Abstract
The relationship between the solubility, crystallinity, and length of the unit chains of plant storage α-glucan was investigated by manipulating the chain length of α-glucans accumulated in a rice mutant. Transgenic lines were produced by introducing a cDNA for starch synthase IIa (SSIIa) from an indica cultivar (SSIIa I, coding for active SSIIa) into an isoamylase1 (ISA1)-deficient mutant (isa1) that was derived from a japonica cultivar (bearing inactive SSIIa proteins). The water-soluble fraction accounted for >95% of the total α-glucan in the isa1 mutant, whereas it was only 35–70% in the transgenic SSIIa I /isa1 lines. Thus, the α-glucans from the SSIIa I /isa1 lines were fractionated into soluble and insoluble fractions prior to the following characterizations. X-ray diffraction analysis revealed a weak B-type crystallinity for the α-glucans of the insoluble fraction, while no crystallinity was confirmed for α-glucans in isa1. Concerning the degree of polymerization (DP) ≤30, the chain lengths of these α-glucans differed significantly in the order of SSIIa I /isa1 insoluble > SSIIa I /isa1 soluble > α-glucans in isa1. The amount of long chains with DP ≥33 was higher in the insoluble fraction α-glucans than in the other two α-glucans. No difference was observed in the chain length distributions of the β-amylase limit dextrins among these α-glucans. These results suggest that in the SSIIa I /isa1 transgenic lines, the unit chains of α-glucans were elongated by SSIIaI, whereas the expression of SSIIaI did not affect the branch positions. Thus, the observed insolubility and crystallinity of the insoluble fraction can be attributed to the elongated length of the outer chains due to SSIIaI.
Key words: Amylopectin, endosperm, isoamylase, phytoglycogen, starch synthase, transgenic rice
Introduction
Starch biosynthesis is catalysed by four known classes of enzymes: ADP-glucose pyrophosphorylases (AGPases), starch synthases (SSs), starch branching enzymes (BEs), and starch debranching enzymes (DBEs) (Smith et al., 1997; Myers et al., 2000; Nakamura, 2002; Ball and Morell, 2003). SS elongates the α-1,4 glucosidic chains of amylopectin and contains the greatest number of isozymes found in green plants. Each SS class plays a distinct role in starch biosynthesis and exhibits tissue and substrate specificities. Several SS genes exist in green plants. Among these, the functions of granule-bound starch synthase I (GBSSI), SSI, SSIIa, and SSIII(a) are relatively well known. GBSSI is involved in elongating amylose and extra long chains (ELCs) of amylopectin (Sano, 1984; Takeda et al., 1987; Hanashiro et al., 2008). SSI generates DP (degree of polymerization) 8–12 chains from DP 6–7 chains that emerge from the branch point in the A and B1 chain of amylopectin (Fujita et al., 2006, 2008). SSIII(a) functions to elongate the long B2–3 chains that connect multiple clusters of amylopectin (Inouchi et al., 1983; Fulton et al., 2002; Ral et al., 2006; Fujita et al., 2007; Borén et al., 2008).
SSII studies have been performed in maize (sugary2: Takeda and Preiss, 1993; Zhang et al., 2004), wheat (SGP-1 null: Yamamori et al., 2000), rice (japonica varieties: Umemoto et al., 2002; Nakamura et al., 2005a ); barley (sex6: Morell et al., 2003), sweet potato (cv. Quick sweet: Katayama et al., 2002; Kitahara et al., 2005), and Arabidopsis (Atss2: Zhang et al., 2008). These SSII-deficient mutants also accumulated a modified amylopectin, which was enriched with short chains with DP ≤12, instead of intermediate length chains with DP 13–24 (mostly B1 chains). The proportion of longer B chains (DP ≥24; B2 and B3) was unchanged in these plant species, except for rug5 in pea embryos (Craig et al., 1998). These findings suggest that SSII(a) performs a uniform function in these plant species. It elongates short chains with DP ≤12 to intermediate length chains within amylopectin clusters. This has a tremendous impact on the gelatinization temperature of starch (Craig et al., 1998; Edwards et al., 1999; Lloyd et al., 1999; Yamamori et al., 2000; Umemoto et al., 2002; Nakamura et al., 2002, 2005a; Zhang et al., 2004). Concerning rice, most indica rice varieties possess active SSIIa. In contrast, most japonica rice cultivars have markedly low or no SSIIa activity caused by three SNPs (single nucleotide polymorphisms) in the SSIIa gene (Nakamura et al., 2002, 2005a).
Recent analyses of ISA1-deficient mutants (sugary1 or isa1 mutants) indicate that the fourth class of enzymes related to starch biosynthesis, designated DBEs, also play an essential role in starch biosynthesis. These mutants accumulate highly branched soluble α-glucan (phytoglycogen) instead of amylopectin in maize (Pan and Nelson, 1984; James et al., 1995), rice (Nakamura et al., 1996, 1997), barley (Burton et al., 2002), and Chlamydomonas (Mouille et al., 1996). James et al. (1995) identified the maize Sugary1 (Su1) gene by transposon tagging of su1 mutants. This gene encodes an ISA-type DBE.
Some allelic isa1 mutant rice lines have been reported and they exhibit different phenotypes of accumulated glucans, either mild or severe phenotypes. The mild phenotype isa1 lines accumulate specific amylopectin that contains an abundance of short chains (sugary-amylopectin) and amylose within the outer region of the endosperm. In contrast, the phytoglycogen is located in the inner region of the endosperm. The severe phenotype isa1 lines accumulate primarily phytoglycogen instead of starch in the whole endosperms (Nakamura et al., 1997; Kubo et al., 1999). The severe isa1 mutants have not been identified in cereal crops other than rice, and an exceptional example has been reported for transient starch synthesis in Arabidopsis leaves (Zeeman et al., 1998). Wong et al. (2003) analysed the structure and physicochemical properties of the α-glucans that accumulate in the rice allelic isa1 lines that exhibit different severities of the sugary1 phenotype. Phytoglycogen was recovered from supernatants using low-speed centrifugation, indicating that phytoglycogen is a soluble α-glucan. Phytoglycogen consists of significantly more short chains of DP ≤10 and fewer chains of 11 ≤DP ≤24 when compared with wild-type amylopectin. In phytoglycogen, the quantity of long chains with DP ≥37 corresponding to B2–3 chains of amylopectin is significantly decreased when compared with amylopectin. Phytoglycogen is composed of multiple components with smaller molecular weights than amylopectin. A greater proportion of short chains compared with normal amylopectin in the isa1 allelic lines contributes to defective A-type crystallinity, and a lower gelatinization temperature and decreased enthalpy when analysed by X-ray diffractometry and differential scanning calorimetry (DSC), respectively (Wong et al., 2003).
Green plants have evolved the capacity to synthesize highly organized branched α-glucans as amylopectin with tandem cluster structure, whereas animals and bacteria continue to produce random branched glycogen. Throughout the long evolution of plants, a wide variety of α-glucan structures can be distinguished. These range from the primitive cyanobacterial glycogen to the highly organized amylopectin typical of green plants. Intermediate α-glucan structures, such as cyanobacteria-starch and semi-amylopectin, have been identified in unique cyanobacteria including Cyanobacterium sp MBIC10216 and in some species of Rhodophyta such as Porphyridium purpureum (Nakamura et al., 2005b ; Deschamps et al., 2008; Shimonaga et al., 2008). Amylopectin and glycogen are both composed of branched α-glucans that contain α-1,4 and α-1,6 glucosidic linkages. However, the solubility and crystallinity of these α-glucans are quite different. The solubility of α-glucans greatly affects the osmotic pressure of the cell and the α-glucan storage mechanism in each organism.
Starches are roughly divided into two types of crystallinity. Cereal endosperm starch displays the A-type X-ray diffraction pattern, and potato starch displays the B-type diffraction pattern. Starches containing amylopectin of relatively short average branch chain lengths (DP 23–29) display the A-type X-ray pattern, while other starches containing amylopectin of relatively longer branch chains (DP 30–44) displays the B-type X-ray pattern (Hizukuri, 1985). Studies concerning the relationship between crystallinity and starch structure have suggested that (i) a chain length of at least DP ≥10 is necessary for the formation of parallel glucan double helices and crystallinity (Gidley and Bulpin, 1987); (ii) a relatively large proportion of short chains with DP ≤9 decreases the crystallinity of starch (Fujita et al., 2003; Wong et al., 2003); and (iii) crystallinity disappears when starch is gelatinized. The specific structural characteristics, namely chain length, branch points, and molecular weight, that are necessary for the insolubility and crystallinity of α-glucans remain to be resolved. However, the tandem cluster structure of amylopectin is recognized as being very important.
To investigate the relationship between the solubility, crystallinity, and length of unit chains of plant storage α-glucans, this study generated transgenic rice (SSIIa I /isa1) exhibiting elongated outer chains of phytoglycogen. These plants were produced by introducing the active SSIIa gene of indica rice (SSIIa I) into the japonica rice sugary-1 mutant (isa1) which contains inactive SSIIa. Analyses of the structure and physicochemical properties of these α-glucans permitted the comparison of a line lacking both SSIIa and ISA1 with a line lacking only ISA1. The requirements for the insolubility and crystallinity of α-glucans are discussed.
Materials and methods
Plant materials
The rice cultivars Nipponbare and Taichung 65 (T65) (japonica cultivars) and IR36 (indica cultivars) were included as wild-type plants in this study. A severe type of the sugary-1 (sug-1) mutant (isoamylase1-deficient mutant, isa1) line, EM914 (Nakamura et al., 1997), was used as the host mutant. EM914 is a product of N-methyl-N-nitrosourea (MNU) mutagenesis of the rice cultivar T65 (Satoh and Omura, 1979). These rice lines were grown during the summer months in a paddy field and greenhouse at Akita Prefectural University.
