Summary
Background
A fundamental process underlying all brain functions is the propagation of spiking activity in networks of excitatory and inhibitory neurons. In the neocortex, although functional connections between pairs of neurons have been studied extensively in brain slices, they remain poorly characterized in vivo, where the high background activity, global brain states, and neuromodulation can powerfully influence synaptic transmission. To understand how spikes are transmitted in cortical circuits in vivo, we used two-photon calcium imaging to monitor ensemble activity and targeted patching to stimulate a single neuron in mouse visual cortex.
Results
Burst spiking of a single pyramidal neuron can drive spiking activity in both excitatory and inhibitory neurons within a ~100 µm radius. For inhibitory neurons, ~30% of the somatostatin interneurons fire reliably in response to a presynaptic burst of ≥ 5 spikes. In contrast, parvalbumin interneurons showed no detectable responses to single-neuron stimulation, but their spiking is highly correlated with the local network activity.
Conclusions
Our results demonstrate the feasibility of mapping functional connectivity at cellular resolution in vivo and reveal distinct operations of two major inhibitory circuits, one detecting single-neuron spike bursts and the other reflecting distributed network activity.
Introduction
Connectivity between neurons is characterized structurally by synaptic contacts and physiologically by the strength and temporal dynamics of synaptic transmission. While high-resolution reconstruction of physical wiring diagrams [1–3] can reveal all potential routes of communication, the functional properties of the synapses dictate what types of signals are actually transmitted to each postsynaptic target. For example, the dynamic properties of synapses can control the routing of recurrent inhibitory signals to different compartments of individual neurons [4] and the gating of excitatory interactions between different brain areas [5]. The temporal interplay between the thalamic input and feedforward inhibition determines the precision of cortical spike timing in response to sensory stimulation [6]. Such dynamic properties of synaptic transmission can enhance the processing power of neuronal circuits by increasing the diversity of signals transmitted from each neuron [7]. In behaving animals, these properties may improve the flexibility of neuronal circuits to meet varying task demands [8].
Synaptic connections between pairs of cortical neurons have been studied extensively in brain slices. Connectivity maps between excitatory neurons have been generated by making simultaneous whole-cell recordings from multiple neurons [9, 10], sometimes following functional characterization of each neuron in vivo [11]. Excitatory connections from pyramidal neurons to the major subtypes of inhibitory neurons, such as the fast-spiking and Martinotti interneurons, have also been examined in great detail, revealing distinct temporal characteristics [12–16]. However, it is unclear to what extent these in vitro findings are applicable in vivo, where the high background activity, global brain states, and neuromodulation can powerfully modulate synaptic properties [17].
In particular, little is known about how spike bursts are transmitted within the cortical circuits in vivo. High-frequency bursts of cortical neurons have been observed in both anesthetized and awake animals [18]. Such bursting activity may play special roles in neural coding because of its reliability in transmitting information and its potency for modifying synapses [19]. Importantly, burst spiking of even a single cortical neuron can have a powerful influence on the animal, from driving sensory perception [20] and whisker movement [21] to switching the global brain state [22]. Presumably, the bursting neuron exerts its global impact by first driving the spiking of other cortical neurons nearby, but how a spike burst is transmitted within the local circuits remains unknown.
In this study, we show that transmission of spike bursts can be measured in vivo with cellular resolution. In layer 2/3 of the mouse visual cortex, we found that burst spiking of a single pyramidal neuron can drive spiking activity in both excitatory and inhibitory neurons within a ~100 µm radius. For inhibitory neurons, while ~30% of the somatostatin (SOM) interneurons can be activated reliably, parvalbumin (PV) interneurons showed no detectable responses to single-pyramidal neuron bursts. Instead, their spiking is highly correlated with the local network activity. Together, these results reveal distinct mechanisms for activating the two inhibitory circuits and provide a framework for understanding how spikes of pyramidal neurons propagate within the local cortical circuits in vivo.
