Abstract
A lysed crude secretory granule fraction from rat islets of Langerhans was shown to process endogenous proinsulin to insulin with a pH optimum of 5.0--6.0. The converting activity in the lysed fraction was not inhibited by serine protease inhibitors (diisopropyl fluorophosphate, soybean trypsin inhibitor, and aprotinin) or metalloprotease inhibitors (EDTA and o-phenanthroline) but was inhibited by some thiol protease reagents (p-chloromercuribenzenesulfonic acid, antipain, and leupeptin) but not by others (N-ethylmaleimide and iodoacetamide). N alpha-p-Tosyl-L-lysyl chloromethyl ketone only mildly inhibited at higher concentrations, whereas L-alanyl-L-lysyl-L-arginyl chloromethyl ketone was a powerful inhibitor. L-Alanyl-L-lysyl-L-arginyl chloromethyl ketone was [125I]iodotyrosylated and used as an affinity labeling agent for the converting activity. Because the crude granule preparation contained contaminating lysosomes the affinity labeling of the granule preparation proteins was compared with that in liver lysosomes purified from rats injected with Triton WR1339. In the crude granule fraction the affinity label bound in a cysteine-enhanced manner to a single 31,500 molecular weight protein, but in purified liver lysosomes the major affinity-labeled protein had a molecular weight of 25,000 and minor 31,500 and 35,000 molecular weight proteins were also labeled. Evidence suggests that these proteins are thiol proteases and that in islets the 31,500 molecular weight thiol protease is involved in the conversion of proinsulin to insulin.
Full text
PDF
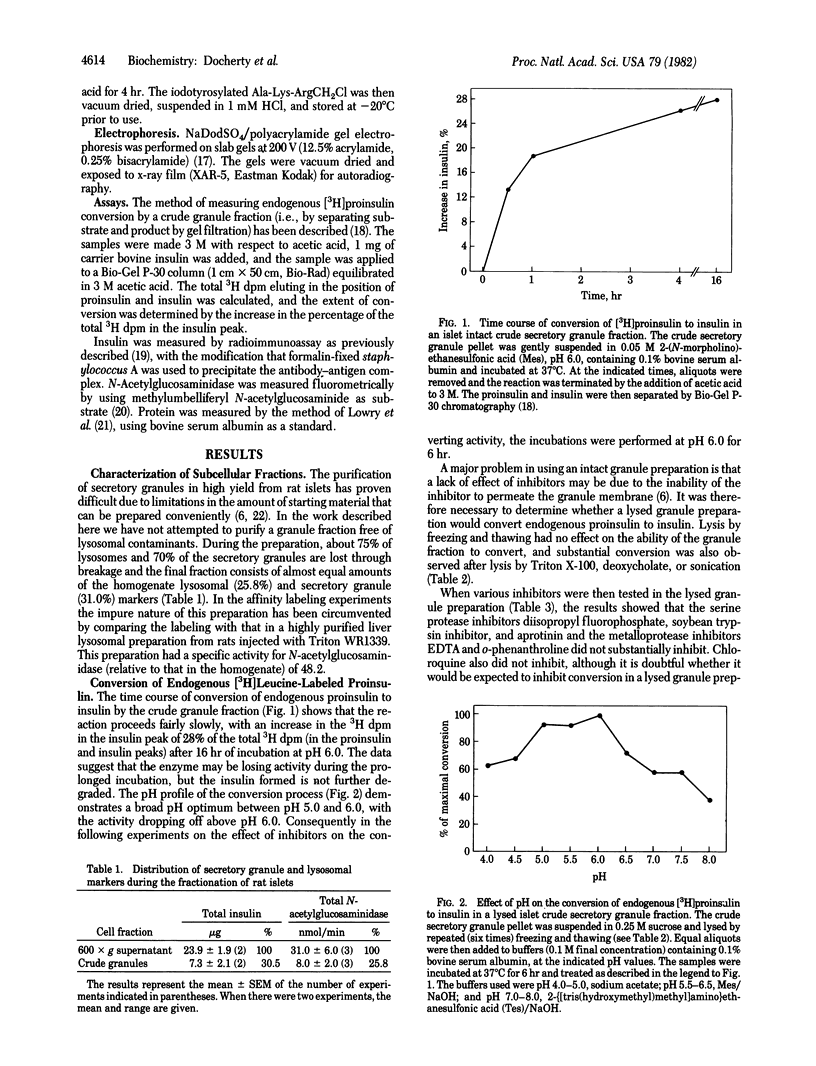
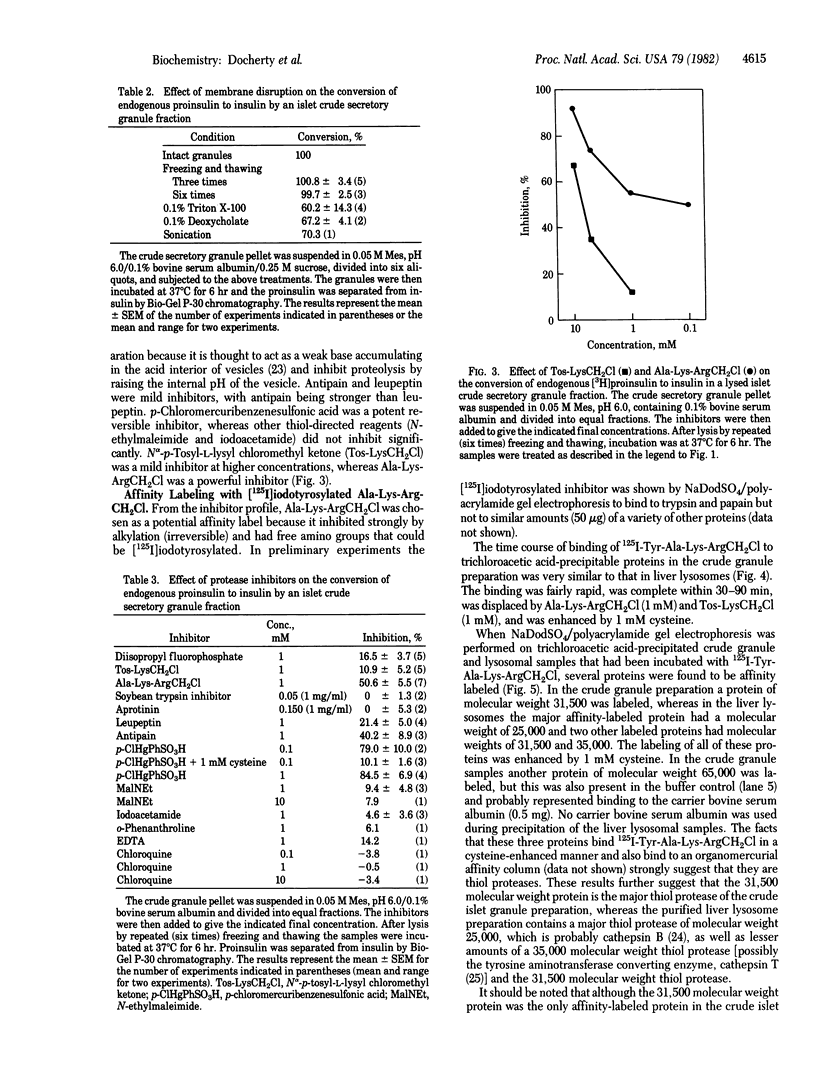
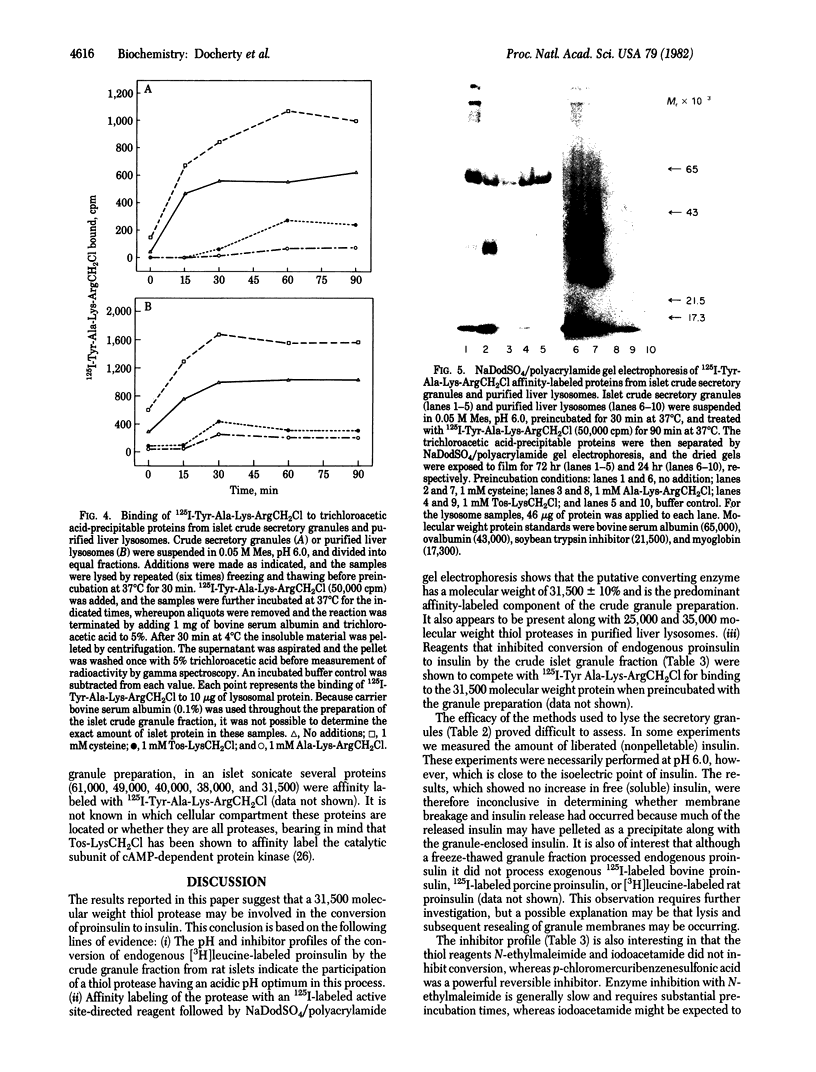

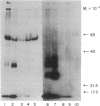