Generation of transgenic rice lines
A DNA construct containing the SSIIa cDNA from indica cultivar IR36 (Nakamura et al., 2005a ) under the control of the rice Wx a promoter (Supplementary Fig. S1 available at JXB online; Utsumi et al., 2011) was introduced into EM914 (isa1) by Agrobacterium tumefaciens EHA105-mediated transformation (Hood et al., 1993). Procedures for rice tissue culture, transformation, and selection were as described previously (Kubo et al., 2005). A total of seven individual T0 progeny lines were isolated from the transformation. Three T1 seeds were randomly chosen from each T0 plant. These seeds were independently analysed for endosperm amylopectin chain length distribution using capillary electrophoresis as described below. Five randomly chosen T1 seeds of four T0 transformed lines were grown, and their seeds (T2) were examined for amylopectin chain length distribution. Western blotting was also conducted using SSIIa antiserum (Nakamura et al., 2005a ). Four homozygous lines (SSIIa I /isa1-#1, #2, #7, and #20) were selected and their seeds (T3) were used for further studies.
Transgenic rice lines were grown during the summer months in a greenhouse at Akita Prefectural University.
Native-PAGE/activity staining and immunoblotting
Native-PAGE/activity staining of DBE and BE was performed using the methods of Fujita et al. (1999) and Yamanouchi and Nakamura (1992), respectively. SS activity staining was performed on 7.5% (w/v) acrylamide slab gels containing 0.8% (w/v) oyster glycogen (G8751, Sigma) according to Nishi et al. (2001) with the modification that 0.5M citrate was included in the reaction mixture.
Preparation of soluble protein, loosely bound protein, and tightly bound protein from the mature endosperm was performed according to the methods of Fujita et al. (2006). Immunoblotting was performed according to the methods of Fujita et al. (1999) using antiserum raised against the peptide fragment APKPKATRSSPIPA of SSIIa in rice cultivars. This peptide sequence is common in indica (Kasalath and IR36) and japonica (Nipponbare and Kinmaze) cultivars (Nakamura et al., 2005a ).
Preparation of stereomicrographs of kernel cross-sections
Dehulled rice seeds, whose embryos were removed at the mature stage, were soaked in distilled water for 16h and cut across the short axis with a razor blade. The cross-sections were stained with 0.5% KI/0.05% I2 solution and observed under a stereo microscope (Olympus SZX7, Tokyo, Japan).
Analysis of the starch granules of endosperm
An estimation of the amount of soluble and insoluble α-glucans from rice endosperm was performed as described in Tanaka et al. (2004) and Fujita et al. (2003), respectively. Total α-glucans from a transgenic rice line (SSIIa I /isa1-#20) and parent mutant (EM914) were prepared by grinding dehulled, seeds, whose embryos had been removed, at the mature stage (~0.5g) with a mortar and a pestle. Soluble and insoluble fractions were prepared from the total α-glucan as follows. A suspension of total α-glucan in 5ml of distilled water was subjected to low-speed (600 g) centrifugation at 20 °C for 10min. The precipitate was washed twice with 5ml of distilled water and the resulting precipitate (i.e. the insoluble fraction) was designated SSIIa I /isa1-#20 insoluble fraction (#20 Insoluble). The first 600 g supernatant was combined with those from the following washes and these soluble fractions were designated SSIIa I /isa1-#20 soluble fraction (#20 Soluble). The insoluble and soluble fractions of #20 were dried under reduced pressure. Measurements of the thermal properties of endosperm starch by differential scanning calorimetry (DSC; DSC-6100, Seiko instrument) and X-ray diffraction were performed as described previously (Fujita et al., 2003, 2006).
Starch granules for scanning electron microscopy (SEM) observation were purified using Percoll (Amersham Biosciences) according to the method of Shimonaga et al. (2008). Two (for T65) to five (for EM914 and #20 transgenic rice lines) dehulled rice seeds, whose embryos had been removed, were ground with a mortar and pestle. The ground tissue was suspended in 0.5ml of distilled water, layered onto 1.5ml of Percoll, and centrifuged at 30 000 g for 20min at 4 °C. The starch pellet was washed with 100% ethanol and dried under pressure. Purified starch granules were coated with gold using a fine coater (JEOL JFC-1200) for 120 s. The morphology of the starch granules was examined by SEM (JEOL-500, Tokyo, Japan). SEM was performed in a secondary electron mode at 15kV. For endosperm observations, dried rice seeds were cut across the short axis with a razor blade. The surface was sputter-coated with gold and observed using SEM with the same conditions described above.
Analyses of α-glucan structure
Phytoglycogen and α-glucan specimens for chain length distribution analyses by high-performance size-exclusion chromatography (HPSEC) were prepared as follows: rice seeds (0.3–1.0g) were ground with a mortar and pestle and the powder was suspended in 3–4ml of distilled water. The suspension was centrifuged at 600 g and 20 °C for 10min. Three volumes of methanol were added to the supernatant, and the mixture was kept at 4 ºC overnight. The precipitate was collected by centrifugation at 3000 g and 4 °C for 10min. The precipitate was washed by suspension in 2ml of ice-cold methanol and centrifugation at 10 000 g and 4 °C for 10min. Sample drying was conducted in a centrifugal vacuum evaporator (designated #20 soluble fraction). For #20, the precipitate that was collected by centrifuging at 600 g was further purified by dissolving in 6ml of 100% dimethylsulphoxide at 37 °C overnight. The sample was then centrifuged twice at 600 g and ambient temperature for 10min. The supernatant was precipitated with 3 vols of methanol as described above and the dried sample was designated #20 insoluble fraction.
The chain length distributions of α-glucans from endosperm were analysed using the fluorescence capillary electrophoresis (FCEP) method of O’Shea and Morell (1996) and Fujita et al. (2001) in a P/ACE MDQ Carbohydrate System (Beckman Coulters, CA, USA). The distributions were also analysed by HPSEC of debranched α-glucans labelled with 2-aminopyridine as previously described (Fujita et al., 2009). The preparation and debranching of β-amylase limit dextrin (β-LD) of the α-glucans was conducted according to Hanashiro et al. (2011), and the chain length distribution was analysed with HPSEC in the same manner as was used for the native α-glucans.
Molecular size separation of starch from wild-type, soluble, and insoluble fractions from the transgenic rice line #20 and total α-glucans from EM914 by Sephacryl S-1000SF chromatography was performed according to the method of Kubo et al. (1999). After chromatography, an aliquot of each fraction was used to measure the carbohydrate content by an enzymatic method (Nakamura and Miyachi, 1982) and to measure the λmax value of the glucan–iodine complex (Fujita et al., 2007). Commercial pullulan standards with defined average molecular weights (Shodex, Showa denko) were used to calibrate the column.
Results
Generation of the SSIIa I /isa1 transgenic rice lines
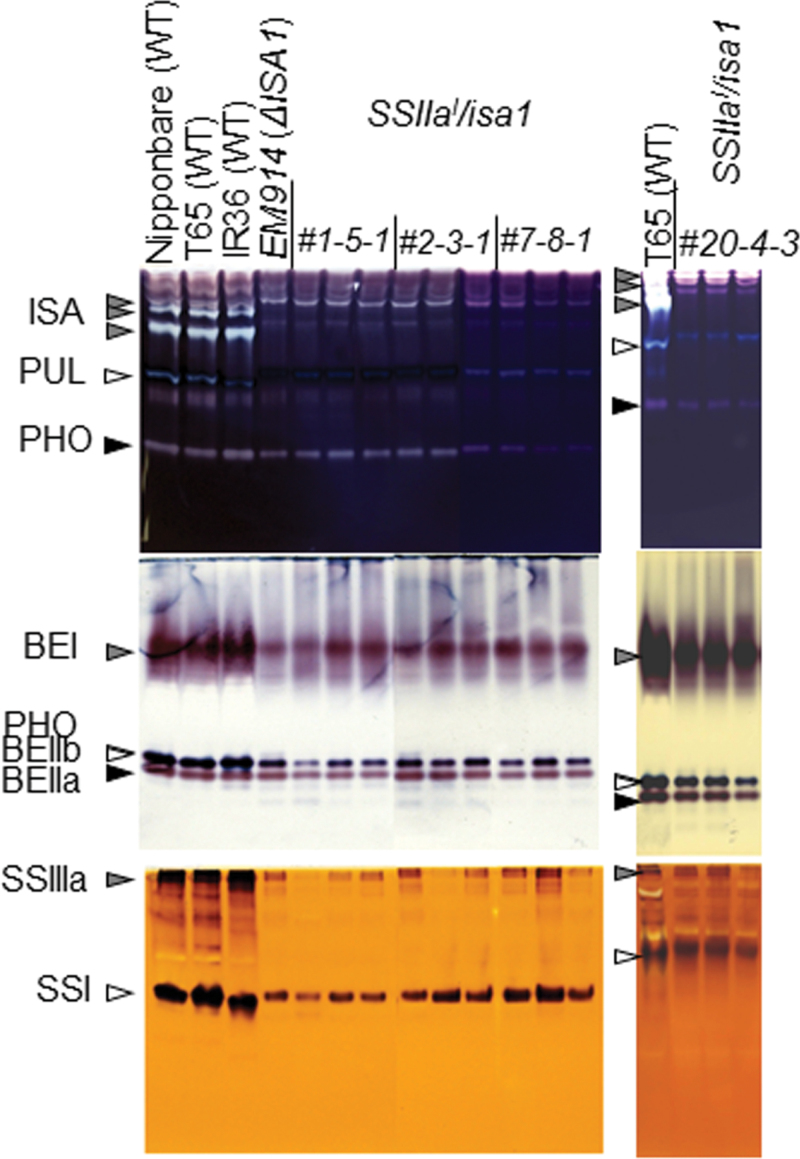
The SSIIa cDNA derived from the indica cultivar IR36 (SSIIa I; Nakamura et al., 2005a ) was introduced into the severe isa1 mutant, EM914 (Nakamura et al., 1997), whose endogenous SSIIa is inactive (Table 4), using the Agrobacterium method. The isozyme activity related to starch biosynthesis in the developing endosperm (~15 d after flowering) of the transgenic rice lines (SSIIa I /isa1) was analysed by native-PAGE/DBE, BE, and SS activity staining (Fig. 1). ISA1 activity was significantly reduced, and a pleiotropic effect of reduced pullulanase (PUL) activity, which is the other type of debranching enzyme, was observed in four SSIIa I /isa1 lines and the host EM914 (Fig. 1; Kubo et al., 1999). The activity of three BE isozymes (BEI, BEIIa, and BEIIb), Pho1, and two SS isozymes (SSI and SSIIIa) was also reduced in these lines compared with wild-type plants (Fig. 1). This result was consistent with the well-known phenotype of the isa1 background, which typically displays significantly reduced starch biosynthesis.