Results
Relationship between spike rate and calcium signal in vivo
We labeled layer 2/3 cells in the primary visual cortex of urethane-anaesthetized adult mouse by bolus injection of the calcium indicator dye Oregon Green 488 BAPTA-1 AM (OGB-1) (see Experimental Procedures). To quantify the relationship between the fluorescence transients and spike rates, we used two-photon imaging [23] to guide cell-attached recordings from three subtypes of cortical neurons.
Excitatory and PV neurons were identified by breeding the loxP-flanked tdTomato reporter mice with the CaMKIIα-Cre and the PV-Cre mice, respectively (Figure 1A, 1C). Simultaneous calcium imaging and cell-attached recordings showed that the fluorescence of both the excitatory and PV neurons increased with the number of action potentials (APs, mean r2 = 0.82 and 0.91, respectively; Figure 1B, 1D, 1I, S1), although the slope of dF/F vs. AP number was much higher for excitatory neurons (0.0185 dF/F per spike) than for PV interneurons (0.0058). We also recorded from the GFP-expressing SOM interneurons in cortical layer 2/3 of the GIN mice, which are predominantly Martinotti cells [24] (Figure 1E). Similar to the excitatory and PV neurons, the dF/F of SOM interneurons (n = 8) also increased with the number of APs (mean r2 = 0.75; slope = 0.0078) (Figure 1F). For all three cell types, the spike-triggered average of the fluorescence signal showed a sharp rise and exponential decay (Figure 1H).
Figure 1. Relationship between fluorescence signal and spike rate for different cortical cell types in vivo.
(A) An excitatory neuron in cortical layer 2/3 of CaMKIIα-Cre × tdTomato mouse was targeted for cell-attached recording. Bottom left, mean spike waveforms of 16 excitatory neurons. The amplitude is normalized for each cell. Scale bar, 2 ms. Middle panel, two-photon image of layer 2/3 cells labeled with OGB-1 (white, note the shadow caused by the pipette) and image of tdTomato-expressing cells (red) taken prior to pipette insertion. Scale bar, 20 µm. Right panel, OGB-1 fluorescence and spikes from an example excitatory neuron. The filtered spike train was smoothed with a Gaussian filter (SD, 0.5 s). (B) OGB-1 fluorescence vs. AP number (based on filtered spike train) for the excitatory neurons (n = 16; 6 identified by tdTomato labeling and 10 putative excitatory neurons, classified based on their spontaneous firing rates; see Experimental Procedures). Gray lines, individual cells; black line, population average; error bar, ± SEM. (C, D) Same as (A) and (B), for PV interneurons (green; n = 10; 8 identified by tdTomato labeling and 2 fast-spiking cells) in PV-Cre × tdTomato mice. (E, F) Same as (A) and (B), for SOM interneurons (red; n = 8; all identified by GFP labeling) in GIN mice. (G) Summary of the spiking properties observed from each cell type using cell-attached recording. Left panel, mean spike waveforms of the excitatory (black), PV (green), and SOM (red) neurons. Scatter plots show the firing rate, spike width, and peak-trough height ratio of all three cell types. Open circle, individual cell; line, population mean. *, p < 0.05; **, p < 0.01 (ANOVA multiple comparison). (H) Mean spike-triggered OGB-1 fluorescence of the excitatory (black), PV (green), and SOM (red) neurons. The elevated baseline for SOM interneurons is likely due to the frequent occurrence of spontaneous spike bursts. (I) Overlay of (B), (D), and (F).
In addition to establishing the relationship between somatic calcium transients and spiking activity for each cell type, these experiments also confirmed that the excitatory and GABAergic neurons have distinct spike widths and waveform asymmetry (Figure 1G). Interestingly, SOM interneurons showed narrow and biphasic spike waveforms similar to PV interneurons, although their spontaneous firing rates were much lower.
