following treatment with rapamycin 1.5mg/kg/day or 4mg/kg/day for 4 days. Panel A, illustrates a representative pattern of expression of VEGF mRNA by in situ hybridization within glomeruli of these treated mice (upper panel). The sense control is shown in the lower panel. The expression was found to be identical to that observed in normal untreated mice (not shown). Panels B and C, illustrate quantitative VEGF expression in glomerular isolates by Western blot analysis. A representative blot and densitometry from one experiment is shown in Panel B and the mean densitometry from 3 different blots is shown in Panel C. VEGF protein levels were reduced in kidneys harvested from the mice that were treated with rapamycin.
DISCUSSION
In this study, we demonstrate that the mTOR signaling pathway is constitutively hyperactive under basal conditions within normal glomeruli, and we show that its activity is prominent in podocytes within the normal kidney. Thus, factor(s) present within the glomerulus may induce or sustain mTOR signaling responses within podocytes, or alternatively, podocytes may constitutively use this pathway to facilitate cellular integrity and normal functional responses.
Recent studies using knockout mice indicate that podocyte-specific interruption of mTORC1 function (via deletion of raptor) results in podocyte effacement, histological evidence of glomerular disease and proteinuria [15; 34; 35]. However, podocyte-specific targeting of mTORC2 (via rictor deletion) alone, which is upstream of mTORC1, does not alter renal function or renal histology under basal conditions [35]. Collectively, these observations indicate that, similar to other cell types [2; 14], mTOR-induced responses play a key role in cell-intrinsic podocyte function. However, they further suggest that mTORC1-inducible responses may be important for basal cellular function. Our studies for the first time show that this pathway is hyperactive in vivo under physiological conditions, and they also suggest that mTOR signaling within podocytes is a requirement for their normal function under basal conditions.
One possibility is that activity of mTORC1 and mTORC2 within podocytes results in the inducible expression of target genes such as vascular endothelial growth factor (VEGF), and that these factor(s) function to sustain glomerular integrity and function. VEGF is produced in large quantities by podocytes, and, it is thought that there is a fine balance to VEGF expression and function within the glomerulus [31; 32]. In addition, current models indicate that VEGF expression by podocytes is important to sustain VEGFR-dependent physiological responses within glomerular endothelial cells [31; 32; 33], either via autocrine effects within podocytes, or via paracrine effects on local endothelial cells [31]. mTOR is well established to mediate VEGF expression [27; 28; 29; 30], and it is also known to function in VEGF receptor-mediated signaling responses [25; 36]. To this end, we were surprised that we failed to observe pmTOR expression and activity within glomerular endothelial cells as a reflection of VEGFR-dependent signaling. This suggested to us that the major function of mTOR in the glomerulus is to regulate mTORC1-inducible VEGF protein expression by podocytes. Consistent with this likelihood, we find that the treatment of mice with rapamycin to pharmacologically inhibit mTORC1 in vivo reduces VEGF protein expression within glomerular isolates.
Nevertheless, it is also possible that our observations indicate that podocytes require mTOR signaling/activity for cell protective responses. However, as discussed above, deletion of podocyte mTORC2 (which is well established to regulate cell protection), in vivo did not result in renal disease in the absence of any injury [34; 35]. Perhaps, therefore, mTORC1-induced responses and/or mTORC1-regulated target genes are important for podocyte function [15; 33].
In summary, in this study, we visualized intense expression of pmTOR in situ in the kidney, notably on podocytes within the normal glomerulus. Our findings indicate that mTOR-induced responses are likely of great importance for normal podocyte function. They also suggest that under normal conditions, the maintenance of mTOR-inducible agonists/factor(s), within the glomerulus is important for normal glomerular homeostasis.
Acknowledgements
We wish to acknowledge Evelyn Flynn for providing technical support in immunocytochemistry.
Footnotes
Conflict of Interest Statement:
This work was funded in part by an investigator-originated grant from Wyeth Pharmaceuticals.