Table 4.
Summary of genotypes, activity levels of SSIIa, ISA, and BEIIb, and phenotypes in lines used in this study and transgenic rice lines containing soluble and insoluble α-glucans
| Lines | References | Genotypes | Activity levels | Dehulled grain weighta (mg) | Insoluble α-glucan (%) | ΔH by DSCb (%) | Crystallinity by X-rayb (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSIIa | ISA (hetero) | ISA (homo) | BEIIb | |||||||||||||||||
| T65 | This study | WT (japonica) | – | + | ++ | ++ | 19.4 | 97.4 | 100 | 100 (A type) | ||||||||||
| IR36 | This study | WT (indica) | + | + | ++ | ++ | 16.8 | 97.0 | 100 | 100 (A type) | ||||||||||
| EM914 | This study | isa1 (japonica) | – | – | – | + | 11.3 | 3.1 | 0 | 0 | ||||||||||
| #20 | This study | OE-SSIIa I / c isa1 | + | – | – | + | 9.3 | 65.6 | 10 | 20 (B type) | ||||||||||
| #G5-1 | Fujita et al. (2003) | DR-ISA1/WT | – | ± | ± | ++ | 21.0 | 83.8 | 50 | 30 (A type) | ||||||||||
| #1-1 | Tanaka et al. (2004) | OE-BEIIb/be2b | – | + | ++ | +++ | 7.7 | 89.2 | 20 | 10 (A type) | ||||||||||
| Wx a :ISA2 | Utsumi et al. (2011) | OE-ISA2/WT | – | +++ | – | + | 10.2 | 92.6 | 0 | 5 (?) | ||||||||||
a Mean of 20 seeds.
b Percentage of the wild type.
c Background
OE, overexpression; DR, down-regulation.
Fig. 1.
Native-PAGE/activity staining of debranching enzyme (DBE), branching enzyme (BE), and starch synthase (SS) in developing endosperm of transgenic and wild-type rice. The ISA (isoamylase), PUL (pullulanase), PHO (phosphorylase), BEI, BEIIa, BEIIb, SSIIIa, and SSI activity bands are indicated by arrowheads. Crude extracts were prepared by adding 3 vols of grinding solution per fresh weight of the developing endosperm. The volume of crude extract applied to the native gels in DBE, BE, and SS were 5, 2, and 8 µl, respectively.
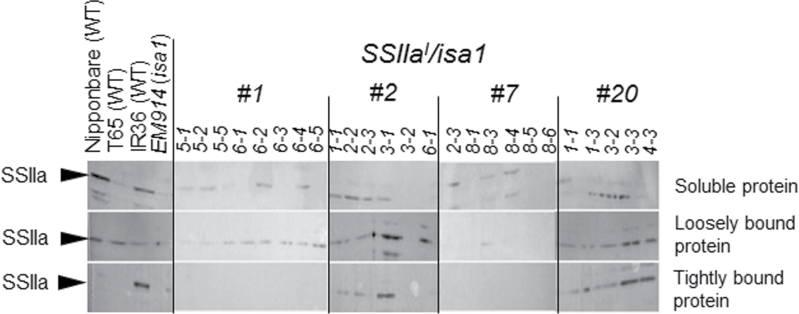
The SSIIa activity band was difficult to detect using native-PAGE/SS activity staining. Therefore, immunoblotting of three different fractions (soluble protein, loosely bound protein, and tightly bound protein) prepared from T3 dry seeds of the SSIIa I /isa1 lines was conducted using an antiserum raised against SSIIa. This immunoblot confirmed the expression of the introduced SSIIa I gene (Fig. 2). SSIIaI, derived from indica rice, was previously detected in the tightly bound protein fraction. Inactive SSIIa, derived from japonica rice, was detected in the soluble protein and/or loosely bound protein fractions (Fig. 2; Nakamura et al., 2005a ). Faint SSIIa bands in individual T3 seeds in #1 and #7 were detected exclusively in the soluble protein and loosely bound protein fractions. Strong SSIIa bands in #2 (1, 2, and 4) and #20 (2, 3, 4, 5, and 6) were detected in the tightly bound protein fraction, as well as in the soluble protein and loosely bound protein fractions (Fig. 2). These results suggest that the introduced SSIIa I gene was highly expressed in the #2 and #20 lines. T3 seeds in the #2 and #20 lines and in the #1 and #7 lines were used for further studies to represent transgenic rice lines expressing the gene at high and low levels, respectively.
Fig. 2.
Immunoblotting of three protein fractions, soluble protein, loosely bound protein, and tightly bound protein, prepared from developing endosperm in transgenic rice lines (SSIIa I /isa1) using antiserum raised against a peptide of OsSSIIa (Nakamura et al., 2005a). The numbers in the lines show the individual T3 seeds.
Characterization of the SSIIa I /isa1 transgenic rice lines
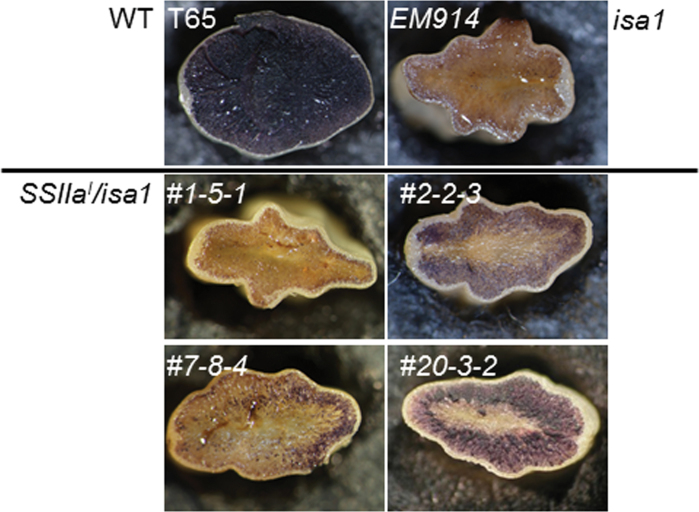
Cross-sections of the mature kernels of SSIIa I /isa1 and the parent lines exhibited blue to purple coloration with iodine solution (Fig. 3). Nearly all of the endosperm cells of isa1 (EM914) did not exhibit such coloration with iodine (Fig. 3; Nakamura et al., 1997). In contrast, endosperm cells of the wild type (T65) were completely stained. This result indicates that the isa1 line accumulates phytoglycogen that contains plenty of short chains in the whole endosperm cells rather than accumulating starch. In contrast, the outer layers of the SSIIa I /isa1 endosperm tissue exhibited blue to purple coloration, whereas the inner region of the kernel did not in the #2 and #20 lines, which had high expression levels of the SSIIa I gene (Fig. 2). On the other hand, very little of the endosperm exhibited purple coloration with iodine in #1 and #7, lines which had low SSIIa I expression levels.
Fig. 3.
Stereomicrographs of iodine-stained cross-sections of water-absorbed mature seeds of transgenic lines, SSIIa I /isa1 (#1, #2, #7, and #20), the host isa1 mutant (EM914), and the wild type (T65 and IR36).
The weight of the dehulled grain from the isa1 line was approximately half (EM914, 58.2%) of the wild-type weight (Table 1). The weights of the SSIIa I /isa1 lines were reduced in comparison with the parent mutant (37.6–54.6% of the wild type), and the values were not related to the level of SSIIa I gene expression.
Table 1.
Dehulled grain weight, and soluble and insoluble α-glucan content in dry seeds of transgenic rice and their parents
| Lines | Dehulled grain weighta (mg) | Soluble α-glucanb (mg) | Insoluble α-glucanb (mg) | % of insoluble fraction of total α-glucan | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taichung 65 | Wild type | 19.4±1.5 | (100)c | 0.38±0.15 | 14.2±0.73 | 97.4 | ||||||
| EM914 | isa1 | 11.3±0.2 | (58.2) | 3.80±0.89 | 0.12±0.06 | 3.1 | ||||||
| #1 | SSIIa I /isa1 | 8.1±1.0 | (41.8) | 2.87±0.38 | 1.04±0.12 | 26.6 | ||||||
| #2 (3)d | SSIIa I /isa1 | 10.6±0.2 | (54.6) | 3.49±0.43 | 3.63±0.53 | 51.0 | ||||||
| #2 (4) | SSIIa I /isa1 | 8.9±1.0 | (45.9) | 1.65±0.39 | 2.54±0.23 | 60.6 | ||||||
| #7 | SSIIa I /isa1 | 7.8±1.3 | (40.2) | 3.26±0.46 | 1.72±0.16 | 34.5 | ||||||
| #20 (4) | SSIIa I /isa1 | 7.3±0.4 | (37.6) | 1.58±0.53 | 2.05±0.33 | 56.5 | ||||||
| #20 (6) | SSIIa I /isa1 | 9.3±1.0 | (47.9) | 1.97±0.30 | 3.72±0.19 | 65.4 |
a Mean ±SE of 20 seeds.
b n=3.
c Percentage of the wild type.
d These numbers correspond to the sample number from Fig. 2, which originated from the same T3 seeds.
α-Glucan samples were separated into soluble and insoluble fractions by centrifugation at 600 g for 10min at 20 °C (see the Materials and methods). The amount of insoluble α-glucan in the wild type (T65) was 97.4% of the total amount of α-glucan contained in the endosperm. In contrast, the amount of insoluble α-glucan in isa1 (EM914) was only 3.1% (Table 1; Wong et al., 2003). The amount of insoluble α-glucan in the SSIIa I /isa1 lines increased in comparison with the parent mutant (26.6–65.4% of the wild type, Table 1). The #2 and #20 lines, which exhibited high SSIIa I gene expression, contained markedly high quantities of insoluble α-glucans (51.0–65.4%). Lines #1 and #7, which exhibited low SSIIa I gene expression, had relatively low quantities of insoluble α-glucans (26.6–34.5%).