Spike bursts drive nearby SOM and putative pyramidal neurons
To probe excitatory connections in the local cortical circuits, we made whole-cell recording from a pyramidal neuron near a labeled interneuron (Figure 2A, 2B). The patched cells were identified as pyramidal neurons by their spiny dendrites (Figure 2C) and low (<2 Hz) spontaneous firing rates. A train of 135 current steps (800 pA, 0.1 s/step, 1 s inter-step interval) was injected to elicit spike bursts (61±11 Hz, SD; n = 26 cells; Figure 2D), and OGB-1 fluorescence of ~ 20 – 40 neighboring cells was imaged.
Figure 2. Imaging spike transmission during single-pyramidal neuron stimulation in vivo.
(A) Schematic of experiment. (B) Two-photon image of the patched neuron (blue) near a SOM interneuron expressing GFP (red), together with other cells labeled with OGB-1 (white), of a heterozygous GIN mouse. (C) Spiny dendrite of a patched pyramidal neuron. (D) Left, schematic of the depolarizing current steps injected into the patched pyramidal neuron during stimulation trials (800 pA, 0.1 s/step, 1 s inter-step interval; gray bars, durations of current steps). Middle and right, peri-stimulus time histogram of the APs recorded from the patched neuron from 135 stimulation or control trials (0 pA, 0.1 s/step, 1 s inter-step interval). (E) OGB-1 fluorescence trace of the same patched pyramidal neuron during stimulation (left). Trial-averaged dF/F were computed from either stimulation (middle) or control (right). Shading, 90% confidence intervals from bootstrap. Gray bar, duration of current step. (F) OGB-1 fluorescence trace and trial-averaged dF/F from a SOM interneuron within the same field of view. (G) OGB-1 fluorescence trace and trial-averaged dF/F from a putative pyramidal neuron from another experiment. This putative pyramidal neuron was located 51 µm lateral and 20 µm more superficial from the patched pyramidal neuron, which was not imaged simultaneously. The traces of (D), (E) and (F) are from the same cells that are shown in (B).
While the majority of the cortical neurons exhibited no obvious change in somatic calcium transients due to the single-pyramidal neuron stimulation, we found some cells with stimulation-induced responses (Figure 2E, 2F and 2G). As expected, the patched pyramidal neuron (Figure 2B, blue) had calcium transients that directly reflected the spiking activity elicited by the stimulation (Figure 2E). The fluorescence rose quickly after the first few current steps and returned to baseline several seconds after termination of the stimulation train. Interestingly, a neighboring SOM interneuron (Figure 2B, red) was also driven, although with a more gradual rise in fluorescence (Figure 2F). As shown by the stimulus-triggered average across all current steps (Figure 2E and 2F, middle and right plots), both the patched pyramidal neuron and the SOM interneuron showed significant time-locked calcium increases in the stimulation but not in control trials (0 pA, 0.1 s/step, 1 s inter-step interval), indicating that this SOM interneuron was driven to fire by the spike burst of the patched pyramidal neuron.
A small number of unidentified cells (black) in the imaged ensemble also exhibited stimulus-locked increases in fluorescence (Figure 2G). For most of these cells, there was no sustained rise in calcium during the stimulation train. Because their fluorescence traces had flat baseline and distinct peaks, typical of identified pyramidal neurons (Figure 1A), and because ~80% of cortical neurons are excitatory, we refer to these unidentified, driven cells as ‘putative pyramidal neurons’. Experiments in CaMKIIα-Cre × tdTomato mice showed an activated cell identified by tdTomato labeling to be an excitatory neuron (Figure S2), indicating that single-neuron stimulation is indeed capable of activating nearby excitatory neurons.
