Abstract
To elucidate the phytohormonal basis of the feedback regulation of leaf senescence induced by potassium (K) deficiency in cotton (Gossypium hirsutum L.), two cultivars contrasting in sensitivity to K deficiency were self- and reciprocally grafted hypocotyl-to-hypocotyl, using standard grafting (one scion grafted onto one rootstock), Y grafting (two scions grafted onto one rootstock), and inverted Y grafting (one scion grafted onto two rootstocks) at the seedling stage. K deficiency (0.03mM for standard and Y grafting, and 0.01mM for inverted Y grafting) increased the root abscisic acid (ABA) concentration by 1.6- to 3.1-fold and xylem ABA delivery rates by 1.8- to 4.6-fold. The K deficiency also decreased the delivery rates of xylem cytokinins [CKs; including the zeatin riboside (ZR) and isopentenyl adenosine (iPA) type] by 29–65% and leaf CK concentration by 16–57%. The leaf ABA concentration and xylem ABA deliveries were consistently greater in CCRI41 (more sensitive to K deficiency) than in SCRC22 (less sensitive to K deficiency) scions under K deficiency, and ZR- and iPA-type levels were consistently lower in the former than in the latter, irrespective of rootstock cultivar or grafting type, indicating that cotton shoot influences the levels of ABA and CKs in leaves and xylem sap. Because the scions had little influence on phytohormone levels in the roots (rootstocks) of all three types of grafts and rootstock xylem sap (collected below the graft union) of Y and inverted Y grafts, it appears that the site for basipetal feedback signal(s) involved in the regulation of xylem phytohormones is the hypocotyl of cotton seedlings. Also, the target of this feedback signal(s) is more likely to be the changes in xylem phytohormones within tissues of the hypocotyl rather than the export of phytohormones from the roots.
Key words: Abscisic acid, cotton, cytokinins, feedback regulation, potassium deficiency
Introduction
The wide adoption of Bt cotton cultivars, noted for greater susceptibility to potassium (K) deficiency (Zhang et al., 2007; Yang et al., 2011), and inadequate input of K fertilizer has brought about an increasing occurrence of premature senescence in Chinese cotton-producing areas (Dong et al., 2006; Tian et al., 2008), characterized by early chlorophyll degradation and reduced photosynthesis in mature leaves (Bednarz and Oosterhuis, 1995; Zhao et al., 2001) during flowering and boll development. The widespread K deficiency caused by the use of higher yielding and faster fruiting cotton cultivars across the Cotton Belt of the USA was also reported about two decades ago (Oosterhuis et al., 1995).
It is generally considered that cytokinins (CKs) and abscisic acid (ABA) are two major phytohormones involved in the initiation and progression of plant senescence. The results of measurement of endogenous CK levels before and during senescence (van Staden et al., 1988; Singh et al., 1992; Gan and Amasino, 1995), external application of CKs (van Staden et al., 1988; Zavaleta-Mancera et al., 1999), and manipulation of endogenous production of CKs in transgenic plants (Gan and Amasino, 1995; Rivero et al., 2007; Ghanem, et al., 2011a ) showed that CKs can inhibit leaf senescence. The gain-of-function analyses also indicated that CK receptors AHK2 and AHK3 participate in regulating the onset of leaf senescence in Arabidopsis (Kim et al., 2006; Riefler et al., 2006). In contrast to CKs, the ABA level increases in senescing leaves (Gepstein and Thimann, 1980; Samet and Sinclair, 1980), and genes coding for proteins involved in ABA synthesis and signalling are up-regulated during leaf senescence (Buchanan-Wollaston et al., 2005; van der Graaff et al., 2006; Jukanti et al., 2008). Furthermore, it has been known that exogenous application of ABA promotes leaf abscission and senescence (Zeevaart and Creelman, 1988), and exogenously applied ABA induces the expression of several SAGs (senescence-associated genes; Weaver et al., 1998), which is consistent with its promotion of leaf senescence.
Nutrient deprivation (1% or 10% full-strength nutrient solution) not only decreased the levels of CKs in shoot and root (Kuiper et al., 1989; Vysotskaya et al., 2009), but also caused an accumulation of ABA in the shoot (Vysotskaya et al., 2009). It is well known that CK levels in roots, shoots, and xylem sap are reduced in nitrogen-starved plants (Takei et al., 2001; Dodd et al., 2004). With respect to K, although increased ABA levels have been measured in grains and flag leaves of wheat (Triticum aestivum) (Haeder and Beringer, 1981), roots, xylem, and phloem sap of castor bean (Ricinus communis L.) (Jeschke et al., 1997; Peuke et al., 2002), and leaves of Arabidopsis (Kim et al., 2009), the physiological consequences of this increase in ABA are less well known. One report (Salama and Wareing, 1979) showed that K deficiency also caused a marked decrease in CK levels in both roots and leaves.
A previous grafting study determined the roles of shoot and root in the regulation of premature leaf senescence induced by K deficiency in cotton (Li et al., 2012). The results showed that the effect of rootstocks on leaf senescence was significant in some cases, but the scion cultivars explained a higher percentage of variation within grafting treatments. It was speculated that the shoot-to-root feedback signal(s) that mediates xylem phytohormone delivery was involved in the shoot regulation of premature senescence of cotton (Li et al., 2012).
Several studies have shown that the root system dominates the root export of CKs via the xylem (Sitton et al., 1967; McKenzie et al., 1998; Dong et al., 2008; Albacete et al., 2009, 2010; Ghanem et al., 2011b ). However, results from studies on grafted pea branching mutants and Arabidopsis branching mutants suggested a feedback regulation of xylem sap CKs by some long-distance signals that move from shoot to root (Beveridge et al., 1997; Foo et al., 2007). Furthermore, the interaction of root and shoot in terms of xylem phytohormone delivery may exist when considering the recirculation of phytohormones between xylem and phloem (Dodd, 2005).
Although premature senescence is a typical symptom of K deficiency in cotton (Bednarz and Oosterhuis, 1995; Wright, 1999; Zhao et al., 2001), there has not been much attention paid to the role of endogenous hormones in the leaf senescence induced by K deficiency. Therefore, the objectives of this study were to (i) examine the effects of K deficiency on the endogenous ABA, zeatin riboside (ZR) + zeatin (Z), and isopentenyl adenosine (iPA) + isopentenyladenine (iP) levels in roots, leaves, and xylem sap of cotton seedlings; (ii) determine whether there is a linkage between leaf senescence induced by K deficiency and changes in endogenous CK and ABA levels in leaves; and (iii) test the hypothesis that the leaf and xylem CK and ABA levels are dependent on feedback signal(s) derived from the shoot by using a grafting study as in previous work (Li et al., 2012). The results should aid our understanding of mechanisms of phytohormone involvement in the feedback regulation of leaf senescence induced by K deficiency, and facilitate the development of approaches to managing this problem.
Materials and methods
Plant material
Two cotton cultivars contrasting in sensitivity to K deficiency were used in the present study: CCRI41, developed by the Cotton Research Institute, Chinese Academy of Agricultural Sciences, which is more likely to senesce under K deficiency, and SCRC22, developed by the Cotton Research Center, Shandong Academy of Agricultural Sciences, which shows relatively late senescence under the same condition.
Growth conditions
The experiment was performed in a growth chamber with 12h light/12h dark at 30±2/22±2 °C, 70–80% humidity, and 600 µmol m–2 s–1 photosynthetically active radiation. Seeds were surface-sterilized by soaking in 9% H2O2 for 30min, rinsed with tap water, and then germinated in K-free sand medium for 4 d in the dark. After germination, uniform seedlings were cultured hydroponically by transferring them to 16 cm×13 cm×16cm plastic pots filled with 2.2 litres of half-strength modified Hoagland’s solution. The constituents of the solution were (mM) 2.5 Ca (NO3)2, 1 MgSO4, 0.5 (NH4)H2PO4, 2×10–4 CuSO4, 1×10–3 ZnSO4, 0.1 Fe Na EDTA, 2×10–2 H3BO3, 5×10–6 (NH4)6Mo7O24, and 1×10–3 MnSO4. The concentration of K in the form of potassium sulphate (K2SO4) in the solution was 0.1mM (mild K deficiency) before grafting and during graft recovery to ensure either a higher survival rate of grafts or faster occurrence of leaf senescence induced by severe K deficiency (0.03mM or 0.01mM) after graft establishment.
Grafting
Standard, Y, and inverted Y grafting of cotton seedlings were performed hypocotyl-to-hypocotyl at the cotyledonary or one-leaf stage as described previously (Li et al., 2012). For each type of grafting, two cultivars were self- and reciprocally grafted; standard grafts are denoted as scion/rootstock, and ‘Y’ and ‘inverted Y’ grafts are denoted as (scion+scion)/rootstock and scion/(rootstock+rootstock), respectively. After establishment under high humidity and low light (80 µmol m–2 s–1), surviving grafts were transferred to severe K-deficient (0.03mM for standard and Y grafts, and 0.01mM for inverted Y grafts because of its two rootstocks providing nutrients for only one scion) solutions to induce premature senescence, with a 2.5mM (K-sufficient) medium as control. One week after establishment, the cotyledons and axillary bud from cotyledonary nodes of rootstock (standard and Y grafts) were removed.
