Abstract
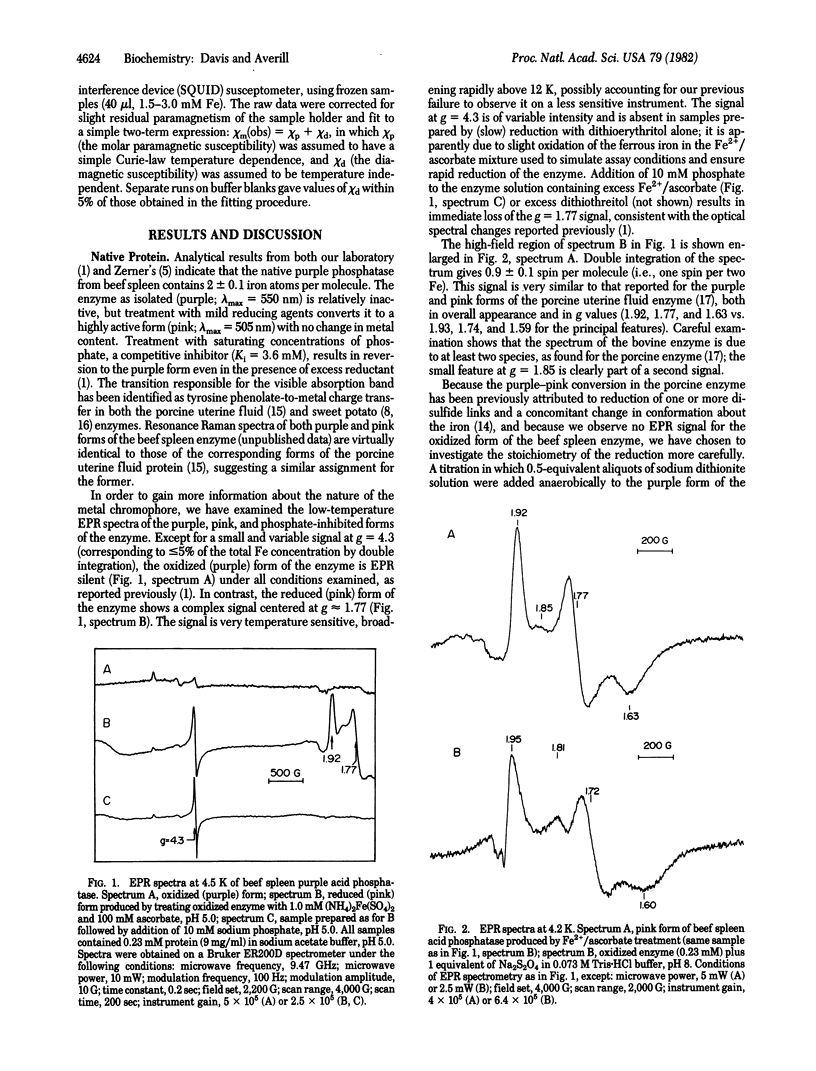
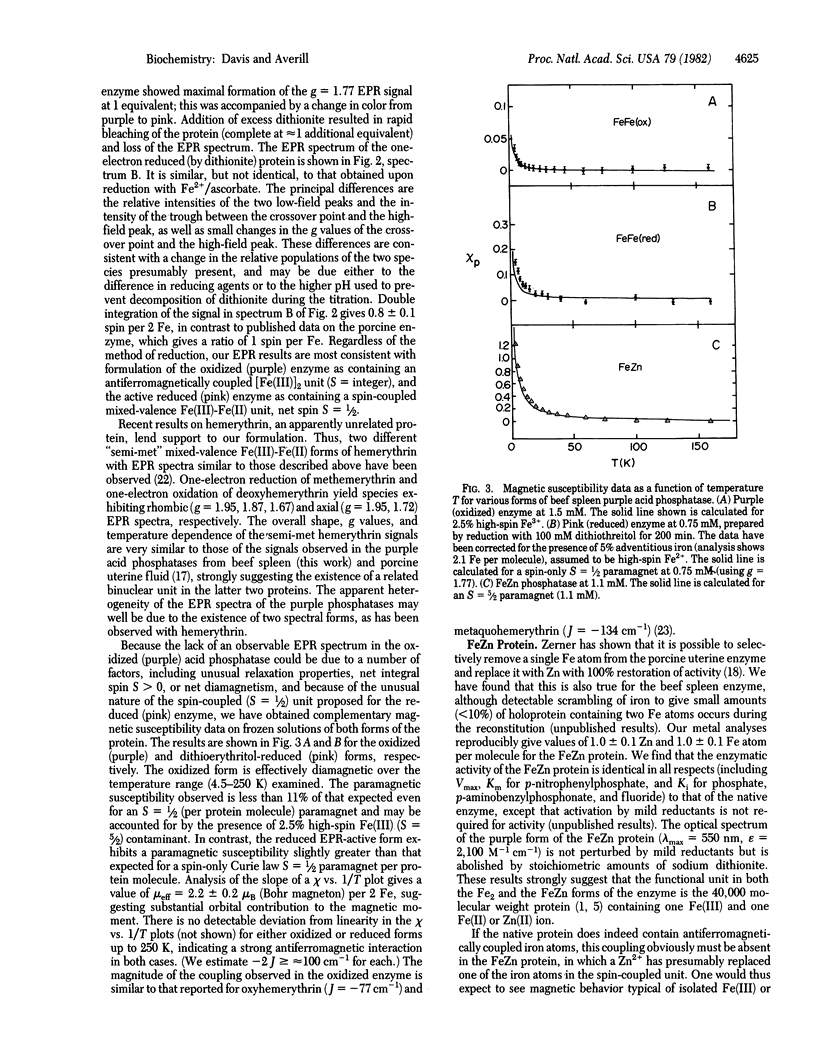
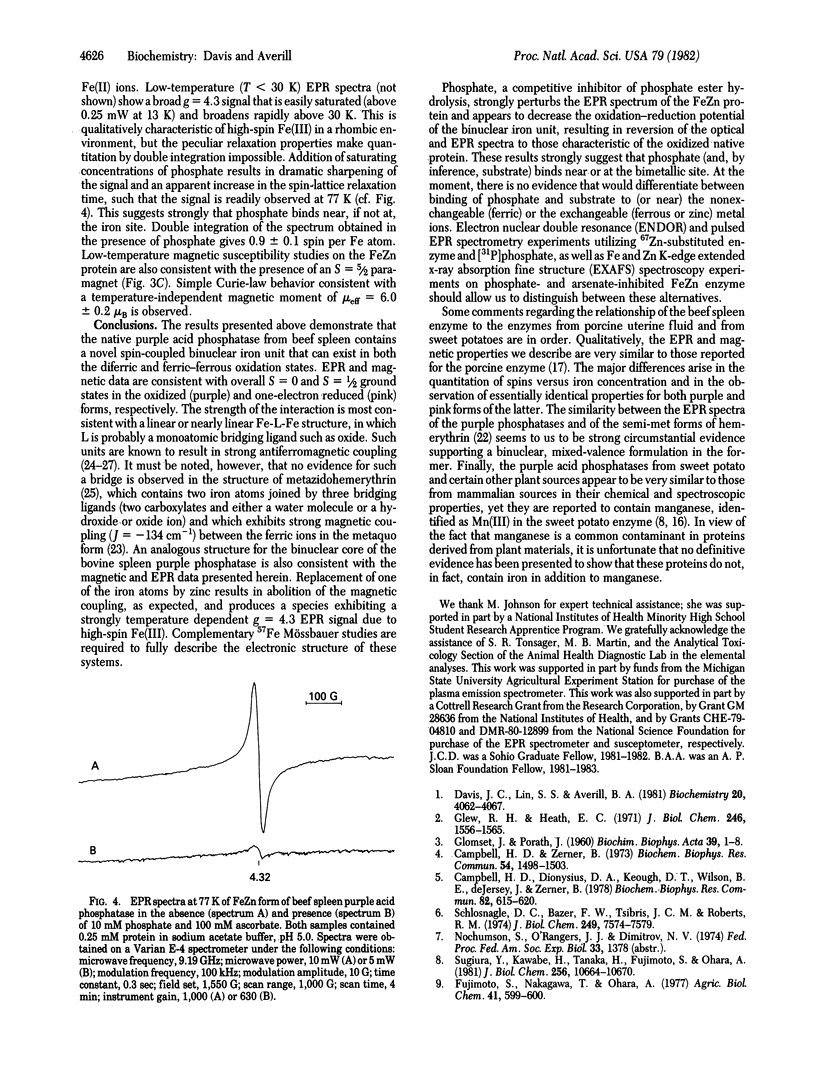
The purple acid phosphatase from beef spleen, which contains two iron atoms per molecule, is EPR silent in its native (oxidized) purple form. Treatment with mild reducing agents results in conversion to a pink, enzymatically active form, which exhibits an unusual EPR signal centered at g approximately equal to 1.77; double integration of the EPR spectrum gives one spin per two iron atoms. A similar EPR spectrum is observed for enzyme reduced anaerobically by one electron, using sodium dithionite. Variable-temperature magnetic susceptibility measurements show that the oxidized and reduced proteins are both antiferromagnetically coupled systems, with S = 0 and 1/2 ground states, respectively. Replacement of one of the iron atoms by zinc produces an FeZn enzyme with full catalytic activity. The FeZn enzyme exhibits a highly temperature dependent g = 4.3 EPR signal, and magnetic susceptibility data are consistent with an S = 5/2 paramagnet. Treatment of the FeZn enzyme with phosphate, a competitive inhibitor, results in sharpening of the EPR spectrum; double integration at 77 K gives one spin per iron. These results strongly suggest the presence of a spin-coupled bimetallic unit at the active site of the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antanaitis B. C., Aisen P., Lilienthal H. R., Roberts R. M., Bazer F. W. The novel "g' = 1.74" EPR spectrum of pink and purple uteroferrin. J Biol Chem. 1980 Dec 10;255(23):11204–11209. [PubMed] [Google Scholar]