The fine structure of α-glucans
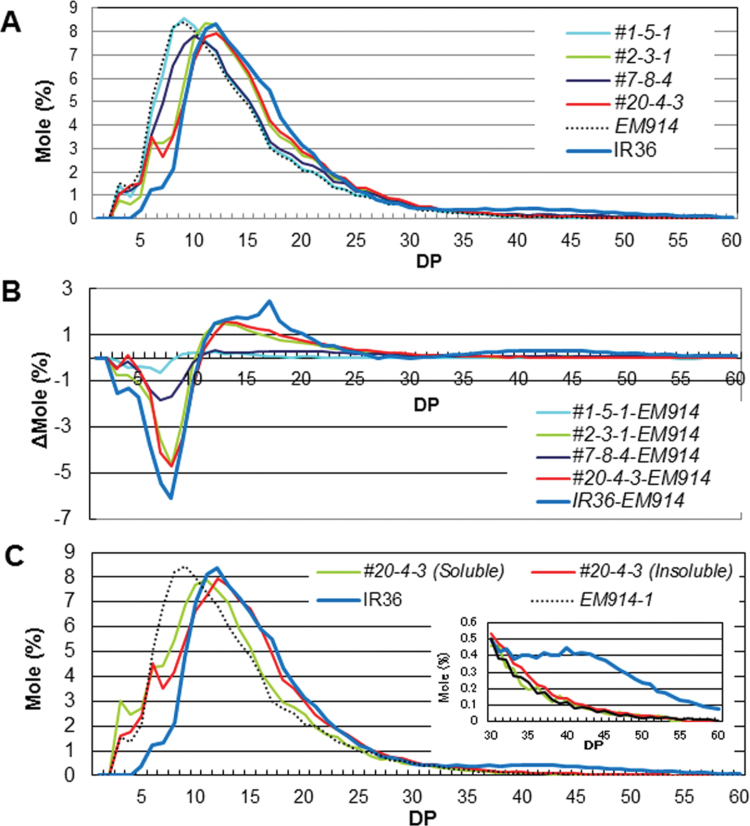
To evaluate the effect of SSIIa I gene expression on the fine structure of phytoglycogen, the chain length distribution of the isoamylolysates of the total α-glucans (see the Materials and methods) in the endosperm was analysed by the FCEP method using the SSIIa I /isa1 and parent mutant lines (Fig. 4). The shorter chains with DP 3–10 and the longer chains with DP ≥11 were significantly increased and decreased, respectively, in the isa1 line when compared with the wild types (Fig. 4A, B; Nakamura et al., 1997; Wong et al., 2003). The shorter chains with DP ≤10 and the intermediate chains with 11 ≤DP ≤30 were decreased and increased, respectively, in the four lines of SSIIa I /isa1 when compared with isa1 (Fig. 4B). Of the SSIIa I /isa1 lines, #2 and #20 exhibited high expression of SSIIa I and high quantities of insoluble α-glucans. For these lines, the extent of these changes in chain length distribution pattern was significantly large. It is worth noting that the long chains with DP ≥33 connecting the amylopectin clusters were significantly low in the four lines of SSIIa I /isa1 as well as in isa1 (Fig. 4A, B; Nakamura et al., 1997; Wong et al., 2003).
Fig. 4.
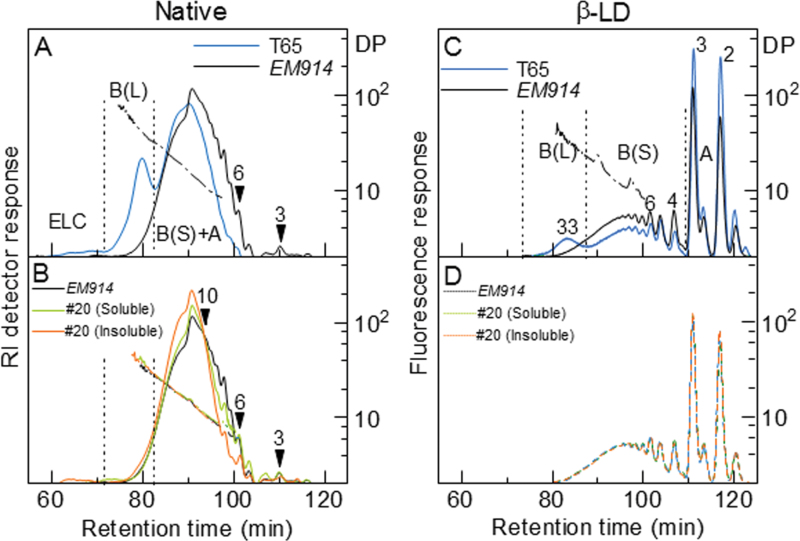
(A) Chain length distribution patterns of endosperm total (soluble and insoluble) α-glucans in the mature endosperm of transgenic rice lines, SSIIa I /isa1 (#1, #2, #7, and #20), EM914, and the wild type (IR36). (B) Differences in the chain length distribution patterns of amylopectin in the mature endosperm of transgenic rice lines, SSIIa I /isa1 (#1, #2, #7, and #20), the wild type (IR36), and the isa1 mutant, EM914. (C) Chain length distribution patterns of soluble (#20 Soluble) and insoluble (#20 Insoluble) α-glucans in the transgenic rice line #20, EM914, and the wild type (IR36). The inset in C indicates the magnification of the pattern in the range of chains with DP 30–60. The numbers on the plots represent the DP values.
The peaks of DP 3 and 11 of the chain length distribution pattern in the soluble fraction were observed in #20, whereas those of DP 6 and 12 were observed in the insoluble fraction in line #20, respectively (Fig. 4C). These results mean that the chain length distribution of insoluble α-glucan in line #20 shifted toward DP 1–3 longer chains when compared with the soluble fraction. This result indicates that the chains of insoluble α-glucans were more elongated by SSIIaI. Consequently, these chains tend to be insoluble in water. The amount of long chains with DP ≥33 in insoluble and soluble α-glucan in line #20 was much lower than that of the wild-type amylopectin, although a slightly higher amount was observed for insoluble α-glucan compared with soluble α-glucan in line #20 (Fig. 4C, inset).
The structure of the soluble and insoluble α-glucans in SSIIa I /isa1-#20 was further analysed. Chain length distributions of the α-glucans were examined by HPSEC after fluorescent labelling of the chains with 2-aminopyridine. This method provides data that are complementary to the FCEP method (Hanashiro et al., 2011). The HPSEC results are shown in Fig. 5 (left panels) and Table 2. In Fig. 5A and B, the elution profiles by refractive index (RI) detection are shown to allow easier recognition of the differences in the long chain fractions. Fluorescence detection (chromatograms not shown) produced results consistent with those obtained by the FCEP method (Fig. 4). The RI profiles were divided into three fractions as shown in Fig. 5A, namely ELC, long B chain [B(L)], and short B [B(S)] plus A chain, at inflection points commonly observed for normal amylopectins. In Fig. 5A, amylopectin from T65 and phytoglycogen from the congenic isa1 mutant line (EM914) are compared. Major differences caused by the loss of ISA1 were: loss of a peak for the B(L) fraction, which corresponds to B2 or B3 chains of amylopectin; and an increased amount of short chains eluted at retention times of ≥90min. These differences indicate that EM914 phytoglycogen has a higher degree of branching with a greater abundance of shorter unit chains. Additionally, considering the role of long B (B2 or B3 of amylopectin with normal cluster structure) chains in the arrangement of clusters in a tandem fashion in an amylopectin molecule, such an organized structure is absent in EM914 phytoglycogen as compared with normal amylopectin (Nakamura et al., 1997; Wong et al., 2003). Figure 5B shows that α-glucans from line #20 endosperm are composed of fewer short chains with DP 3–11 than those of EM914. A significant reduction was observed in different DP ranges, including DP 7–11 for the soluble fraction and DP 3–10 for the insoluble fractions in line #20. The extent of the reduction of these short chains was larger in the insoluble fractions than in the soluble fraction of line #20. In the DP range of ≥12, including the B(L) and ELC fractions, the amount of unit chains was larger in the insoluble fraction than in the soluble fraction of line #20 or in EM914 (Fig. 5A, Table 2)
Fig. 5.
Chain length distributions of α-glucans and their β-amylase limit dextrins (β-LDs) analysed by HPSEC after labelling with 2-aminopyridine. Solid and dashed line, RI detector (left panels) or fluorescence detector (right panels) response; dash-dot-dash line, DP; number with arrowhead, DP at the specified elution position. These DPs were determined either directly from the ratio of detector responses (RI/fluorescence) or by comparisons with authentic malto-oligosaccharides with known DPs (in the range of DP ≤6). Detector responses are normalized by weight (left panels) or moles (right panels). (A) Amylopectin from the wild-type japonica line T65 and phytoglycogen from the congenic isa1 mutant line EM914. (B) isa1 phytoglycogen and soluble and insoluble α-glucan of SSIIa I /isa1-#20. (C) β-LDs of T65 and EM914. (D) β-LDs of EM914 and soluble and insoluble α-glucan of SSIIa I /isa1-#20.
Table 2.
Chain length distributions of rice α-glucansa
| Sample | Fraction | |||||
|---|---|---|---|---|---|---|
| ELC | B(L) | B(S)+A | ||||
| Mole (%) | ||||||
| T65 | <0.1 | 9.0±0.1 | 90.9±0.2 | |||
| EM914 | –b | 1.7±0.2 | 98.3±0.2 | |||
| #20 (Soluble) | <0.1 | 1.7±0.1 | 98.2±0.1 | |||
| #20 (Insoluble) | <0.1 | 2.9±0.2 | 97.0±0.1 | |||
| Weight (%) | ||||||
| T65 | 2.0±0.1 | 22.8±0.4 | 75.2±0.4 | |||
| EM914 | 0.6±0.5 | 4.9±0.2 | 94.5±0.3 | |||
| #20 (Soluble) | 0.2±0.2 | 4.9±0.7 | 94.9±0.8 | |||
| #20 (Insoluble) | 1.1±0.7 | 7.1±0.7 | 91.8±0.1 |
a Values are means ±SD (n=3 or 5). Each fraction is designated as shown in Fig. 5A. The B(L) and B(S)+A fractions are equivalent to the B2+B3 and B1+A fraction, respectively, of the designation for normal amylopectin.
b Not detected.