Sampling of xylem sap
At the 8–9 leaf stage (~32 d after severe K deficiency treatment as above), xylem sap was collected basically according to Noodèn et al. (1990). Ten grafts per treatment were decapitated 5–10mm above the graft union to collect scion xylem sap, and another 10 grafts per treatment were cut below the graft union (~5mm away) to collect rootstock xylem sap. The cut surface was wiped with distilled water to remove disrupted cells and residual cell elements, then a flexible silicon tube (length 15mm, internal diameter 2mm) was placed ~5–10mm over the stump and tied tightly in place. The collection period started at 10:00h and lasted for 24h in the dark each time. After collection, the sap volume was quickly determined to calculate the sap flow rate over the collection period, and then the sap was freeze-dried (SIM FD5-6, LA, USA) and stored in the dark at –40 °C. The xylem ABA and CK delivery rates were calculated by multiplying phytohormone concentrations by the xylem sap flow rates.
Extraction and purification of phytohormone in leaves and roots
The fourth main-stem leaf from the apex and the whole roots were harvested before and after xylem sap collection to determine CKs and ABA. About 0.5g of fresh samples was extracted and homogenized in 2ml of 80% methanol (containing 40mg l–l butylated hydroxytoluene as an antioxidant). The extract was incubated at 4 °C for 48h, and then centrifuged at 4000rpm for 15min at 4 °C. The supernatant was passed through C18 Sep-Pak cartridges (Waters Corp., Millford, MA, USA), and the phytohormone fraction was eluted with 10ml of 100% (v/v) methanol and then 10ml of ether. The eluate was dried down by pure N2 at 20 °C, and then stored at –40 °C.
Quantification of ABA and CKs by enzyme-linked immunosorbent assay (ELISA)
Freeze-dried xylem sap and N2-dried extracts of leaf and root samples were dissolved in 2.0ml of phosphate-buffered saline (PBS) containing 0.1% (v/v) Tween-20 and 0.1% (w/v) gelatin (pH 7.5) to quantify free ABA, ZR, and iPA by ELISA following the protocol described in Zhao et al. (2006). The mouse monoclonal antigen and antibodies against free ABA, ZR, and iPA were produced at the Center of Crop Chemical Control, China Agricultural University, China, according to Weiler et al. (1981). As the anti-ZR antibody also detects Z, the CKs quantified by this antibody are described as ZR-type CKs. Similarly, the anti-iPA antibody detects iP and iPR, hence the CKs quantified by this antibody are described as iPA-type CKs. Calculations of the ELISA data were performed as described in Weiler et al. (1981). The recovery percentage obtained by using internal standards during extraction and analysis was all >90%.
Leaf photosynthesis measurement
In a separate experiment with four replicates and four plants for each replicate (Li et al., 2012), the photosynthesis rate (Pn) of the fourth main-stem leaf from the apex (functional leaf) was measured by a Li-6400 portable photosynthesis system (LI-COR, Lincoln, NE, USA) with 1000 µmol m–2 s–1 quantum flux and 500 µmol mol–1 CO2 concentration.
Data analysis
Three replicates were used for each grafting treatment, and each replicate consisted of 10 plants for either rootstock or scion xylem sap samples and 20 plants for leaf and root samples. A similar trend of results was found in three independent repeat experiments; thus data were pooled across repeats. Analysis of variance (ANOVA) was performed using SAS statistical software (V8, SAS Institute Inc., Cary, NC, USA) to determine the significance of the effects of K supply, rootstock, and scion, and their interaction under K deficiency. Treatment means were compared using Duncan’s multiple range test.
Results
Spatial pattern of ABA and CKs in cotton seedlings
As shown in Figs 1, 3, and 5, the ABA concentrations in cotton leaves were much higher than those in roots. The iPA-type concentrations in roots and leaves were higher than those of the ZR-type, whereas the xylem iPA-type delivery rate was much lower than that of the ZR-type (Figs 2, 4, 6), indicating that the ZR-type is the dominant type of CKs in cotton xylem sap, as in other plants (Singh et al., 1992; Beveridge et al., 1997; Foo et al., 2007).
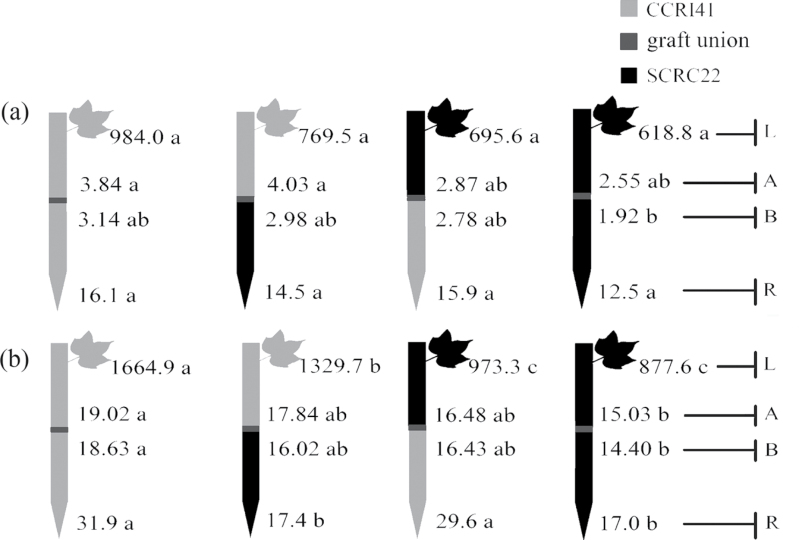
Fig. 1.
Effect of K deficiency on ABA levels of cotton standard graft (scion/rootstock) at the 8–9 leaf stage. Grafting was performed hypocotyl-to-hypocotyl at the 1-leaf stage of the rootstock and the cotyledonary stage of the scion. Grafts were maintained in nutrient solution with 0.1mM K during establishment, and transferred to solutions with either 2.5mM (a) or 0.03mM K (b) after establishment. The ABA concentrations (ng g–1 FW) in roots (R) and the youngest fully expanded leaf (L), and xylem ABA delivery rates (ng plant–1 24h–1) below (B) and above the graft union (A) were determined. For each K level, means of the same sampling part (i.e. roots, leaf, and xylem sap collected both below and above the graft union) followed by the same letter are not significantly different according to Duncan’s multiple range test, P < 0.05, n=4.
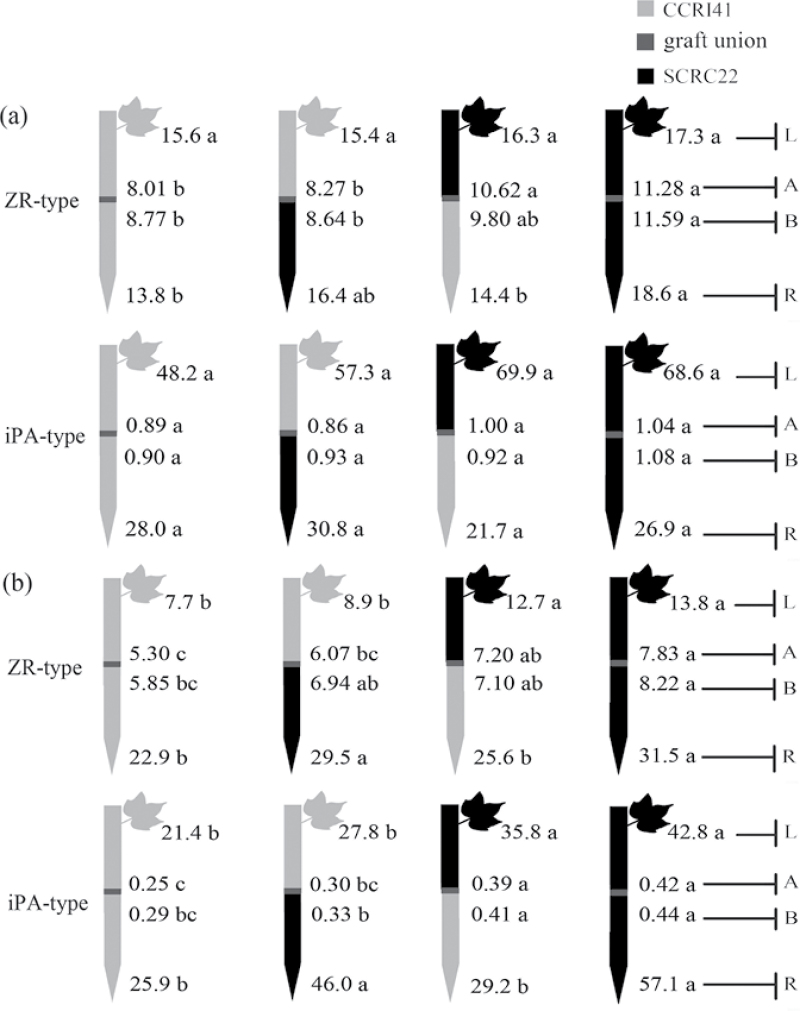
Fig. 2.
Effect of K deficiency on cytokinin (including the ZR-and iPA-type) levels of cotton standard graft (scion/rootstock) at the 8–9 leaf stage. Grafting was performed hypocotyl-to-hypocotyl at the 1-leaf stage of the rootstock and the cotyledonary stage of the scion. Grafts were maintained in nutrient solution with 0.1mM K during establishment, and transferred to solutions with either 2.5mM (a) or 0.03mM K (b) after establishment. The ZR- and iPA-type concentrations (ng g–1 FW) in roots (R) and the youngest fully expanded leaf (L), and xylem ZR- and iPA-type delivery rates (ng plant–1 24h–1) below (B) and above the graft union (A) were determined. For each K level, means of the same sampling part (i.e. roots, leaf, and xylem sap collected both below and above the graft union) followed by the same letter are not significantly different according to Duncan’s multiple range test, P < 0.05, n=4.