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Blix P. M., Rubenstein A. H., Tager H. S. Iodotyrosylation of peptides using tertiary-Butyloxycarbonyl-L-[125I]iodotyrosine N-hydroxysuccinimide ester. Anal Biochem. 1980 Mar 15;103(1):70–76. doi: 10.1016/0003-2697(80)90238-9. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartino G. N., Doebber T. W., Miller L. L. Pepstatin-insensitive proteolytic activity of rat liver lysosomes. Isolation and purification of a new pepstatin-insensitive proteolytic enzyme from rat liver lysosomes. J Biol Chem. 1977 Nov 10;252(21):7511–7516. [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Fletcher D. J., Noe B. D., Bauer G. E., Quigley J. P. Characterization of the conversion of a somatostatin precursor to somatostatin by islet secretory granules. Diabetes. 1980 Aug;29(8):593–599. doi: 10.2337/diab.29.8.593. [DOI] [PubMed] [Google Scholar]
- Fletcher D. J., Quigley J. P., Bauer G. E., Noe B. D. Characterization of proinsulin- and proglucagon-converting activities in isolated islet secretory granules. J Cell Biol. 1981 Aug;90(2):312–322. doi: 10.1083/jcb.90.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinot C., Gautheron D. C., Galante Y., Hatefi Y. Labeling of thiols involved in the activity of complex V of the mitochondrial oxidative phosphorylation system. J Biol Chem. 1981 Jul 10;256(13):6776–6782. [PubMed] [Google Scholar]
- Gohda E., Pitot H. C. A new thiol proteinase from rat liver. J Biol Chem. 1981 Mar 10;256(5):2567–2572. [PubMed] [Google Scholar]
- Howell S. L., Fink C. J., Lacy P. E. Isolation and properties of secretory granules from rat islets of Langerhans. I. Isolation of a secretory granule fraction. J Cell Biol. 1969 Apr;41(1):154–161. doi: 10.1083/jcb.41.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judah J. D., Quinn P. S. Calcium ion-dependent vesicle fusion in the conversion of proalbumin to albumin. Nature. 1978 Jan 26;271(5643):384–385. doi: 10.1038/271384a0. [DOI] [PubMed] [Google Scholar]