Chain length distributions of the β-amylase limit dextrins (β-LDs) of the α-glucans
Chain length distributions of the β-amylase limit dextrins (β-LDs) of the α-glucans were analysed to examine the effect(s), if any, of the mutations and the introduction of the SSIIa I gene on the branched structure of these α-glucans [Fig. 5 (right panels) and Table 3]. Chromatograms on a molar basis (by fluorescence detection) are shown in Fig. 5C and D. With regard to the B chains, the chain length of β-LDs indicates the position of the outermost branch point of the B chain. Similar to the native α-glucans (Fig. 5A), the elution profiles were divided into three fractions [B(L), B(S), and A] according to the characteristic inflection points in the elution profiles (Fig. 5C). Consistent with the results shown in Fig. 5B, the β-LDs of the phytoglycogens of isa1 and the soluble and insoluble α-glucans of SSIIa I /isa1 did not exhibit a peak near the retention time of 83min. This peak is typically detected for normal amylopectin as a peak of long B (B2 and B3) chains. In agreement with the indispensable role of isoamylase in constructing the normal cluster structure (Nakamura, 2002; Zeeman et al., 2010), the absence of such long B chains in these isa1 lines implies that a characteristic feature of normal amylopectin, where multiple clusters are connected to each other, is absent in the phytoglycogens and the soluble and insoluble α-glucans from SSIIa I /isa1 transformant #20. Figure 5D shows that the β-LDs of α-glucans from EM914 and its transformant SSIIa I /isa1 (#20 Soluble and Insoluble) contain nearly identical chain length distributions despite the significantly different chain length distributions prior to the exhaustive trimming with β-amylase (Fig. 5B). The amount of each fraction is summarized in Table 3. No significant differences were observed in the amounts of any fractions between the soluble fraction in line #20 and EM914. However, slight differences were observed between the insoluble fraction in line #20 and the others regarding the amount of the A and B(S) fractions. The α-glucan of the insoluble fraction in line #20 contained slightly more A chains and concomitantly fewer short B chains.
Table 3.
Chain length distributions of β-LDs of rice α-glucansa
| Sample | Fluorescence peak area (%) | |||||
|---|---|---|---|---|---|---|
| B (L) | B (S) | A | ||||
| T65 | 9.4±0.4 | 33.9±0.4 | 56.7±0.5 | |||
| EM914 | 4.0±0.1 | 51.8±0.3 | 44.2±0.3 | |||
| #20 (Soluble) | 3.9±0.1 | 51.6±0.4 | 44.5±0.5 | |||
| #20 (Insoluble) | 4.1±0.1 | 50.2±0.2* | 45.7±0.2* | |||
a Values represent means ±SD (n=3 or 4). Each fraction is designated as shown in Fig. 5C. For T65 amylopectin, the B(L) and B(S) fractions are equivalent to the B2+B3 and B1 fraction, respectively, of the designation for normal amylopectins. An asterisk indicates that the values are significantly different between EM914 and #20 (insoluble and soluble) (by t-test with Bonferroni correction, P < 0.0167).
Molecular size separation of α-glucans of SSIIa I /isa1 transgenic rice
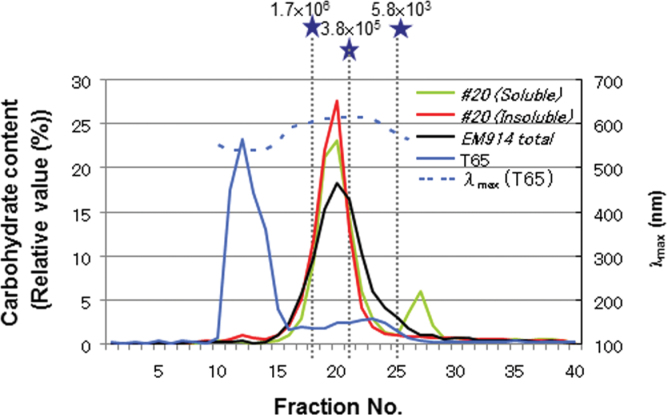
To determine the rough molecular weight of the α-glucans in the SSIIa I /isa1 line (#20), whole α-glucans (non-digested) were dissolved in 1 N NaOH and used for gel filtration column chromatography (Sephacryl-S1000). Based on the λmax values of the α-glucan–iodine complexes, the fractions containing the majority, if not all, of the amylopectin and amylose of the wild-type starch (T65) eluted in fractions 10–15 and 17–27, respectively (Fig. 6). The molecular weight of amylopectin was much greater than 1.7×106, as determined from the pullulan standard, and was estimated to be larger than 108 using the HPSEC-MALLS-RI method (Fujita et al., 2003; Wong et al., 2003). In contrast, the main peak of total α-glucans (almost phytoglycogen) in EM914 was detected in fraction 20, much smaller than that of the amylopectin (Fig. 6; Nakamura et al., 1997). The molecular weight of phytoglycogen was between 3.8×105 and 1.7×106 based on the pullulan standard. Although the amount of molecules eluted in fractions ≤18 was slightly higher in the insoluble fraction than in the soluble fraction in line #20, the vast majority of molecules were eluted in the same range of fractions, around fraction 20, in both cases. Therefore, in terms of average molecular weight and its distribution, the α-glucans of soluble and insoluble fractions in line #20 are not significantly different. The second peak that appeared at fraction 27 in regards to the soluble fraction was most probably a mixture of small oligo- or monosaccharides.
Fig. 6.
Size separation of α-glucans of SSIIa I /isa1-#20, the wild type (T65), and the host isa1 (EM914) by Sephacryl S-1000SF gel filtration chromatography. Solid lines, carbohydrate content by the enzymatic method (left axis); dashed lines, λmax of the T65 starch and iodine complex (right axis); grey dashed lines and stars, commercial pullulan standards with defined average molecular weights.
Crystallinity and granular structure of α-glucans of SSIIa I /isa1 transgenic rice
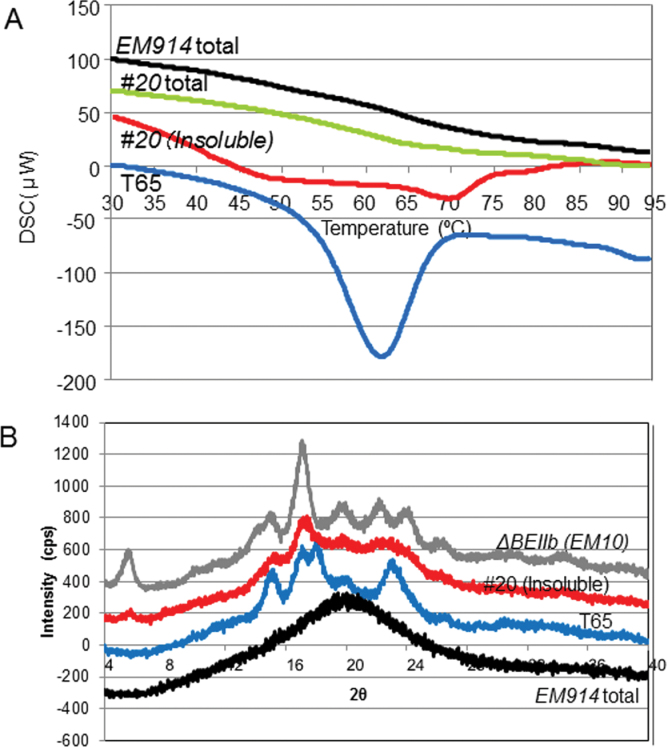
The X-ray diffraction method is commonly used to determine the degree of crystallinity. In this study, crystallinity was estimated by the DSC method by the smaller scale measurement as well as the X-ray diffraction method (Fig. 7). The total α-glucans of isa1 and #20 (EM914 total and line #20 total) did not show an obvious ΔH peak, although an apparent ΔH peak at 62 °C was observed in the starch of T65 (Fig. 7A; Wong et al., 2003). In contrast, the insoluble α-glucans in line #20 [#20 (Insoluble)] showed obvious deviation of the measured thermogram from a baseline at ca. 50 °C and 70 °C (Fig. 7A).
Fig. 7.
(A) Differential scanning calorimetric (DSC) curve of rice powder of SSIIa I /isa1-#20 (#20 total), isa1 (EM914 total), the wild type (T65), and the insoluble fraction of #20 (#20 Insoluble). (B) X-ray diffraction pattern of the insoluble fraction of #20 (#20 Insoluble), rice powder of EM914 (EM914 total), and purified starch from T65 and the be2b mutant (EM10). (This figure is available in colour at JXB online.)
Normal rice starches typically show A-type crystallinity. The crystallinity of phytoglycogen in severe sug-1 mutants, such as EM914, was absent (Fig. 7B). In contrast, X-ray diffraction analysis of the insoluble α-glucans in line #20 revealed a weak B-type diffraction pattern (Fig. 7B). These results suggest that although the crystalline amount may be small, the insoluble α-glucans in line #20 did exhibit crystallinity. However, the packing of the double helices is quite different between wild-type starch and the insoluble α-glucans in line #20.