In total, we patched and stimulated 26 pyramidal neurons and examined 1189 possible connections, including 17 pyramidal-SOM pairs. To quantify the effect of stimulation, for each neuron we calculated the difference between the mean fluorescence within 200 ms windows before and after each current injection (ΔdF/Fpost-pre, see Experimental Procedures). For SOM interneurons (red), 29% (5/17) were driven by the stimulation with significant trial-averaged ΔdF/Fpost-pre (Figure 3A). Of the putative pyramidal neurons, however, a much smaller fraction (1.7%, 20/1152) was driven by burst spiking of the patched neuron. We refer to these neurons with significant responses as “functionally connected” cells.
Figure 3. Temporal dynamics of the functional connections.
(A) The two types of functional connections under study (red, pyramidal-SOM; black, pyramidal-putative pyramidal). An imaged neuron was classified as functionally connected to the stimulated, patched pyramidal neuron if ΔdF/Fpost-pre > 3 × SEM (see Experimental Procedures). (B) Magnified views of fluorescence traces in response to single-pyramidal neuron stimulation (800 pA, 0.1 s/step, 1 s inter-step interval). Traces are from the same cells shown in (E) – (G) in Figure 2. (C) Trial-averaged fluorescence response, averaged across the 5 SOM interneurons driven by single-pyramidal neuron stimulation. Response of each SOM interneuron was normalized by its peak-to-peak amplitude (bin width = 10 ms; gray bar, duration of current step). Note the delay of fluorescence transient relative to stimulation onset. Because the imaging frame rate (15.6 Hz) and frequency of stimulation (0.91 Hz) are not multiples of each other, over many trials, fluorescence was sampled at many time points around the current step and the effective temporal resolution is higher than 15 Hz. Dotted line, mean spike-triggered dF/F of SOM interneurons from Fig. 1H with the triggered spike aligned to onset of single-cell stimulation. (D) ΔdF/Fpost-pre vs. number of APs elicited in the patched neuron, mean of the 5 SOM interneurons. Dashed line, expected dF/F for 1 AP/trial in SOM neurons, based on the fitted slope of the population average (red line) in Fig. 1F. (E) ΔdF/Fpost-pre of each trial over the first 120 stimulation trials. Gray, mean of the 5 SOM interneurons. Red, linear fit. (F) Mean ΔdF/Fpost-pre for trials in UP or DOWN state. The state of each trial was determined from the membrane potential of the patched neuron immediately preceding stimulation (see Experimental Procedures). Gray lines, individual cells; red, population. (G – J), Similar to (B – E) for the 20 pyramidal-putative pyramidal connections. Dashed line in (H), expected dF/F for 1 AP/trial in pyramidal neurons, based on fitted slope of black line in Fig. 1B. For (C) – (E) and (G) – (I), error bar, ± SEM.
Temporal dynamics of the functional connections
Although a subset of both the SOM interneurons and putative pyramidal neurons were driven to fire by single-pyramidal neuron stimulation, these functional connections exhibited different characteristics. The SOM interneurons were activated reliably in nearly every trial, whereas the putative pyramidal neurons showed calcium transients only in a small subset of the trials (Figure 3B). Trial-averaged dF/F showed that the SOM interneurons responded with a considerable delay (t50 = 65 ms) (Figure 3C and S3A), and the response amplitude (measured by ΔdF/Fpost-pre) increased with the number of APs evoked in the patched neuron with a threshold of ~ 5 APs (Figure 3D and S3B). Both the delay and AP threshold are consistent with the facilitating pyramidal-Martinotti synapses found in vitro [13–15]. In contrast, for the pyramidal-putative pyramidal connections, the fluorescence rose immediately after each stimulation (t50 = 15 ms) (Figure 3G and S3A), and the response amplitude showed no further increase above a threshold of ~5 APs (Figure 3H). Over repeated stimulation, the response reduced slightly for the SOM interneurons (slope = (−5±2) × 10−5, zero-crossing at 237th trial, p = 0.03, t-test) (Figure 3E) but not for the putative pyramidal neurons (slope = (1±2) × 10−5, p = 0.74, t-test) (Figure 3I).