There were no significant differences in the xylem ABA and CK delivery rates (the product of phytohormone concentration in the xylem sap and sap flow rate) between rootstock (collected below the graft union) and scion (collected above the graft union) within a standard graft (Figs 1, 2), implying that the graft union itself had little influence on phytohormone delivery as previously demonstrated (Holbrook et al., 2002; Dodd et al., 2008). In addition, the sum of the xylem phytohormone levels of the two scions was more than that of the rootstock within a Y graft (Figs 3, 4), and the xylem phytohormone level in the scion was less than that of the sum of the two rootstocks within an inverted Y graft (Figs 5, 6), suggesting that the scion xylem phytohormone deliveries were independent of the xylem of the rootstocks.
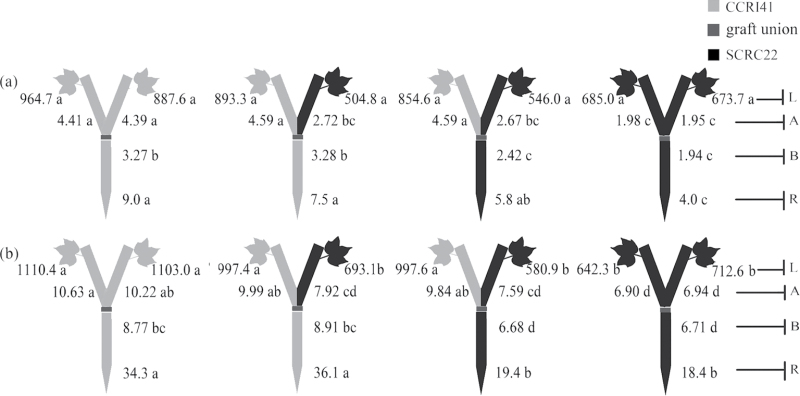
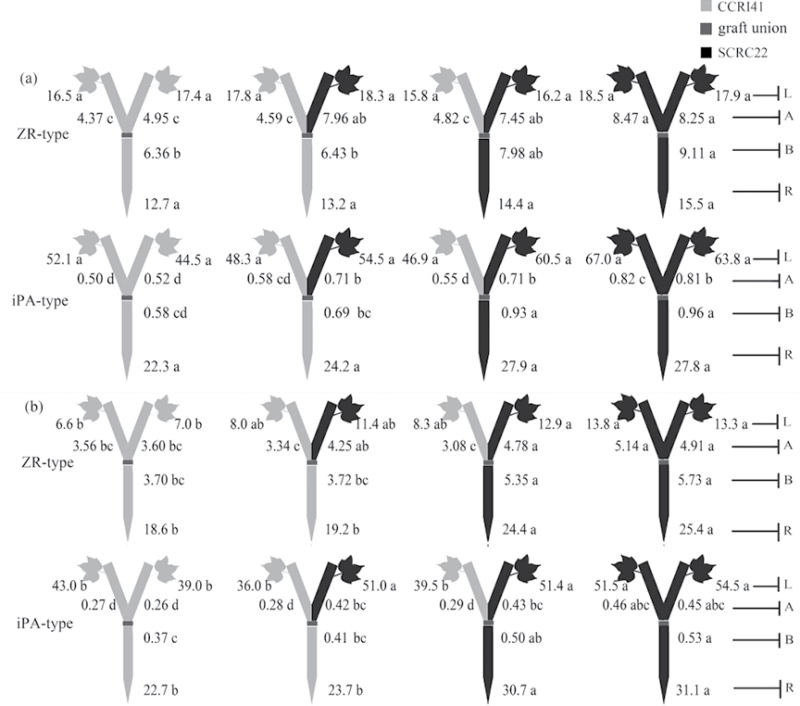
Fig. 3.
Effect of K deficiency on ABA levels of cotton Y graft (scion+scion/rootstock) at the 8–9 leaf stage. Grafting was performed hypocotyl-to-hypocotyl at the 1-leaf stage of the rootstock and the cotyledonary stage of the scion. Grafts were maintained in nutrient solution with 0.1mM K during establishment, and transferred to solutions with either 2.5mM (a) or 0.03mM K (b) after establishment. The ABA concentrations (ng g–1 FW) in roots (R) and the youngest fully expanded leaf (L), and xylem ABA delivery rates (ng plant–1 24h–1) below (B) and above the graft union (A) were determined. For each K level, means of the same sampling part (i.e. roots, leaf, and xylem sap collected both below and above the graft union) followed by the same letter are not significantly different according to Duncan’s multiple range test, P < 0.05, n=4.
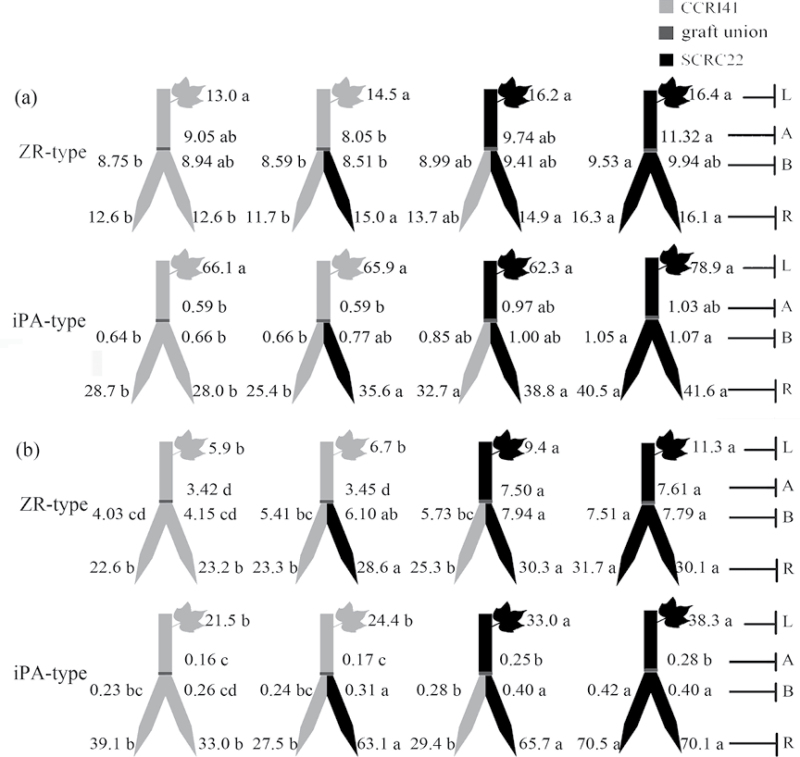
Fig. 5.
Effect of K deficiency on ABA levels of cotton inverted Y graft (scion/rootstock+rootstock) cotton at the 8–9 leaf stage. Grafting was performed hypocotyl-to-hypocotyl at the 1-leaf stage of both the rootstock and scion. Grafts were maintained in nutrient solution with 0.1mM K during establishment, and transferred to solutions with either 2.5mM (a) or 0.01mM K (b) after establishment. The ABA concentrations (ng g–1 FW) in roots (R) and the youngest fully expanded leaf (L), and xylem ABA delivery rates (ng plant–1 24h–1) below (B) and above the graft union (A) were determined. For each K level, means of the same sampling part (i.e. roots, leaf, and xylem sap collected both below and above the graft union) followed by the same letter are not significantly different according to Duncan’s multiple range test, P < 0.05, n=4.
ABA and CK levels in standard grafts
ABA
Under K sufficiency, there were no significant differences between SCRC22 and CCRI41 in either root (rootstocks) ABA concentrations or leaf (scion) ABA concentrations (Fig. 1a). Also, SCRC22 had similar xylem ABA delivery rates to CCRI41 both in rootstocks and in scions (Fig. 1a).
When submitted to K deficiency, the root ABA concentrations and xylem ABA delivery rates across grafts significantly (P < 0.001) increased by 1.6- and 4.6-fold, respectively, whereas the leaf ABA concentrations changed little (Fig. 1b; Table 1). Compared with SCRC22 self-grafts, CCRI41 self-grafts had 88, 90, and 28% greater ABA levels in roots, leaves, and xylem sap, respectively. In addition, it was observed that although the SCRC22 rootstock could reduce the ABA levels in leaves and xylem sap of CCRI41 scions compared with CCRI41 self-grafts, the corresponding values were greater than those of SCRC22 self-grafts. Similarly, the CCRI41 rootstock had a tendency to enhance the ABA levels in leaves and xylem sap of SCRC22 scions compared with SCRC22 self-grafts, but the corresponding values were lower than those of CCRI41 self-grafts (Fig. 1b). These results suggest a feedback regulation of leaf ABA concentrations and xylem ABA delivery rates by scion cultivars. Furthermore, the scion did not affect the rootstock in terms of root ABA concentrations under K deficiency (Fig. 1b; Table 1).
Table 1.