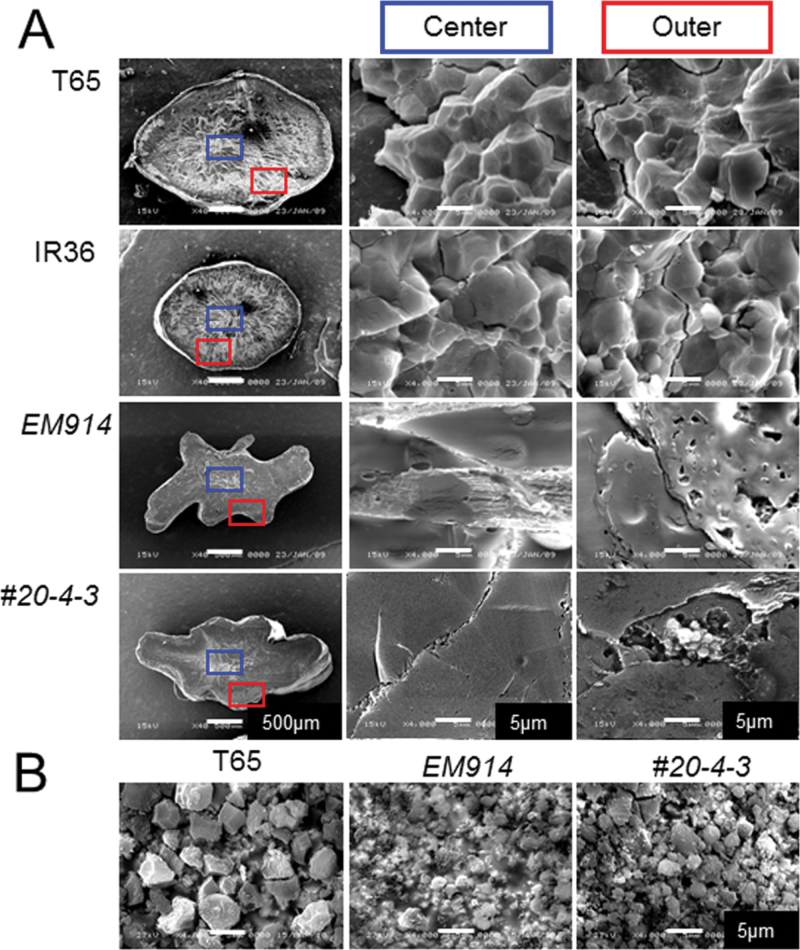
To analyse whether the chain elongation of phytoglycogen by SSIIaI affects the distinct granular structure of α-glucans, cross-sections of rice seeds were observed by SEM (Fig. 8A). The inner and outer portions of the isa1 (EM914) cross-section did not show a granular structure, although polygonal granules with sharp edges were observed in both portions of the wild type (T65 and IR36). On the other hand, some sections of the outer portion of SSIIa I /isa1 contained granular structures that were much smaller than those of the wild type, although no granule structure was detected in the inner portion. For more detailed SEM observations, Percoll-purified granules of the insoluble fraction in line #20, the wild type, and isa1 were examined (Fig. 8B). The granules of isa1 were much smaller than those of the wild type (Fig. 8B; Wong et al., 2003). Interestingly, the Percoll-purified granules from the insoluble fraction in line #20 appeared as a mixture of large granules of a size equivalent to that of T65 and small granules with a size similar to that of isa1.
Fig. 8.
Scanning electron microscopy (SEM) observations of the centre and outer regions in the cross-sections of maturing seeds (A) and starch granules (B) of transgenic rice (SSIIa I /isa1- #20), isa1 (EM914), and the wild type (T65 and IR36) purified using Percoll. (This figure is available in colour at JXB online.)
Discussion
The structure of elongated phytoglycogen by active SSIIa I
The characteristics of the phytoglycogen structure that accumulates in isa1 (sug-1) mutants indicate that it is devoid of B2 chains and enriched in short chains (Figs 4, 5; Nakamura et al., 1997; Wong et al., 2003). Compared with amylopectins, the molar ratio of long and short B chains was drastically altered in the phytoglycogens, reduced to less than half and increased by 1.5-fold, respectively (Table 3). These structural changes should result in reduced molecular weight (Fig. 6) and the disruption of the cluster structure typical of normal amylopectin. These results along with previous reports of ISA1-deficient mutant analyses in maize, rice, Chlamydomonas, and Arabidopsis (James et al., 1995; Mouille et al., 1996; Nakamura et al., 1997; Zeeman et al., 1998; Wong et al., 2003) strongly suggest that ISA1 function is important for the maintenance of amylopectin structure via removal of improper branch points (Nakamura, 2002; Zeeman et al., 2010). Moreover, phytoglycogen is soluble in water and does not exhibit purple coloration with iodine (Table 1, Fig. 3; Nakamura et al., 1997). In this study, structural alterations of phytoglycogen (isa1) were attempted by introducing SSIIaI (SSIIa I /isa1) for the production of elongated chains. Since the elongated phytoglycogens exhibit different chain lengths, the structural requirements for the solubility of the α-glucan molecules was investigated at the molecular level, and the crystalline structure was investigated at the granular level.
In the SSIIa I /isa1 lines, in which SSIIa I was highly expressed, more than half of the total α-glucans were insoluble (Table 1) and the outer layers of the endosperm tissue of these lines exhibit purple coloration with iodine (Fig. 3). The reason for the localized staining is not clear, but it might be related to the manner of development of the rice endosperm cell. Additionally, the chain length distribution pattern and gel filtration pattern of the #20 line of SSIIa I /isa1 was shifted to the right by FCEP analysis (Fig. 4A) and to the left by HPSEC analysis of debranched starch (Fig. 5A), respectively. Notably, the extent of the change in amylopectin chain length distribution among the four lines of SSIIa I /isa1 was positively correlated with the extent of SSIIa I expression (Figs 2, 4A, B). These results indicated that the chains are most probably elongated by the active SSIIaI. Meanwhile it cannot be excluded that indirect effects caused by the introduction of SSIIaI on other synthetic enzymes are responsible for the observed chain elongation in glucans from the SSIIaI-expressing lines. The same possibilities similarly apply to the crystallinity observed for the SSIIaI-expressing lines. In contrast to the changes in short chain fractions, the long B2+ chains with DP ≥33 that connect cluster structures were significantly reduced in the SSIIa I /isa1 lines when compared with normal starches as well as phytoglycogen by FCEP (Fig. 4A, B) and HPSEC analysis (Fig. 5A, B). Additionally, the structure of the β-LDs of SSIIa I /isa1 was similar to that of the β-LDs of phytoglycogen (Fig. 5C, D), indicating that the branch points and branch frequency of the α-glucans in SSIIa I /isa1-#20 were nearly identical to those of the parental phytoglycogen, although slight differences were observed between insoluble and soluble α-glucans in line #20 or EM914 in the amount of the A and B(S) fractions (Table 3). These results strongly suggest that SSIIaI elongates the outer chains of phytoglycogen, but does not affect the location of the branch points (Fig. 9). This also indicates that the chains elongated by SSIIaI are unlikely to be substrates for BEs.
Fig. 9.
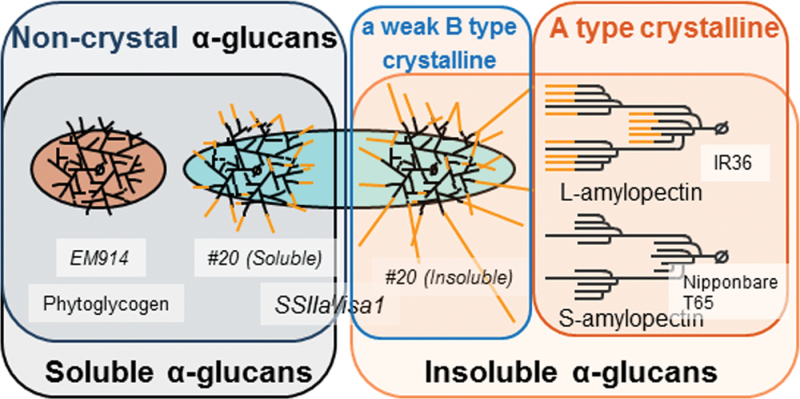
Schematic representation of the α-glucans in this study, phytoglycogen in EM914, soluble and insoluble α-glucan in the transgenic rice line (#20), and L- and S-amylopectin in indica and japonica rice, respectively. Insoluble α-glucan in line #20 is more elongated than the soluble α-glucan and phytoglycogen. Amylopectin exhibits A-type crystallinity, whereas phytoglycogen and α-glucan in SSIIa I /isa1 have no (phytoglycogen and soluble α-glucan) or a weak B-type (insoluble α-glucan) crystallinity.
Following the separation of water-suspended α-glucans of line #20 by 600 g centrifugation, further structural characterizations revealed that the outer chains of the insoluble α-glucans of line #20 (#20 Insoluble) were longer than those of the soluble α-glucans (#20 Soluble) (Figs 4C, 5B). The amount of longer chains with DP ≥33 and ELCs was increased in the insoluble fraction compared with the soluble fraction in line #20 and the phytoglycogen of the host plant (Figs 4C, 5B, Table 2). In contrast, the molecular weights of the whole α-glucans (not-debranched) were not significantly different between the insoluble and soluble fraction in line #20 and isa1 phytoglycogen. Moreover, the insoluble fraction in #20 exhibited weak B-type crystallinity, which is quite different from the A-type crystallinity of normal rice starches (Fig. 8B). Additionally, the insoluble fraction in #20 showed a low, but definite, endothermy by DSC measurement (Fig. 8A). Putaux et al. (2006) produced modified oyster glycogens whose external chains were extended by recombinant amylosucrase from Neisseria polysaccharea. The λmax value of the iodine complex of the products was 614nm, corresponding to the average chain length of DP 127. The elongated chains formed double-helical segments by intra- and interchain entanglement, resulting in strong B-type crystallinity. In this study, outer chains partially elongated by SSIIaI were able to extend longer than DP 30. This caused weak B-type crystallinity (Hizukuri, 1985) and a small ΔH by DSC.
Solubility and crystallinity of α-glucans
The approach of this study was to vary the suite of enzymes present in rice endosperm that are responsible for starch biosynthesis. This was conducted as a means to vary the structure of the α-glucans that are synthesized. The genotypes of the SSIIa I /isa1 transgenic lines used in this study (Table 4) were the same as those of a presumed sug-1 mutant of indica rice, although such a mutant has not been identified to date, or as a maize su1 mutant line with the exception of the GBSSI genotype (typical japonica rice cultivars are gbss1 leaky mutants). Most of the maize su1 mutant lines contain insoluble, starch-like α-glucans as well as phytoglycogen (Dinges et al., 2001). This may be related to the active SSIIa in maize. In contrast, the complete loss of both ISA1 and SSIIa (severe isa1 mutant lines such as EM914) activity impaired the production of insoluble α-glucans.