- Kemmler W., Peterson J. D., Steiner D. F. Studies on the conversion of proinsulin to insulin. I. Conversion in vitro with trypsin and carboxypeptidase B. J Biol Chem. 1971 Nov 25;246(22):6786–6791. [PubMed] [Google Scholar]
- Kemmler W., Steiner D. F., Borg J. Studies on the conversion of proinsulin to insulin. 3. Studies in vitro with a crude secretion granule fraction isolated from rat islets of Langerhans. J Biol Chem. 1973 Jul 10;248(13):4544–4551. [PubMed] [Google Scholar]
- Kettner C., Shaw E. Inactivation of trypsin-like enzymes with peptides of arginine chloromethyl ketone. Methods Enzymol. 1981;80(Pt 100):826–842. doi: 10.1016/s0076-6879(81)80065-1. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P. Cathepsin L. A new proteinase from rat-liver lysosomes. Eur J Biochem. 1977 Apr 1;74(2):293–301. doi: 10.1111/j.1432-1033.1977.tb11393.x. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Gani V., Jiménez J. S., Shaltiel S. Affinity labeling of the catalytic subunit of cyclic AMP-dependent protein kinase by N alpha-tosyl-L-lysine chloromethyl ketone. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3073–3077. doi: 10.1073/pnas.76.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lernmark A., Nathans A., Steiner D. F. Preparation and characterization of plasma membrane-enriched fractions from rat pancreatic islets. J Cell Biol. 1976 Nov;71(2):606–623. doi: 10.1083/jcb.71.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y. P., Gainer H. Characterization of pro-opiocortin-converting activity in purified secretory granules from rat pituitary neurointermediate lobe. Proc Natl Acad Sci U S A. 1982 Jan;79(1):108–112. doi: 10.1073/pnas.79.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R. R., Hamilton J. W., Kent G. N., Shofstall R. E., Cohn D. V. The degradation of proparathormone and parathormone by parathyroid and liver cathepsin B. J Biol Chem. 1979 Jun 10;254(11):4428–4433. [PubMed] [Google Scholar]