References
- 1.Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23:744–55. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall MN. mTOR-what does it do? Transplant Proc. 2008;40:S5–8. doi: 10.1016/j.transproceed.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Su B, Jacinto E. Mammalian TOR signaling to the AGC kinases. Crit Rev Biochem Mol Biol. 2011;46:527–47. doi: 10.3109/10409238.2011.618113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 7.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, Sessa WC, Qin J, Zhang P, Su B, Jacinto E. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–43. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 10.Contreras AG, Dormond O, Edelbauer M, Calzadilla K, Hoerning A, Pal S, Briscoe DM. mTOR-understanding the clinical effects. Transplant Proc. 2008;40:S9–S12. doi: 10.1016/j.transproceed.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 12.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Mondino A, Mueller DL. mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol. 2007;19:162–172. doi: 10.1016/j.smim.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dormond O, Madsen JC, Briscoe DM. The Effects of mTOR-Akt Interactions on Anti-apoptotic Signaling in Vascular Endothelial Cells. J Biol Chem. 2007;282:23679–86. doi: 10.1074/jbc.M700563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogo AB. The targeted podocyte. J Clin Invest. 2011;121:2142–5. doi: 10.1172/JCI57935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walid S, Eisen R, Ratcliffe DR, Dai K, Hussain MM, Ojakian GK. The PI 3-kinase and mTOR signaling pathways are important modulators of epithelial tubule formation. J Cell Physiol. 2008;216:469–79. doi: 10.1002/jcp.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahl PR, Le Hir M, Vogetseder A, Arcaro A, Starke A, Waeckerle-Men Y, Serra AL, Wuthrich RP. Mitotic activation of Akt signalling pathway in Han:SPRD rats with polycystic kidney disease. Nephrology (Carlton) 2007;12:357–63. doi: 10.1111/j.1440-1797.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 18.Edelstein CL. Mammalian target of rapamycin and caspase inhibitors in polycystic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2008;3:1219–1226. doi: 10.2215/CJN.05611207. [DOI] [PubMed] [Google Scholar]
- 19.Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N, Arns W, Pavenstadt H, Gaedeke J, Buchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU. Everolimus in patients with autosomal dominant polycystic kidney disease. The New England journal of medicine. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 20.Perico N, Antiga L, Caroli A, Ruggenenti P, Fasolini G, Cafaro M, Ondei P, Rubis N, Diadei O, Gherardi G, Prandini S, Panozo A, Bravo RF, Carminati S, De Leon FR, Gaspari F, Cortinovis M, Motterlini N, Ene-Iordache B, Remuzzi A, Remuzzi G. Sirolimus therapy to halt the progression of ADPKD. Journal of the American Society of Nephrology : JASN. 2010;21:1031–1040. doi: 10.1681/ASN.2009121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wuthrich RP. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. The New England journal of medicine. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 22.Kumar D, Konkimalla S, Yadav A, Sataranatarajan K, Kasinath BS, Chander PN, Singhal PC. HIV-associated nephropathy: role of mammalian target of rapamycin pathway. The American journal of pathology. 2010;177:813–821. doi: 10.2353/ajpath.2010.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budde K, Gaedeke J. Tuberous Sclerosis Complex-Associated Angiomyolipomas: Focus on mTOR Inhibition. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2011;59:276–283. doi: 10.1053/j.ajkd.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–70. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Dormond O, Contreras AG, Meijer E, Datta D, Flynn E, Pal S, Briscoe DM. CD40-induced signaling in human endothelial cells results in mTORC2- and Akt-dependent expression of vascular endothelial growth factor in vitro and in vivo. J Immunol. 2008;181:8088–95. doi: 10.4049/jimmunol.181.11.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 29.El-Hashemite N, Walker V, Zhang H, Kwiatkowski DJ. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 2003;63:5173–7. [PubMed] [Google Scholar]
- 30.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–14. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sison K, Eremina V, Baelde H, Min W, Hirashima M, Fantus IG, Quaggin SE. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol. 2010;21:1691–701. doi: 10.1681/ASN.2010030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF--a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol. 2007;106:p32–7. doi: 10.1159/000101798. [DOI] [PubMed] [Google Scholar]
- 33.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–16. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Ruegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121:2181–96. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mor A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstadt H, Kerjaschki D, Cohen CD, Hall MN, Ruegg MA, Inoki K, Walz G, Huber TB. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121:2197–209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. EXS. 1997;79:233–269. doi: 10.1007/978-3-0348-9006-9_10. [DOI] [PubMed] [Google Scholar]