Quadruple mutants lacking all four DBE proteins (ISA1, 2, 3, and PUL) in Arabidopsis are devoid of starch granules and instead accumulate phytoglycogen (Streb et al., 2008). On the other hand, the additional loss of the chloroplastic α-amylase AMY3 partially reverts the phenotype of the quadruple DBE mutant, restoring starch granules. In maize, in contrast to the single mutant parents, double mutant endosperms affected in both SSIII and ISA2 were starch deficient and accumulated phytoglycogen as shown in ISA1-deficient mutant lines (Lin et al., 2012). These previous reports and the results in this study implied that although DBEs are important in starch biosynthesis, other enzymes such as SSIIa in rice, SSIII and SSIIa in maize, and α-amylase in Arabidopsis are also indispensable for the distribution of granular and soluble α-glucans.
Transgenic rice lines with increased soluble α-glucans in the endosperm (Table 4) were previously produced by several means: antisense inhibition of ISA1 (Fujita et al., 2003), overexpression of ISA2 (Utsumi et al., 2011), and overexpression of BEIIb (Tanaka et al., 2004).
In the endosperm of rice line #G5-1a, 16.2% soluble α-glucans accumulated. This was mediated by a 94% reduction in ISA1 activity using an antisense transgenic approach (Fujita et al., 2003). These soluble α-glucans exhibited a low molecular weight comparable with phytoglycogen and contained numerous short chains as compared with amylopectin. In contrast, the molecular weight of the insoluble α-glucans was similar to that of normal amylopectin. Even so, the short chains with DP ≤9 and long B2+ chains with around DP ≥40 connecting the amylopectin clusters in soluble α-glucans were significantly increased and decreased, respectively, as compared with amylopectin (Fujita et al., 2003).
In the endosperm of rice line #1-1 (Tanaka et al., 2004), 10.8% of the soluble α-glucans accumulated in response to overexpression of the OsBEIIb gene in rice be2b. The DP ≥40 chains and high molecular weight α-glucans in the soluble fraction of #1-1 were significantly reduced when compared with the wild type. However, a marked increase in short chains with DP ≤14 was observed (Tanaka et al., 2004).
Soluble α-glucans of Wx a :ISA2, a transgenic rice line overexpressing the OsISA2 gene under the control of the Wx a promoter, were 7- to 8-fold higher relative to the wild type. However, the OsISA2 repressed lines exhibited nearly the same level as wild-type plants. In contrast, the insoluble α-glucans of Wx a :ISA2 did not exhibit any ΔH peak by DSC analysis (Utsumi et al., 2011).
ISA1, the most critical enzyme for the construction of normal cluster structure (Nakamura, 2002; Zeeman et al., 2010), does exist in the transgenic rice lines #G5-1a (6% of the wild type) and #1-1. In contrast, ISA1 activity in the #20 line of this study was near zero, as derived from the parent isa1 (Fig. 2). In the case of Wx a :ISA2, functional ISA1 activity appears to be significantly decreased. Overexpression of ISA2 and the resulting excess amount of ISA2 protein caused ISA1-2, a non-functional hetero-oligomer, to become dominant over the functional ISA1 homo-oligomer (Utsumi et al., 2011). The DSC endothermic peak and the A-type crystallinity (Table 4) of the α-glucans were sustained in #G5-1a and #1-1, while they were not observed in SSIIa I /isa1 (#20 total) and Wx a :ISA2. These results indicate that functional ISA1 activity is indispensable for the crystallinity of α-glucans in rice, through removal of improper branch chains that otherwise interfere with the formation of double helices, even though the outer chains are elongated by SSIIaI.
The relationships among starch granule structure, water solubility, and molecular structure are still obscure. It is apparent that the normal starch molecular structure can build rigid starch granules, although the reduction of ISA1 activity leads to irregular and small granules (Fig. 8; Boyer et al., 1977; Zeeman et al., 1998; Burton et al., 2002; Fujita et al., 2003; Wong et al., 2003; Utsumi et al., 2011). In contrast, small particles are formed by phytoglycogen and elongated glycogen via recombinant amylosucrase (Fig. 8; Putaux et al., 2006). Moreover, the size of the starch granules of even normal starches depends upon the plant species. Further studies are necessary to characterize the regulation of the granule structure of α-glucans.
In summary, this study shed light on relationships among structures, water solubility, and crystallization of plant storage α-glucans (Fig. 9). Phytoglycogen produced in EM914, an isa1 mutant, was present in a highly branched, soluble form with no measurable crystallinity and did not form water-insoluble granules. Differences in the chain length of the outer chains in the line #20 insoluble glucan, which is affected either by the slightly increased amount of relatively long chains and/or by elongation of relatively short chains by ~3 residues, is critically important for whether or not the glucan chains crystallize, and consequently the crystallinity influences the solubility of the glucans. The line #20 insoluble glucan occurred in endosperm in a small granule form (particle or aggregate? Fig. 8B) but still not in a granular form like normal starch. The degree of branch frequency and branch point location are indispensable for normal crystallinity and granule formation as seen in wild-type starches. Considering solely the occurrence of crystallization, however, they are not necessarily indispensable as shown by the example of the insoluble SSIIa I /isa1 α-glucan with a weak B-type crystallinity in this study. This finding for the SSIIa I /isa1 glucan and elongated glycogen via recombinant amylosucrase (Putaux et al., 2006) further indicates that crystallization occurred even in the absence of long B chains, which generally are required to connect cluster units, implying that the arrangement of unit chains in a cluster fashion and/or tandemly connected clusters is not an essential requirement for crystallization itself. The same finding also suggests that crystallization and granule formation are not different reflections of the same phenomena. Crystallization at least seems to be a physical process independent of granule formation, while the former might be a necessary condition for the latter.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Construction of the transgenic rice line, SSIIa I /isa1.
Acknowledgements
This work was partly supported by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry and a Grant-in Aid for Scientific Research (B) (19380007). The authors are grateful to Professor Jay-Lin Jane (Iowa State University), Professor Yasuhito Takeda (Kagoshima University), Dr Kimiko Itoh (Niigata University), and Dr Sayuri Akuzawa (Tokyo University of Agriculture) for helpful discussions. The authors also express gratitude to Professor Hikaru Satoh (Kyushu University) for providing the sugary-1 mutant line (EM914), and to Ms Aiko Nishi (Kyushu University) and Mr Yoshinori Furukawa (Kagoshima University) for technical support.
Glossary
Abbreviations:
- β-LD
β-amylase limit dextrin
- DP
degree of polymerization
- DSC
differential scanning calorimeter
- ISA
isoamylase
- PAGE
polyacrylamide gel electrophoresis
- SDS
sodium dodecyl sulphate
- SEM
scanning electron microscopy.
References
- Ball SG, Morell MK. 2003. From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule Annual Review of Plant Biology 54 207–233 [DOI] [PubMed] [Google Scholar]
- Borén M, Glaring MA, Ghebremedhin H, Olsson H, Blennow A, Jansson C. 2008. Molecular and physicochemical characterization of the high-amylose barley mutant Amo1 Journal of Cereal Science 47 79–89 [Google Scholar]
- Boyer CD, Daniels RR, Shannon JC. 1977. Starch granules (amyloplast) development in endosperm of several Zea mays L. genotypes affecting kernel polysaccharides American Journal of Botany 64 50–56 [Google Scholar]
- Burton RA, Jenner H, Carrangis L, et al. 2002. Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity The Plant Journal 31 97–112 [DOI] [PubMed] [Google Scholar]
- Craig J, Lloyd JR, Tomlinson K, Barber L, Edwards A, Wang TL, Martin C, Hedley CL, Smith AM. 1998. Mutations in the gene encoding starch synthase II profoundly alter amylopectin structure in pea embryos The Plant Cell 10 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps P, Colleoni C, Nakamura Y, et al. 2008. Metabolic symbiosis and the birth of the plant kingdom Molecular Biology and Evolution 25 536–548 [DOI] [PubMed] [Google Scholar]
- Dinges JR, Colleoni C, Myers AM, James MG. 2001. Molecular structure of three mutations at the maize sugary1 locus and their allele-specific phenotypic effects Plant Physiology 125 1406–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A, Fulton DC, Hylton CM, Jobling SA, Gidley M, Rossner U, Martin C, Smith AM. 1999. A combined reduction in activity of starch synthases II and III of potato has novel effects on the starch of tubers The Plant Journal 17 251–261 [Google Scholar]
- Fujita N, Goto S, Yoshida M, Suzuki E, Nakamura Y. 2008. The function of rice starch synthase I expressed in E. coli Journal of Applied Glycoscience 55 167–172 [Google Scholar]
- Fujita N, Hasegawa H, Taira T. 2001. The isolation and characterization of a waxy mutant of diploid wheat (Triticum monococcum L.) Plant Science 160 595–602 [DOI] [PubMed] [Google Scholar]
- Fujita N, Kubo A, Francisco PB, Jr, Nakakita M, Harada K, Minaka N, Nakamura Y. 1999. Purification, characterization, and cDNA structure of isoamylase from developing endosperm of rice Planta 208 283–293 [DOI] [PubMed] [Google Scholar]
- Fujita N, Kubo A, Suh S–D, Wong K–S, Jane J–L, Ozawa K, Takaiwa F, Inaba Y, Nakamura Y. 2003. Antisense inhibition of isoamylase alters the structure of amylopectin and the physicochemical properties of starch in rice endosperm Plant and Cell Physiology 44 607–618 [DOI] [PubMed] [Google Scholar]