- Campbell H. D., Dionysius D. A., Keough D. T., Wilson B. E., de Jersey J., Zerner B. Iron-containing acid phosphatases: comparison of the enzymes from beef spleen and pig allantoic fluid. Biochem Biophys Res Commun. 1978 May 30;82(2):615–620. doi: 10.1016/0006-291x(78)90919-1. [DOI] [PubMed] [Google Scholar]
- Campbell H. D., Zerner B. A low-molecular-weight acid phosphatase which contains iron. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1498–1503. doi: 10.1016/0006-291x(73)91155-8. [DOI] [PubMed] [Google Scholar]
- Davis J. C., Lin S. S., Averill B. A. Kinetics and optical spectroscopic studies on the purple acid phosphatase from beef spleen. Biochemistry. 1981 Jul 7;20(14):4062–4067. doi: 10.1021/bi00517a018. [DOI] [PubMed] [Google Scholar]
- Dawson J. W., Gray H. B., Hoenig H. E., Rossman G. R., Schredder J. M., Wang R. H. A magnetic susceptibility study of hemerythrin using an ultrasensitive magnetometer. Biochemistry. 1972 Feb 1;11(3):461–465. doi: 10.1021/bi00753a026. [DOI] [PubMed] [Google Scholar]
- Erbes D. L., Burris R. H. The kinetics of methyl viologen oxidation and reduction by the hydrogenase from Clostridium pasteurianum. Biochim Biophys Acta. 1978 Jul 7;525(1):45–54. doi: 10.1016/0005-2744(78)90198-5. [DOI] [PubMed] [Google Scholar]
- Gaber B. P., Sheridan J. P., Bazer F. W., Roberts R. M. Resonance Raman scattering from uteroferrin, the purple glycoprotein of the porcine uterus. J Biol Chem. 1979 Sep 10;254(17):8340–8342. [PubMed] [Google Scholar]