Summary of analysis of variance of ABA, ZR-, and iPA-type levels in the cotton grafting experiment P-values are presented for each main effect or interaction.
| Effect or interaction | Root | Xylem sap below the graft union | Xylem sap above the graft union | Leaf | |
|---|---|---|---|---|---|
| Standard grafting | |||||
| ABA | Ka | <0.001 | <0.001 | <0.001 | 0.061 |
| S-scionb | 0.452 | 0.046 | 0.027 | 0.020 | |
| S-rootstockc | 0.096 | 0.129 | 0.881 | 0.690 | |
| S-scion×rootstockd | 0.541 | 0.276 | 0.591 | 0.308 | |
| D-scione | 0.168 | 0.067 | 0.002 | <0.001 | |
| D-rootstockf | <0.001 | 0.033 | 0.057 | 0.010 | |
| D-scion×rootstockg | 0.315 | 0.756 | 0.825 | 0.060 | |
| ZR-type | K | <0.001 | <0.001 | <0.001 | <0.001 |
| S-scion | 0.097 | 0.003 | <0.001 | 0.172 | |
| S-rootstock | 0.002 | 0.129 | 0.322 | 0.623 | |
| S-scion×rootstock | 0.295 | 0.085 | 0.651 | 0.470 | |
| D-scion | 0.024 | 0.031 | <0.001 | 0.018 | |
| D-rootstock | <0.001 | 0.051 | 0.040 | 0.392 | |
| D-scion×rootstock | 0.734 | 0.984 | 0.813 | 0.956 | |
| iPA-type | K | 0.009 | <0.001 | <0.001 | <0.001 |
| S-scion | 0.166 | 0.613 | 0.005 | 0.037 | |
| S-rootstock | 0.262 | 0.574 | 0.864 | 0.505 | |
| S-scion×rootstock | 0.719 | 0.711 | 0.320 | 0.389 | |
| D-scion | 0.070 | <0.001 | <0.001 | 0.002 | |
| D-rootstock | <0.001 | 0.094 | 0.043 | 0.030 | |
| D-scion×rootstock | 0.286 | 0.923 | 0.621 | 0.873 | |
| Y grafting | |||||
| ABA | K | <0.001 | <0.001 | <0.001 | 0.546 |
| S-rootstock | <0.001 | <0.001 | 0.374 | 0.770 | |
| D-rootstock | <0.001 | <0.001 | 0.850 | 0.281 | |
| ZR-type | K | <0.001 | <0.001 | <0.001 | <0.001 |
| S-rootstock | 0.015 | <0.001 | 0.662 | 0.305 | |
| D-rootstock | <0.001 | <0.001 | 0.657 | 0.097 | |
| iPA-type | K | <0.001 | <0.001 | <0.001 | 0.004 |
| S-rootstock | 0.084 | <0.001 | 0.251 | 0.248 | |
| D-rootstock | <0.001 | <0.001 | 0.159 | 0.641 | |
| Inverted Y grafting | |||||
| ABA | K | <0.001 | <0.001 | <0.001 | 0.308 |
| S-scion | 0.118 | 0.089 | 0.021 | <0.001 | |
| D-scion | 0.279 | 0.681 | <0.001 | <0.001 | |
| ZR-type | K | <0.001 | <0.001 | <0.001 | <0.001 |
| S-scion | 0.080 | 0.081 | 0.001 | 0.045 | |
| D-scion | 0.017 | <0.001 | <0.001 | <0.001 | |
| iPA-type | K | <0.001 | <0.001 | <0.001 | <0.001 |
| S-scion | 0.100 | 0.002 | <0.001 | 0.707 | |
| D-scion | 0.742 | <0.001 | <0.001 | <0.001 | |
a K deficiency (0.03mM for standard and Y grafts, and 0.01mM for inverted Y grafts) relative to control (2.5mM) was imposed on cotton grafts after establishment
b Scion effect under K sufficiency (S-scion).
c Rootstock effect under K sufficiency (S-rootstock).
d Scion×rootstock under K sufficiency (S-scion×rootstock).
e Scion effect under K deficiency (D-scion).
f Rootstock effect under K deficiency (D-rootstock).
g Scion×rootstock under K deficiency (D-scion×rootstock).
CKs
Under K sufficiency, the ZR- and iPA-type concentrations in roots and leaves of SCRC22 tended to be higher than those of CCRI41, but the differences were not significant in most cases. In addition, the xylem ZR-type delivery rates in SCRS22 scions were significantly greater than those in CCRI41 scions regardless of rootstock cultivars. However, there were no significant variations in the xylem iPA-type delivery rates between scions of the two cultivars.
When exposed to K deficiency, the roots showed 73% (P < 0.001) more ZR-type concentrations across grafts compared with K sufficiency, and a slight but significant (P = 0.009) increase occurred in iPA-type concentrations (Fig. 2b; Table 1). Nonetheless, the ZR-type levels in leaves and xylem sap decreased by 32% and 29% (P < 0.001), and the iPA-type levels decreased 48% and 63% (P < 0.001), respectively. The ZR- and iPA-type levels in leaves and xylem sap of reciprocal grafts were altered insignificantly compared with self-grafts with the same scions as reciprocal grafts, suggesting a feedback regulation by scion cultivars (Fig. 2b). The same cultivar rootstock had similar root ZR- or iPA-type concentrations regardless of scion cultivar, indicating little influence of scion on rootstock. Considering genotypic variations, SCRC22 rootstocks across self- and reciprocal grafts showed 26% and 87% greater ZR- and iPA-type concentrations in roots (Fig. 2b). Also, SCRC22 scions showed 61% and 60% greater ZR- and iPA-type concentrations in leaves, and 26% and 42% greater ZR- and iPA-type delivery rates in xylem sap (averaged across above and below the graft union) than CCRI41 scions.
ABA and CK levels in Y grafts
ABA
Under K sufficiency, although there were no typical symptoms of premature senescence, genotypic variations in root and xylem ABA levels were observed between the two cultivars (Fig. 3a). Compared with SCRC22, CCRI41 rootstocks had greater ABA levels not only in roots but also in xylem sap (collected below the graft union) regardless of scion cultivars. Also, the xylem ABA delivery rates in CCRI41 scions were 93% significantly greater than those of SCRC22 scions regardless of rootstock cultivars. There were no significant differences in the leaf ABA concentrations between CCRI41and SCRC22 scions.
Under K deficiency, the ABA levels significantly increased by 3.1-fold in roots, 1.9-fold in rootstock xylem sap, and 1.6-fold in scion xylem sap compared with K sufficiency (Fig. 3b; Table 1). However, there was no significant alteration in leaf ABA concentrations. CCRI41 rootstocks showed 86% and 32% greater ABA levels in roots and xylem sap than SCRC22 rootstocks; and its scions had consistently greater ABA levels in leaves and xylem sap than SCRC22 scions, even if they were grafted together onto the same rootstock, clearly suggesting a feedback regulation by scion cultivar. The scions did not affect root and xylem ABA levels in rootstocks (Fig. 3b).
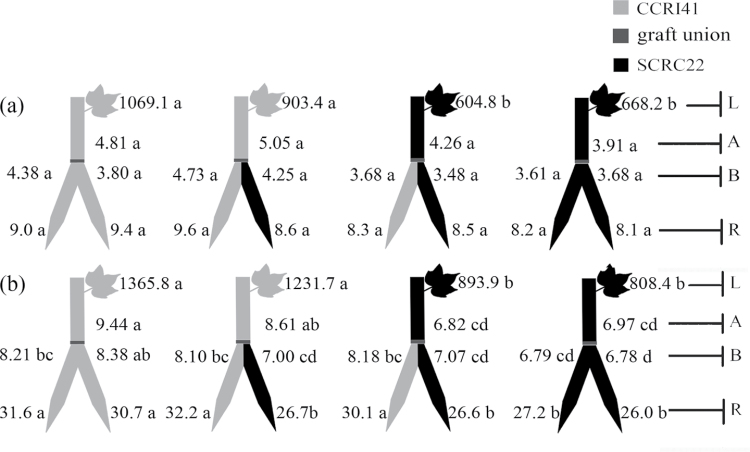
CKs
Under K sufficiency, there were no significant differences in root ZR- and iPA-type concentrations between SCRC22 and CCRI41 rootstocks. However, the xylem ZR- and iPA-type delivery rates of SCRC22 rootstocks were much greater than those of CCRI41 rootstocks in most cases, regardless of scion cultivars. Similarly, SCRC22 scions showed greater xylem ZR- and iPA-type levels than CCRI41 scions, even if they were grafted together onto the same rootstock (Fig. 4a). No significant differences in leaf ZR- and iPA-type concentrations between the two cultivars were observed.
Fig. 4.
Effect of K deficiency on cytokinin (including the ZR-and iPA-type) levels of cotton Y graft (scion+scion/rootstock) at the 8–9 leaf stage. Grafting was performed hypocotyl-to-hypocotyl at the 1-leaf stage of the rootstock and the cotyledonary stage of the scion. Grafts were maintained in nutrient solution with 0.1mM K during establishment, and transferred to solutions with either 2.5mM (a) or 0.03mM K (b) after establishment. The ZR- and iPA-type concentrations (ng g–1 FW) in roots (R) and the youngest fully expanded leaf (L), and xylem ZR- and iPA-type delivery rates (ng plant–1 24h–1) below (B) and above the graft union (A) were determined. For each K level, means of the same sampling part (i.e. roots, leaf, and xylem sap collected both below and above the graft union) followed by the same letter are not significantly different according to Duncan’s multiple range test, P < 0.05, n=4.