- Fujita N, Toyosawa Y, Utsumi Y, et al. 2009. Characterization of PUL-deficient mutants of rice (Oryza sativa L.) and the function of PUL on the starch biosynthesis in the rice endosperm. Journal of Experimental Botany 60 1009–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Yoshida M, Asakura N, Ohdan T, Miyao A, Hirochika H, Nakamura Y. 2006. Function and characterization of starch synthase I using mutants in rice Plant Physiology 140 1070–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Yoshida M, Kondo T, et al. 2007. Characterization of SSIIIa-deficient mutants of rice: the function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm Plant Physiology 144 2009–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton DC, Edwards A, Pilling E, et al. 2002. Role of granule-bound starch synthase in determination of amylopectin structure and starch granule morphology in potato Journal of Biological Chemistry 277 10834–10841 [DOI] [PubMed] [Google Scholar]
- Gidley MJ, Bulpin PV. 1987. Crystallisation of malto-oligosaccharides as models of the crystalline from of starch: minimum chain-length requirement for the formation of double helices Carbohydrate Research 161 291–300 [Google Scholar]
- Hanashiro I, Higuchi T, Aihara S, Nakamura Y, Fujita N. 2011. Structure of starches from rice mutants deficient in the starch synthase isozyme SSI or SSIIIa Biomacromolecules 12 1621–1628 [DOI] [PubMed] [Google Scholar]
- Hanashiro I, Itoh K, Kuratomi Y, Yamazaki M, Igarashi T, Matsugasako J, Takeda Y. 2008. Granule-bound starch synthase I is responsible for biosynthesis of extra-long unit chains of amylopectin in rice Plant and Cell Physiology 49 925–933 [DOI] [PubMed] [Google Scholar]
- Hizukuri S. 1985. Relationship between the distribution of the distribution of the chain length of amylopectin and crystalline structure of starch granules Carbohydrate Research 141 295–306 [Google Scholar]
- Hood EE, Gelvin SB, Melchers LS, Hoekema A. 1993. New Agrobacterium helper plasmids for gene transfer to plants Transgenic Resserch 2 208–218 [Google Scholar]
- Inouchi N, Glover DV, Takaya T, Fuwa H. 1983. Development changes in fine structure of starches of several endosperm mutants of maize Starch/Stärke 35 371–376 [Google Scholar]
- James MG, Robertson DS, Myers AM. 1995. Characterization of the maize gene sugary1, a determinant of starch composition in kernels The Plant Cell 7 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Komae K, Kohyama K, Kato T, Tamiya S, Komaki K. 2002. New sweet potato line having low gelatinization temperature and altered starch structure Starch/Stärke 54 51–57 [Google Scholar]
- Kitahara K, Fukunaga S, Katayama K, Takahata Y, Nakazawa Y, Yoshinaga M, Suganuma T. 2005. Physicochemical properties of sweetpotato starches with different gelatinization temperatures Starch/Stärke 57 473–479 [Google Scholar]
- Kubo A, Fujita N, Harada K, Matsuda T, Satoh H, Nakamura Y. 1999. The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm Plant Physiology 121 399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Rahman S, Utsumi Y, et al. 2005. Complementation of sugary-1 phenotype in rice endosperm with the wheat isoamylase1 gene supports a direct role for isoamylase1 in amylopectin biosynthesis Plant Physiology 137 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Huang B, Zhang M, Zhang X, Rivenbark J, Lappe RL, James MG, Myers AM, Hennen–Bierwagen TA. 2012. Functional interactions between starch synthase III and isoamylase-type starch-debranching enzyme in maize endosperm. Plant Physiology 158 679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR, Landschutze V, Kossmann J. 1999. Simultaneous antisense inhibition of two starch-synthase isoforms in potato tubers leads to accumulation of grossly modified amylopectin Biochemical Journal 338 515–521 [PMC free article] [PubMed] [Google Scholar]
- Morell MK, Kosar–Hashemi B, Cmiel M, Samuel MS, Chandler P, Rahman S, Buleon A, Batey IL, Li Z. 2003. Barley sex6 mutants lack starch synthase IIa activity and contain a starch with novel properties The Plant Journal 34 173–185 [DOI] [PubMed] [Google Scholar]
- Mouille G, Maddelein M–L, Libessart N, Talaga P, Decq A, Delrue B, Ball S. 1996. Pre-amylopectin processing: a mandatory step for starch biosynthesis in plants The Plant Cell 8 1353–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AM, Morell MK, James MG, Ball SG. 2000. Recent progress toward understanding biosynthesis of the amylopectin crystal Plant Physiology 122 989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y. 2002. Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue Plant and Cell Physiology 43 718–725 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Francisco PB, Jr, Hosaka Y, Sato A, Sawada T, Kubo A, Fujita N. 2005. a Essential amino acids of starch synthase IIa differentiate amylopectin structure and starch quality between japonica and indica rice varieties Plant Molecular Biology 58 213–227 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kubo A, Shimamune T, Matsuda T, Harada K, Satoh H. 1997. Correlation between activities of starch debranching enzyme and α-polyglucan structure in endosperms of sugary-1 mutants of rice The Plant Journal 12 143–153 [Google Scholar]
- Nakamura Y, Miyachi S. 1982. Effect of temperature on starch degradation in Chlorella vulgaris 11h cells Plant and Cell Physiology 31 303–309 [Google Scholar]
- Nakamura Y, Sakurai A, Inaba Y, Kimura K, Iwasawa N, Nagamine T. 2002. The fine structure of amylopectin in endosperm from Asian cultivated rice can be largely classified into two classes Starch 54 117–131 [Google Scholar]
- Nakamura Y, Takahashi J–I, Sakurai A, et al. 2005. b Some cyanobacteria synthesize semi-amylopecin type α-polyglucans instead of glycogen Plant and Cell Physiology 46 539–545 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Umemoto T, Ogata N, Kuboki Y, Yano M, Sasaki T. 1996. Starch debranching enzyme (R-enzyme or pullulanase) from developing rice endosperm: purification, cDNA and chromosomal localization of the gene Planta 199 209–218 [DOI] [PubMed] [Google Scholar]
- Nishi A, Nakamura Y, Tanaka N, Satoh H. 2001. Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm Plant Physiology 127 459–472 [PMC free article] [PubMed] [Google Scholar]
- O’Shea MG, Morell MK. 1996. High resolution slab gel electrophoresis of 8-amino-1,3, 6-pyrenetrisulfonic acid (APTS) tagged oligosaccharides using a DNA sequencer Electrophoresis 17 681–688 [DOI] [PubMed] [Google Scholar]
- Pan D, Nelson OE., Jr 1984. A debranching enzyme deficiency in endosperms of the sugary1 mutants of maize Plant Physiology 74 324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaux J–L, Potocki–Véronèse G, Remaud–Simeon M, Buleon A. 2006. α-d-Glucan-based dendritic nanoparticles prepared by in vitro enzymatic chain extension of glycogen Biomacromolecules 7 1720–1728 [DOI] [PubMed] [Google Scholar]
- Ral J–P, Colleoni C, Wattebled F, et al. 2006. Circadian clock regulation of starch metabolism establishes GBSSI as a major contributor to amylopectin synthesis in Chlamydomonas reinhardtii Plant Physiology 142 305–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y. 1984. Differential regulation of waxy gene expression in rice endosperm Theoretical and Applied Genetics 68 467–473 [DOI] [PubMed] [Google Scholar]
- Satoh H, Omura T. 1979. . Induction of mutation by treatment of fertilized egg cell with N-methyl-N-nitrosourea in rice Journal of the Faculty of Agriculture, Kyushu University 24 165–174 [Google Scholar]
- Shimonaga T, Konishi M, Oyama Y, et al. 2008. Variation in storage α-glucans of the Porphyridiales (Rhodophyta) Plant and Cell Physiology 49 103–116 [DOI] [PubMed] [Google Scholar]
- Smith AM, Denyer K, Martin C. 1997. The synthesis of the starch granule Annual Review of Plant Physiology and Molecular Biology 48 67–87 [DOI] [PubMed] [Google Scholar]
- Streb S, Delatte T, Umhang M, Eicke S, Schorderet M, Reinhardt D, Zeeman SC. 2008. Starch granule biosynthesis in Arabidopsis is abolished by removal of all debranching enzymes but restored by the subsequent removal of an endoamylase The Plant Cell 20 3448–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Hizukuri S, Juliano BO. 1987. Structures of rice amylopectins with low and high affinities for iodine Carbohydrate Reserch 168 79–88 [Google Scholar]
- Takeda Y, Preiss J. 1993. Structure of B90 (sugary) and W64A (normal) maize starches Carbohydrate Research 240 265–275 [Google Scholar]
- Tanaka N, Fujita N, Nishi A, Satoh H, Hosaka Y, Ugaki M, Kasawaki S, Nakamura Y. 2004. The structure of starch can be manipulated by changing the expression levels of starch branching enzyme IIb in rice endosperm Plant Biotechnology Journal 2 507–516 [DOI] [PubMed] [Google Scholar]
- Umemoto T, Yano M, Satoh H, Shomura A, Nakamura Y. 2002. Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties Theortical and Applied Genetics 104 1–8 [DOI] [PubMed] [Google Scholar]
- Utsumi Y, Utsumi C, Sawada T, Fujita N, Nakamura Y. 2011. Functional diversity of isoamylase (ISA) oligomers: the ISA1 homo-oligomer is essential for amylopectin biosynthesis in rice endosperm Plant Physiology 156 61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K–S, Kubo A, Jane J–L, Harada K, Satoh H, Nakamura Y. 2003. Structures and properties of amylopectin and phytoglycogen in the endosperm of sugary-1 mutants of rice Journal of Cereal Science 37 139–149 [Google Scholar]
- Yamamori M, Fujita S, Hayakawa K, Matsuki J, Yasui T. 2000. Genetic elimination of a starch granule protein, SGP-1, of wheat generates an altered starch with apparent high amylose Theoretical and Applied Genetics 101 21–29 [Google Scholar]
- Yamanouchi H, Nakamura Y. 1992. Organ specificity of isoforms of starch branching enzyme (Q-enzyme) in rice Plant and Cell Physiology 33 985–991 [Google Scholar]
- Zeeman SC, Kossmann J, Smith AM. 2010. Starch: its metabolism, evolution, and biotechnological modification in plants Annual Review of Plant Biology 61 209–234 [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Umemoto T, Lue W–L, Au–Yeung P, Martin C, Smith AM, Chen J. 1998. A mutant of Arabidopsis lacking a chloroplastic isoamylase accumulates both stach and phytoglycogen The Plant Cell 10 1699–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Colleoni C, Ratushna V, Sirghie–Colleoni M, James MG, Myers AM. 2004. Molecular characterization demonstrates that the Zea mays gene sugary2 codes for the starch synthase isoform SSIIa Plant Molecular Biology 54 865–879 [DOI] [PubMed] [Google Scholar]
- Zhang X, Szydlowski N, Delvallé D, D’Hulst C, James MG, Myers AM. 2008. Overlapping functions of the starch synthase SSII and SSIII in amylopectin biosynthesis in Arabidopsis BMC Plant Biology 8 96–113 [DOI] [PMC free article] [PubMed] [Google Scholar]