- Nolan C., Margoliash E., Peterson J. D., Steiner D. F. The structure of bovine proinsulin. J Biol Chem. 1971 May 10;246(9):2780–2795. [PubMed] [Google Scholar]
- Patzelt C., Labrecque A. D., Duguid J. R., Carroll R. J., Keim P. S., Heinrikson R. L., Steiner D. F. Detection and kinetic behavior of preproinsulin in pancreatic islets. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1260–1264. doi: 10.1073/pnas.75.3.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. H., Welbourne W. P., Mako M., Melani F., Steiner D. F. Comparative immunology of bovine, porcine and human proinsulins and C-peptides. Diabetes. 1970 Aug;19(8):546–553. doi: 10.2337/diab.19.8.546. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Tager H. S., Rubenstein A. H., Steiner D. F. Methods for the assessment of peptide precursors. Studies insulin biosynthesis. Methods Enzymol. 1975;37:326–345. doi: 10.1016/s0076-6879(75)37030-4. [DOI] [PubMed] [Google Scholar]
- Trouet A. Isolation of modified liver lysosomes. Methods Enzymol. 1974;31:323–329. doi: 10.1016/0076-6879(74)31034-8. [DOI] [PubMed] [Google Scholar]
- Virji M. A., Vassalli J. D., Estensen R. D., Reich E. Plasminogen activator of islets of Langerhans: modulation by glucose and correlation with insulin production. Proc Natl Acad Sci U S A. 1980 Feb;77(2):875–879. doi: 10.1073/pnas.77.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoi O. O., Seldin D. C., Spragg J., Pinkus G. S., Austen K. F. Sequential cleavage of proinsulin by human pancreatic kallikrein and a human pancreatic kininase. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3612–3616. doi: 10.1073/pnas.76.8.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zühlke H., Kohnert K. D., Jahr H., Schmidt S., Kirschke H., Steiner D. F. Proteolytic and transhydrogenolytic activities in isolated pancreatic islets of rats. Acta Biol Med Ger. 1977;36(11-12):1695–1703. [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]