When subjected to K deficiency, the ZR-type levels in roots across grafts increased by 58% (P < 0.001) compared with K sufficiency, and iPA-type levels changed slightly but significantly (Fig. 4b; Table 1). However, the ZR-type levels in xylem sap of rootstocks, xylem sap of scions, and leaves decreased by 39, 36, and 41% (P < 0.001); and the iPA-type levels decreased by 38, 43 (P < 0.001), and 16% (P = 0.004), respectively. Compared with CCRI41 self-graft, the rootstock of the SCRC22 self-graft had 37% and 55% greater ZR-type, and 37% and 43% greater iPA-type levels, in roots and xylem sap, respectively (Fig. 4b). When one of two scions of a self-graft was replaced by the other cultivar, the ZR- and iPA-type levels in both SCRC22 or CCRI41 rootstocks changed little. SCRC22 scions had more ZR- and iPA-type CKs in leaves and xylem sap than CCRI41 scions, even if they were grafted together onto the same rootstock (insignificant for the ZR type; Fig. 4b). The mean values of leaf ZR- and iPA-type concentrations and xylem ZR- and iPA-type delivery rates across SCRC22 scions were 72% and 32%, and 41% and 60% more, respectively, than those across CCRI41 scions.
ABA and CK levels in inverted Y grafts
ABA
Under K sufficiency, there were no significant differences in root and xylem ABA levels between CCRI41 and SCRC22 rootstocks, and in xylem ABA delivery rates between CCRI41 and SCRC22 scions. Nevertheless, CCRI41 scions showed greater leaf ABA concentrations than SCRC22 scions regardless of rootstock (Fig. 5a).
Under K deficiency, the ABA levels in roots and xylem sap across rootstocks strongly increased by 2.3- and 1.9-fold (P < 0.001) compared with K sufficiency; and those in xylem sap across scions increased by 78% (P < 0.001); no significant changes were observed in leaf ABA concentrations (Fig. 5b; Table 1). CCRI41 rootstocks showed significantly greater ABA levels in roots and xylem sap than SCRC22 rootstocks whether in self- or reciprocal grafts (except xylem ABA delivery rates in reciprocal grafts). In addition, the ABA levels in leaves and xylem sap of CCRI41 scions were more than those of SCRC22 scions, even if they were separately grafted onto the same combination of rootstocks, displaying the characteristic of feedback regulation. The mean values of ABA in leaves and xylem sap of CCRI41 scions were 53% and 31% more than those of SCRC22 scions.
CKs
Under K sufficiency, SCRC22 rootstocks had greater ZR- and iPA-type levels in roots and xylem sap than CCRI41 rootstocks, but not all differences were significant (Fig. 6a). Similarly, the xylem ZR- and iPA-type delivery rates in SCRC22 scions tended to be higher than those in CCRI41 scions. However, the leaf ZR- and iPA-type concentrations of SCRC22 scions were equivalent to those of CCRI41 scions.
Fig. 6.
Effect of K deficiency on cytokinin (including the ZR-and iPA-type) levels of cotton inverted Y graft (scion+scion/rootstock) cotton at the 8–9 leaf stage. Grafting was performed hypocotyl-to-hypocotyl at the 1-leaf stage of both the rootstock and scion. Grafts were maintained in nutrient solution with 0.1mM K during establishment, and transferred to solutions with either 2.5mM (a) or 0.01mM K (b) after establishment. The ZR- and iPA-type concentrations (ng g–1 FW) in roots (R) and the youngest fully expanded leaf (L), and xylem ZR- and iPA-type delivery rates (ng plant–1 24h–1) below (B) and above the graft union (A) were determined. For each K level, means of the same sampling part (i.e. roots, leaf, and xylem sap collected both below and above the graft union) followed by the same letter are not significantly different according to Duncan’s multiple range test, P < 0.05, n=4.
Under K deficiency, the ZR- and iPA-type levels in roots across grafts increased by 90% and 47% (P < 0.001) but decreased by 33% and 63% (P < 0.001) in rootstock xylem sap, 42% and 73% (P < 0.001) in scion xylem sap, and 45% and 57% (P < 0.001) in leaves (Fig. 6b). SCRC22 rootstocks had more ZR- and iPA-type CKs in roots and xylem sap than CCRI41 rootstocks, even if they were grafted together with the same scion (Fig. 6b). In addition, SCRC22 scions showed more ZR- and iPA-type CKs in leaves and xylem sap than CCRI41 scions, even if they were separately grafted onto the same combination of rootstocks (Fig. 6b), suggesting a feedback regulation by shoot cultivars. The mean ZR- and iPA-type levels in leaves and xylem sap of SCRC22 scions were 64% and 55% (leaves), and 1.2-fold and 61% (xylem sap) more than those of CCRI41 scions.
Discussion
Potassium deficiency increased ABA levels in roots and xylem sap but decreased CK levels in xylem sap and leaves of cotton seedlings
It was observed that the flow rates of xylem sap under K deficiency were lower than those under K sufficiency in the present study (data not shown) since K deficiency decreases root hydraulic conductance (Cabanero and Carvajal, 2007), which may result in the overestimation of the xylem phytohormone concentration (Dodd, 2005). Therefore, the phytohormone delivery rate (the product of xylem phytohormone concentration and sap flow rate) was used to compare the effects of different K supplies on xylem phytohormone levels.
In the present study, K deficiency (0.01mM or 0.03mM) increased root ABA concentrations by 1.6- to 3.1-fold, and xylem ABA flux by 1.8- to 4.6-fold across grafting types and cultivars, as compared with adequate K supply (2.5mM). These results agreed with those of Peuke et al. (2002) showing that low K supply increased the deposition of ABA in the roots (1.9-fold), and root-to-shoot ABA signal in the xylem (4.6-fold). However, there was no significant change in leaf ABA concentrations across cultivars regardless of grafting type under K deficiency (Table 1), possibly due to the high degradation of xylem-sourced ABA in leaves (Zhang et al., 1997; Holbrook et al., 2002; Peuke et al., 2002).
The responses of ZR- and iPA-type CKs to K deficiency were different among parts of cotton seedlings. The levels of ZR- and iPA-type CKs decreased in leaves and xylem sap under K deficiency, but increased in the root tissue (albeit insignificantly for the iPA type in Y and inverted Y grafts). Salama and Wareing (1979) studied the effects of low supply of nitrogen (N), phosphorus (P), and K on endogenous CKs in sunflower (Helianthus annuus), and found that not only low N and low P but also low K decreased the levels of CKs in the roots. The difference between the present results and those of Salama and Wareing (1979) may be due to interspecific differences (cotton versus sunflower) in responses to K deficiency and the different intensities of K deficiency applied (~1/100 versus 1/10).
Recent studies showed potential co-regulation of the ABA and CK status in plants. Changes in ABA status can regulate shoot CK concentrations via altering cytokinin oxidase activity (Vysotskaya et al., 2009). Root overexpression of the ipt gene can decrease ABA accumulation in roots, xylem sap, and the mature leaf of salinized plants (Ghanem et al., 2011a ). In the present study, significant negative relationships of ABA with ZR- and iPA-type CKs were also found in the organs determined (data not shown). However, no causal relationship, or direct interaction, between them can be discerned.
ABA and CKs are responsible for premature senescence induced by K deficiency
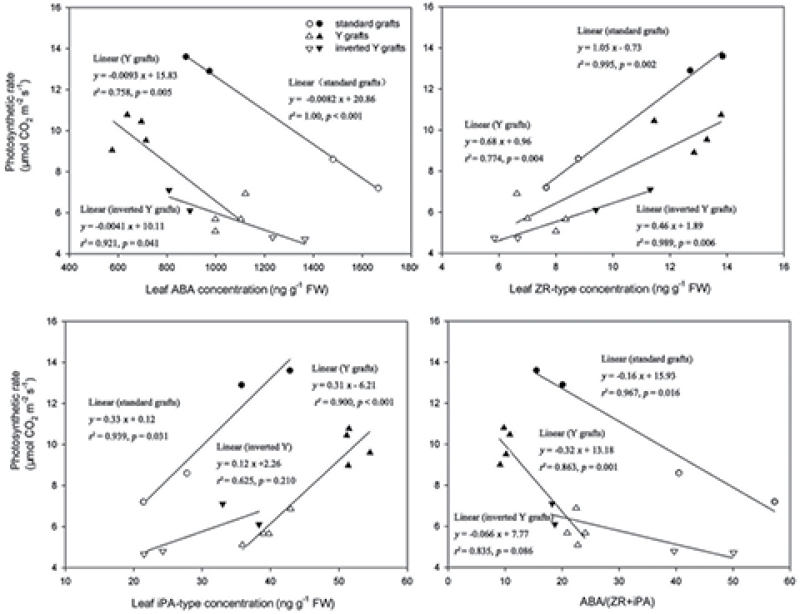
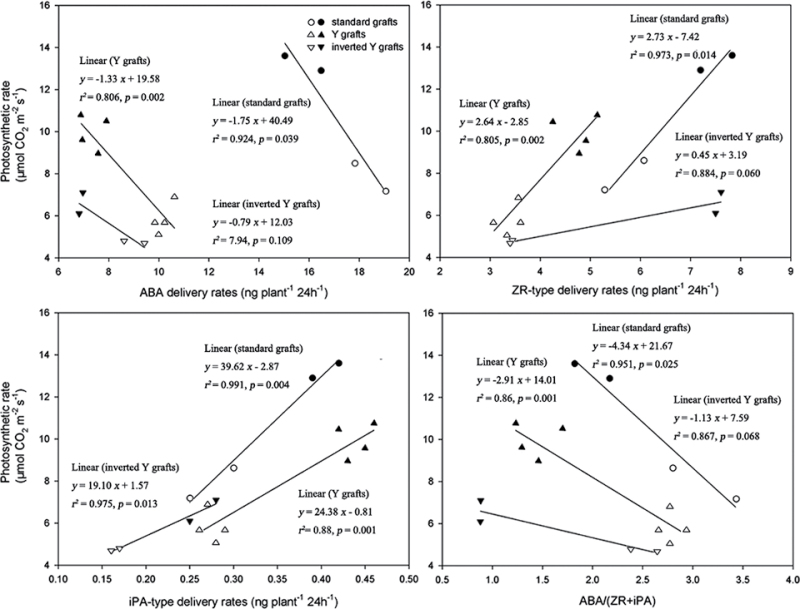
Ghanem et al. (2008) and Albacete et al. (2010) have demonstrated that CKs in tomato was the hormonal parameter best related to photosystem II efficiency (F v/F m, indicative of leaf senescence) under salinity. In the present study, a close negative correlation of photosynthesis rate (Pn) in the fourth main-stem leaf from the apex (Li et al., 2012), indicative of leaf senescence, with leaf ABA concentrations, scion xylem ABA delivery rates, and ratios of ABA/(ZR+iPA) type in leaves and xylem sap under K deficiency were found; a close positive correlations was also noted between Pn and CK levels in leaves and xylem sap (Figs 7, 8).