- Glew R. H., Heath E. C. Studies on the extracellular alkaline phosphatase of Micrococcus sodonensis. I. Isolation and characterization. J Biol Chem. 1971 Mar 25;246(6):1556–1565. [PubMed] [Google Scholar]
- Keough D. T., Dionysius D. A., de Jersey J., Zerner B. Iron-containing acid phosphatases: characterization of the metal-ion binding site of the enzyme from pig allantoic fluid. Biochem Biophys Res Commun. 1980 May 30;94(2):600–605. doi: 10.1016/0006-291x(80)91274-7. [DOI] [PubMed] [Google Scholar]
- Muhoberac B. B., Wharton D. C., Babcock L. M., Harrinton P. C., Wilkins R. G. EPR spectroscopy of semi-methemerythrin. Biochim Biophys Acta. 1980 Dec 16;626(2):337–345. doi: 10.1016/0005-2795(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Schlosnagle D. C., Bazer F. W., Tsibris J. C., Roberts R. M. An iron-containing phosphatase induced by progesterone in the uterine fluids of pigs. J Biol Chem. 1974 Dec 10;249(23):7574–7579. [PubMed] [Google Scholar]
- Schlosnagle D. C., Sander E. G., Bazer F. W., Roberts R. M. Requirement of an essential thiol group and ferric iron for the activity of the progesterone-induced porcine uterine purple phosphatase. J Biol Chem. 1976 Aug 10;251(15):4680–4685. [PubMed] [Google Scholar]
- Sugiura Y., Kawabe H., Tanaka H., Fujimoto S., Ohara A. Purification, enzymatic properties, and active site environment of a novel manganese(III)-containing acid phosphatase. J Biol Chem. 1981 Oct 25;256(20):10664–10670. [PubMed] [Google Scholar]