Fig. 7.
Relationships between leaf ABA, ZR-, and iPA-type concentrations, and ABA/(ZR+iPA) ratios (x) with photosynthetic rate (Pn) of the youngest fully expanded leaf (the fourth leaf from the top of the plant) grown under K deficiency (0.03mM for standard and Y grafts, and 0.01mM for inverted Y grafts). Open and filled circles denote CCR141 and SCRC22 scions of standard grafts, respectively; open and filled triangles denote CCR141 and SCRC22 scions of Y grafts; and open and filled inverted triangles denote CCR141 and SCRC22 scions of inverted Y grafts. Each point represents the mean of a grafting treatment averaged across three or four replicates, and each replicate consisted of 4–20 plants. The linear regressions were fitted with Sigmaplot 11.0.
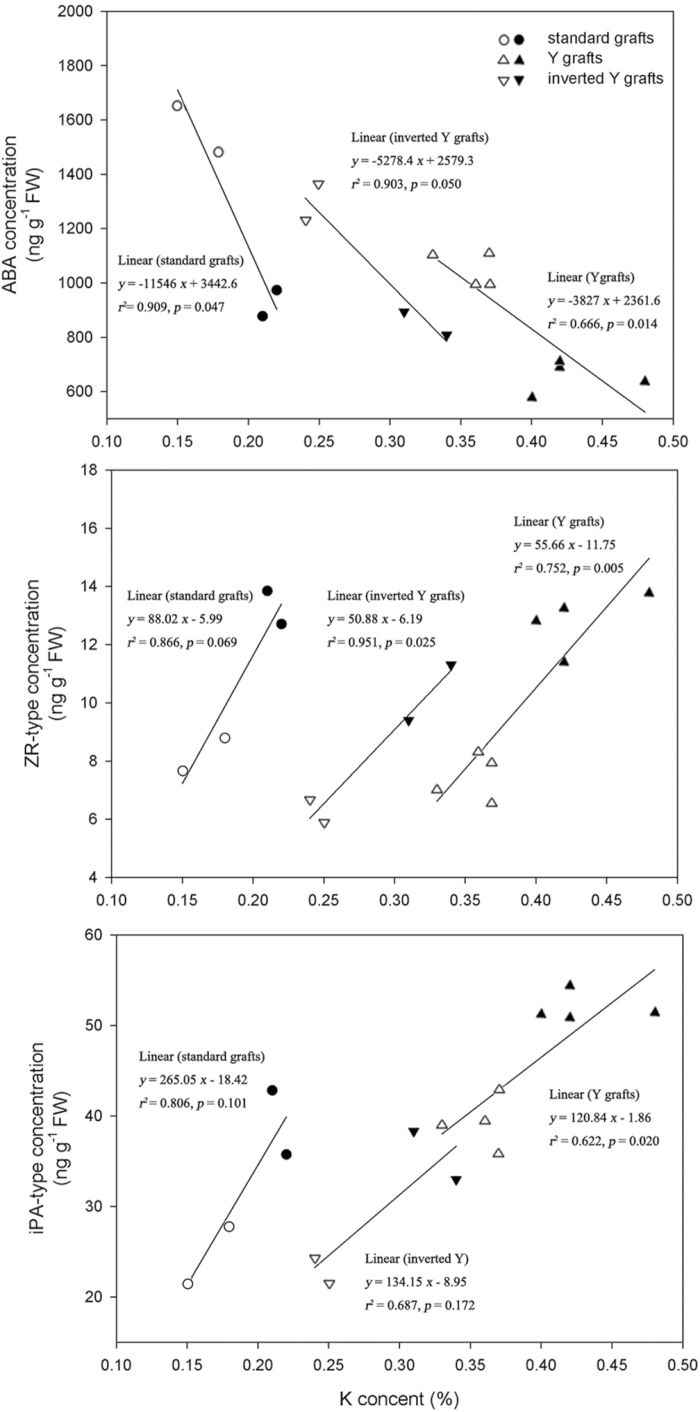
In previous work (Li et al., 2012), a close positive relationship between leaf K content and senescence induced by K deficiency was observed. In order to investigate whether leaf K content influences leaf senescence via phytohormones, K content in the fourth main-stem leaf from the apex (Li et al., 2012) was correlated with ABA and CK concentrations in the same leaf under K deficiency. There was a significant negative relationship between K content and ABA concentration and a significant positive relationship between K content and ZR-type concentration (except standard grafting; Fig. 9). In addition, significant relationships were found between K deliveries and phytohormone levels in rootstock xylem sap of standard (except iPA-type CKs) and inverted Y grafts and in scion xylem sap of Y grafts (data not shown). However, there were no significant relationships between root K content and ABA and CK concentrations in root tissues.
Fig. 9.
Relationships between K content (x) and ABA, ZR-, and iPA-type concentrations in the youngest fully expanded leaf (the fourth leaf from the top of plant) grown under K deficiency (0.03mM for standard and Y grafts, and 0.01mM for inverted Y grafts). Open and filled circles denote CCR141 and SCRC22 scions of standard grafts, respectively; open and filled triangles denote CCR141 and SCRC22 scions of Y grafts; and open and filled inverted triangles denote CCR141 and SCRC22 scions of inverted Y grafts. Each point represents the mean of a grafting treatment averaged across three or four replicates, and each replicate consisted of 4–20 plants. The linear regressions were fitted with Sigmaplot 11.0.
Feedback regulation of ABA and CK levels in leaves and scion xylem sap (collected above the graft union) is guaranteed by the different grafting types
Grafting is a useful tool to test whether there was a feedback regulation of some physiological responses, including leaf senescence. For the seedlings exposed to K deficiency, the rootstocks have a tendency to alter phytohormone levels in leaves and scion xylem sap in some cases (Table 1), even significantly for the leaf ABA concentration of a CCRI41 scion grafted onto a SCRC22 rootstock (compared with CCRI41 self-graft; Fig. 1b), but the scion cultivars explained a higher percentage of variation within grafting treatments under K deficiency irrespective of grafting types. No interactions were found between rootstock and scion in the standard grafting experiment regardless of plant organs and phytohormone types (Table 1), indicating that rootstock and scion are autonomous to a great extent in terms of ABA and CK levels.
The levels of ZR- and iPA-type CKs were consistently lower in leaves and xylem of CCRI41 scions than those of SCRC22 scions, and the leaf ABA concentrations and scion xylem ABA flux were consistently greater in grafts with CCRI41 as scions than those with SCRC22 as scions. These results revealed that the cotton shoot can modify (i.e. feedback regulate) the import of phytohormones from the root, as reported in Beveridge et al. (1997) and Foo et al. (2007) for pea and Arabidopsis, and the leaf phytohormone concentrations. Furthermore, it was noticed that it is the xylem phytohormone concentration rather than sap volume that was regulated by shoot cultivars. With respect to seedlings grown under K sufficiency, although significant genotypic differences in scion xylem CK flux were observed in some cases, the leaf CK concentrations were similar between CCRI41 and SCRC22 scions (Figs 2a, 4a, 6a; Table 1).
Consistent with the present results, Gan and Amasino (1995) and Faiss et al. (1997) found that the CK-overproducer rootstock failed to delay leaf senescence via a grafting study. Due to the absence of data for xylem CKs in grafts which they performed, it remained unclear whether or not there was a feedback regulation of CKs exported from roots by the shoots. However, the present results are different from the classic notion that the roots play an important role in regulation of leaf senescence by delivering hormones to shoots (van Staden et al., 1988; McKenzie et al., 1998; Dong et al., 2008; Albacete et al., 2009, 2010; Ghanem et al., 2011b ), reflecting the diversity and complexity of the long-distance signalling system in higher plants. As for ABA, studies with reciprocal grafts of wild-type plants and ABA-deficient mutants indicated that the mutant rootstocks had no/little effect on ABA concentrations in xylem sap (Dodd et al., 2009) and in the leaves (Holbrook et al., 2002) of wild-type scions, which are similar to those in the present study.
The site of feedback regulation of xylem ABA and CK levels is the hypocotyl
Xylem sap was collected below and above the graft union, and the phytohormone levels were measured separately. This permits the site of feedback regulation to be deduced more precisely. The three types of grafting were all performed hypocotyl-to-hypocotyl. Because scions did not influence the root (rootstock) ABA and CK concentrations (Figs 1b, 2b, 3b, 4b, 5b, 6b), it is postulated that the site of feedback regulation of xylem phytohormones by scion lies beyond the root tissue. For standard grafts, no significant differences in xylem ABA and CK delivery between rootstock (collected below the graft union) and scion (collected above the graft union) within a graft were found (Figs 1b, 2b), suggesting that the site of feedback regulation of xylem phytohormones in standard grafts is most probably the rootstock hypocotyl (from the root–shoot junction to the graft union). In terms of Y and inverted Y grafts, the scion(s) did not significantly affect the xylem ABA and CK flux in rootstock(s) (collected below the graft union) (Figs 3b, 4b, 5b, 6b), thus implying that the main site of feedback regulation of xylem phytohormones by scion(s) is the scion hypocotyl (above the graft union), and the target of feedback signal(s) is more likely to be the changes in xylem phytohormone levels within hypocotyl tissues rather than the export of phytohormones from the roots.
It is considered that when/after xylem phytohormoes passed through the graft union from rootstock(s) to scion(s) in Y and inverted Y grafts, they experienced great changes in metabolism, and/or exchanges with xylem parenchyma cells via the action of feedback signal(s). This presumption can at least partly explain the imbalance between rootstock(s) and scion(s) within either a Y graft or an inverted Y graft in terms of xylem phytohormone delivery rates (Figs 3–6), and is supported by the literature. Sauter and Hartung (2002) demonstrated that the lateral transport of ABA in the stem (from the stem parenchyma to the xylem, and vice versa) may contribute to modifying the xylem ABA levels, and xylem sap can be enriched with ABA sourced from xylem parenchyma cells by the higher xylem pH (Li et al., 2011). Moreover, it is known that CKs may be modulated along their transport pathway, for example by movement from xylem to parenchyma cells in the stem or petioles (Singh et al., 1992). Future studies need to confirm the site of feedback regulation of xylem phytohormones further by using interstock grafting and top grafting (on the main stem above the cotyledons, as opposed to the hypocotyl in the present study), and the physiological and molecular mechanisms underlying the changes in xylem phytohormones levels within hypocotyl tissues.
The mechanism of feedback regulation of leaf ABA and CK concentrations remains unclear
Theoretically, the level of phytohormones in leaf is regulated at diverse steps, including de novo synthesis, activation, conjugation, and degradation, as well as local and long-distance transport. Since the differences in xylem CKs levels between CCRI41 and SCRC22 scions were similar to those differences in leaf CKs concentrations under K deficiency, we cannot exclude the xylem contribution to leaf phytohormone status by long-distance transport in the present study.
The capacity of the leaf to biosynthesize ABA and CKs has been well demonstrated (Miyawaki et al., 2004; Endo et al., 2008). Furthermore, both ABA inactivation (the conjugation with glucose by ABA glucosyltransferase to form ABA glucose ester; Xu et al., 2002) and CK degradation by cytokinin oxidase (Vysotskaya et al., 2009) can occur in the leaf. Therefore, further metabolic studies such as on de novo synthesis, activation, conjugation, and degradation, and local and long-distance transport need to be undertaken to find the exact mechanism underlying the feedback regulation of leaf phytohormone concentrations.
Conclusion
K deficiency strongly increased ABA levels in roots and xylem sap of cotton seedlings and decreased those of ZR- and iPA-type CKs in xylem sap and leaves. Correlation analysis indicated that ABA, ZR-, and iPA-type levels in leaves and scion xylem sap (collected above the graft union) under K deficiency were closely associated with leaf senescence. The results of both standard (one scion grafted onto one rootstock) and Y (two scions grafted onto one rootstock) or inverted Y (one scion grafted onto two rootstocks) grafting showed that the ABA and CK levels in leaves and scion xylem sap were feedback regulated by scion cultivars under K deficiency. The main action site of basipetal feedback signal(s) involved in xylem phytohormones is the rootstock hypocotyl (below the graft union) in standard grafting and the scion hypocotyl (above the graft union) in Y and inverted Y grafting. The target of this feedback signal(s) is more likely to be the changes in xylem phytohormones within hypocotyl tissues rather than the export of phytohormones from the roots. The mechanism of feedback regulation of leaf ABA and CK concentrations remains unclear. The results of this study have provided an improved understanding of communication between shoot and root, and are beneficial for the development of approaches to managing this problem.
Acknowledgements
Fig. 8.
Relationships between ABA, ZR-, and iPA-type delivery rates, and ABA/(ZR+iPA) ratios (x) in scion xylem sap (collected above the graft union) and photosynthetic rate (Pn) of the youngest fully expanded leaf (the fourth leaf from the top of the plant) grown under K deficiency (0.03mM for standard and Y grafts, and 0.01mM for inverted Y grafts). Open and filled circles denote CCR141 and SCRC22 scions of standard grafts, respectively; open and filled triangles denote CCR141 and SCRC22 scions of Y grafts; and open and filled inverted triangles denote CCR141 and SCRC22 scions of inverted Y grafts. Each point represents the mean of a grafting treatment averaged across three or four replicates, and each replicate consisted of 4–20 plants. The linear regressions were fitted with Sigmaplot 11.0.
Acknowledgments
This research was supported by the NSFC (National Natural Science Foundation of China, 30571118 and 30971708) and the Program for New Century Excellent Talents in University of China (NCET-08-0533). We thank the Cotton Research Institute, Chinese Academy of Agricultural Sciences, Anyang, Henan, and the Cotton Research Center, Shandong Academy of Agricultural Sciences, Jinan, Shandong for providing cotton seeds.
References
- Albacete A, Ghanem ME, Dodd IC, Pérez-Alfocea F. 2010. Principal component analysis of hormone profiling data suggests an important role for cytokinins in regulating leaf growth and senescence of salinized tomato Plant Signaling and Behavior 5 45–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albacete A, Martinez-Andujar C, Ghanem ME, Acosta M, Sanchez-Bravo J, Asins MJ, Cuartero J, Lutts S, Dodd IC, Pérez-Alfocea F. 2009. Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato Plant, Cell and Environment 32 928–938 [DOI] [PubMed] [Google Scholar]
- Bednarz CW, Oosterhuis DM. 1995. Plant potassium partitioning during progression of deficiency symptoms in cotton (Gossypium hirsutum). Better Crops Potash and Phosphate Institute 79 12–14 [Google Scholar]
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C. 1997. The shoot controls zeatin riboside export from pea roots. Evidence from the branching mutant rms4 The Plant Journal 11 339–345 [Google Scholar]
- Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, Leaver CJ. 2005. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis The Plant Journal 42 567–585 [DOI] [PubMed] [Google Scholar]
- Cabanero FJ, Carvajal M. 2007. Different cation stresses affect specifically osmotic root hydraulic conductance, involving aquaporins, ATPase and xylem loading of ions in Capsicum annuum, L. plants Journal of Plant Physiology 164 1300–1310 [DOI] [PubMed] [Google Scholar]
- Dodd IC. 2005. Root-to-shoot signalling: assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta Plant and Soil 274 251–270 [Google Scholar]
- Dodd IC, Egea G, Davies WJ. 2008. Accounting for sap flow from different parts of the root system improves the prediction of xylem ABA concentration in plants grown with heterogeneous soil moisture Journal of Experimental Botany 59 4083–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd IC, Ngo C, Turnbull CGN, Beveridge CA. 2004. Effects of nitrogen supply on xylem cytokinin delivery, transpiration and leaf expansion of pea genotypes differing in xylem-cytokinin concentration Functional Plant Biology 31 903–911 [DOI] [PubMed] [Google Scholar]
- Dodd IC, Theobald JC, Richer SK, Davies WJ. 2009. Partial phenotypic reversion of ABA-deficient flacca tomato (Solanum lycopersicum) scions by a wild-type rootstock: normalizing shoot ethylene relations promotes leaf area but does not diminish whole plant transpiration rate Journal of Experimental Botany 60 4029–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HZ, Li WJ, Tang W, Li ZH, Zhang DM. 2006. Effects of genotypes and plant density on yield, yield components and photosynthesis in Bt transgenic cotton Journal of Agronomy and Crop Science 192 132–139 [Google Scholar]
- Dong HZ, Niu YH, Li WJ, Zhang DM. 2008. Effects of cotton rootstock on endogenous cytokinins and abscisic acid in xylem sap and leaves in relation to leaf senescence Journal of Experimental Botany 59 1295–1304 [DOI] [PubMed] [Google Scholar]
- Endo A, Sawada Y, Takahashi H, et al. 2008. Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells Plant Physiology 147 1984–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiss M, Zalubìlová J, Strnad M, Schmülling T. 1997. Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants The Plant Journal 12 401–415 [DOI] [PubMed] [Google Scholar]
- Foo E, Morris SE, Parmenter K, Young N, Wang H, Jones A, Rameau C, Turnbull CGN, Beveridge CA. 2007. Feedback regulation of xylem cytokinin content is conserved in pea and Arabidopsis Plant Physiology 143 1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan SS, Amasino RM. 1995. Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270 1986–1988 [DOI] [PubMed] [Google Scholar]
- Gepstein S, Thimann KV. 1980. Changes in the abscisic acid content of oat leaves during senescence Proceedings of the National Academy of Sciences, USA 77 2050–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem ME, Albacete A, Martínez-Andújar C, Acosta M, Romero-Aranda R, Dodd IC, Lutts S, Pérez-Alfocea F. 2008. Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.) Journal of Experimental Botany 59 3039–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem ME, Albacete A, Smigocki AC, et al. 2011. a Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants Journal of Experimental Botany 62 125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem ME, Hichri I, Smigocki AC, Albacete A, Fauconnier ML, Diatloff E, Martinez-Andujar C, Lutts S, Dodd IC, Pérez-Alfocea F. 2011. b Root-targeted biotechnology to mediate hormonal signalling and improve crop stress tolerance Plant Cell Reports 30 807–823 [DOI] [PubMed] [Google Scholar]
- Haeder HE, Beringer H. 1981. Influence of potassium nutrition and water stress on the content of abscisic acid in grains and flag leaves of wheat during grain development Journal of the Science of Food and Agriculture 32 552–556 [Google Scholar]
- Holbrook NM, Shashidhar VR, James RA, Munns R. 2002. Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying Journal of Experimental Botany 53 1503–1514 [PubMed] [Google Scholar]
- Jeschke WD, Peuke AD, Pate JS, Hartung W. 1997. Transport, synthesis and catabolism of abscisic acid (ABA) in intact plants of castor bean (Ricinus communis L.) under phosphate deficiency and moderate salinity Journal of Experimental Botany 48 1737–1747 [Google Scholar]
- Jukanti AK, Heidlebaugh NM, Parrott DL, Fischer IA, McInnerney K, Fischer AM. 2008. Comparative transcriptome profiling of near-isogenic barley (Hordeum vulgare) lines differing in the allelic state of a major grain protein content locus identifies genes with possible roles in leaf senescence and nitrogen reallocation New Phytologist 177 333–349 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ryu H, Woo HR, Lim PO, Lee IC, Sheen J, Nam HG, Hwang I. 2006. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis Proceedings of the National Academy of Sciences, USA 106 814–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Shin R, Schachtman DP. 2009. A nuclear factor regulates abscisic acid responses in Arabidopsis Plant Physiology 151 1433–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper D, Kuiper PJC, Lambers H, Schuit J, Staal M. 1989. Cytokinin concentration in relation to mineral nutrition and benzyladenine treatment in Plantago major ssp. pleiosperma Physiologia Plantarum 75 511–517 [Google Scholar]
- Li B, Wang Y, Zhang ZY, Wang BM, Eneji AE, Duan LS, Li ZH, Tian XL. 2012. Cotton shoot plays a major role in mediating senescence induced by potassium deficiency Journal of Plant Physiology 169 327–335 [DOI] [PubMed] [Google Scholar]
- Li BB, Feng ZG, Xie M, Sun MZ, Zhao YX, Liang LY, Liu GJ, Zhang JH, Jia WS. 2011. Modulation of the root-sourced ABA signal along its way to the shoot in Vitis riparia×Vitis labrusca under water deficit Journal of Experimental Botany 62 1731–1741 [DOI] [PubMed] [Google Scholar]
- McKenzie MJ, Mett V, Reynolds PHS, Jameson PE. 1998. Controlled cytokinin production in transgenic tobacco using a copper-inducible promoter Plant Physiology 116 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Matsumoto-Kitano M, Kakimoto T. 2004. Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate The Plant Journal 37 128–138 [DOI] [PubMed] [Google Scholar]
- Noodèn LD, Singh S, Letham DS. 1990. Correlation of xylem sap cytokinin levels with monocarpic senescence in soybean Plant Physiology 93 33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhuis DM. 1995. Potassium nutrition of cotton with particular reference to foliar fertilization. In: Constable GA, Forrester NW, eds. World Cotton Research Conference Brisbane, Australia: CSIRO; 133–146 [Google Scholar]
- Peuke AD, Jeschke WD, Hartung W. 2002. Flows of elements, ions and abscisic acid in Ricinus communis and site of nitrate reduction under potassium limitation Journal of Experimental Botany 53 241–250 [DOI] [PubMed] [Google Scholar]
- Riefler M. 2006. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism The Plant Cell 18 40–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E. 2007. Delayed leaf senescence induces extreme drought tolerance in a flowering plant Proceedings of the National Academy of Sciences, USA 104 19631–19636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama AMSEA, Wareing PF. 1979. Effects of mineral nutrition on endogenous cytokinins in plants of sunflower (Helianthus annuus L.) Journal of Experimental Botany 30 971–981 [Google Scholar]
- Samet JS, Sinclair TR. 1980. Leaf senescence and abscisic acid in leaves of field-grown soybean Plant Physiology 66 1164–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter A, Hartung W. 2002. The contribution of internode and mesocotyl tissues to root-to-shoot signalling of abscisic acid Journal of Experimental Botany 53 297–302 [DOI] [PubMed] [Google Scholar]
- Singh S, Letham DS, Palni LMS. 1992. Cytokinin biochemistry in relation to leaf senescence. VIII. Translocation, metabolism and biosynthesis of cytokinins in relation to sequential leaf senescence of tobacco Physiologia Plantarum 86 398–406 [Google Scholar]
- Sitton D, Itai C, Kende H. 1967. Decreased cytokinin production in the roots as a factor in shoot senescence Planta 73 296–300 [DOI] [PubMed] [Google Scholar]
- Takei K, Sakakibara H, Taniguchi M, Sugiyama T. 2001. Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: implication of cytokinin species that induces gene expression of maize response regulator Plant and Cell Physiology 42 85–93 [DOI] [PubMed] [Google Scholar]
- Tian XL, Wang GW, Yang FQ, Yang PZ, Duan LS, Li ZH. 2008. Differences in tolerance to low-potassium supply among different types of cultivars in cotton (Gossypium hirsutum) Acta Agronomica Sinica 34 1770–1780 [Google Scholar]
- van der Graaff E, Schwacke R, Schneider A, Desimone M, Flügge UI, Kunze R. 2006. Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence Plant Physiology 141 776–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staden J, Cook E, Noodén LD. 1988. Cytokinins and senescence. In: Noodén LD, Leopold A, eds. Senescence and aging in plants London: Academic Press; 281–328 [Google Scholar]
- Vysotskaya LB, Korobova AV, Veselov SY, Dodd IC, Kudoyarova GR. 2009. ABA mediation of shoot cytokinin oxidase activity: assessing its impacts on cytokinin status and biomass allocation of nutrient-deprived durum wheat Functional Plant Biology 36 66–72 [DOI] [PubMed] [Google Scholar]
- Weaver LM, Gan SS, Quirino B, Amasino RM. 1998. A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment Plant Molecular Biology 37 455–469 [DOI] [PubMed] [Google Scholar]
- Weiler EW, Jourdan PS, Conrad W. 1981. Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay Planta 153 561–571 [DOI] [PubMed] [Google Scholar]
- Wright PR. 1999. Premature senescence of cotton (Gossypium hirsutum L.): predominantly a postassium disorder caused by an imbalance of source and sink Plant and Soil 211 231–239 [Google Scholar]
- Xu ZJ, Nakajima M, Suzuki Y, Yamaguchi I. 2002. Cloning and characterization of the abscisic acid-specific glucosyltransferase gene from adzuki bean seedlings Plant Physiology 129 1285–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FQ, Wang GW, Zhang ZY, Eneji AE, Duan LS, Li ZH, Tian XL. 2011. Genotypic variations in potassium uptake and utilization in cotton Journal of Plant Nutrition 34 83–91 [Google Scholar]
- Zavaleta-Mancera HA, Franklin KA, Ougham HJ, Thomas H, Scott IM. 1999. Regreening of senescent Nicotiana leaves. I. Reappearance of NADPH-protochlorophyllide oxidoreductase and light-harvesting chlorophyll a/b binding protein Journal of Experimental Botany 50 1677–1682 [Google Scholar]
- Zeevaart JAD, Creelman RA. 1988. Metabolism and physiology of abscisic acid Annual Review of Plant Physiology and Plant Molecular Biology 39 39–473 [Google Scholar]
- Zhang J, Jia W, Zhang DP. 1997. Effect of leaf water status and xylem pH on metabolism of xylem-transported abscisic acid Plant Growth Regulation 21 51–58 [Google Scholar]
- Zhang ZY, Tian XL, Duan LS, Wang BM, He ZP, Li ZH. 2007. Differential responses of conventional and Bt-transgenic cotton (Gossypium hirsutum L.) to potassium deficiency Journal of Plant Nutrition 30 659–671. [Google Scholar]
- Zhao D, Oosterhuis DM, Bednarz CW. 2001. Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants Photosynthetica 39 103–109 [Google Scholar]
- Zhao J, Li G, Yi GX, Wang BM, Deng AX, Nan TG, Li ZH, Li QX. 2006. Comparison between conventional indirect competitive enzyme-linked immunosorbent assay (icELISA) and simplified icELISA for small molecules Analytica Chimica Acta 571 79–85 [DOI] [PubMed] [Google Scholar]