To examine the influence of cortical state, we used the membrane potential of the patched neuron as a proxy for UP (depolarized) and DOWN (hyperpolarized) states (see Experimental Procedures). For both the connected SOM interneurons and putative pyramidal neurons, the response was higher during the UP than the DOWN state (Figure 3F and 3J), although the difference was not significant (SOM, p = 0.27; putative pyramidal, p = 0.17, paired t-test).
PV interneurons are less sensitive to single-pyramidal neuron burst
In addition to the SOM interneurons, another major class of inhibitory neurons in the neocortex is the fast-spiking PV-expressing cells, known to play important roles in generating oscillations [25] and in promoting neuronal synchrony [12]. To test the effects of single-neuron stimulation on PV interneurons in vivo, we patched nearby pyramidal neurons and used the same stimulation protocol (800 pA, 0.1 s/step, 1 s inter-step interval) (Figure 4A and 4B) to measure spike transmission in the pyramidal-PV pairs (n = 20).
Figure 4. PV interneurons are less sensitive to single-pyramidal neuron stimulation.
(A) Schematic of experiment. (B) Two-photon image of the patched neuron (blue) near a PV interneuron expressing tdTomato (green), together with other cells labeled with OGB-1 (white) of a PV-Cre × tdTomato mouse. (C) OGB-1 fluorescence traces of the patched pyramidal neuron (blue) and PV interneuron (green) during stimulation trials (800 pA, 0.1 s/step, 1 s inter-step interval; gray bars, durations of current steps). (D) Trial-averaged dF/F were computed from 135 trials of stimulation or control (0 pA, 0.1 s/step, 1 s inter-step interval). Shading, 90% confidence intervals from bootstrap. (E) Trial-averaged dF/F during stimulation for the patched pyramidal neuron. The traces of (C), (D), and (E) are from the same cells that are shown in (B). Note that despite their proximity, there was no contamination of the PV trace by the stimulus-locked signal in the patched cell.
In contrast to the SOM interneurons, of which ~30% was driven by single-pyramidal neuron stimulation, none of the PV interneurons showed significant trial-averaged ΔdF/Fpost-pre. Although the PV interneurons had large fluctuations in their intracellular calcium concentrations during both control and stimulation periods (Figure 1C and 4C), these fluctuations were not time-locked to the current injection steps in the patched neuron. The trial-averaged fluorescence was similar between the stimulation and control (0 pA, 0.1 s/step, 1 s inter-step interval) conditions in both the mean and variance (Figure 4D). Note that in this particular example, the somas of the PV interneuron and the patched pyramidal neuron were immediately adjacent to each other (Figure 4B). The lack of stimulus-locked fluorescence change in the PV interneuron (Figure 4D) despite the large response in the neighboring pyramidal neuron (Figure 4E) testifies to the absence of signal contamination among the imaged neurons.
Note that the absence of PV interneuron responses to single-cell stimulation does not indicate a lack of synaptic connectivity, as spiking of a single layer 2/3 pyramidal neuron is known to induce synaptic responses in fast-spiking interneurons in vitro [12, 16]. Instead, this result shows that single-pyramidal-neuron stimulation exerts much less influence on the spiking of PV interneurons than of SOM interneurons and some of the pyramidal neurons. To further assess the influence of pyramidal neuron stimulation, we estimated the number of added APs in the PV and SOM interneurons due to each trial of single-pyramidal neuron stimulation by dividing the mean ΔdF/Fpost-pre during stimulation by the measured slope of dF/F as a function of AP number (Figure 1D and 1F, respectively). For the 20 PV interneurons imaged, the mean number of added APs per stimulation trial was 0.00±0.06 (SEM; Figure S4A). In contrast, for the 17 SOM interneurons tested, the mean was 0.25±0.13 AP/trial (SEM; Figure S4B), even though the fluorescence response to spikes was comparable between the SOM (0.0078 dF/F per spike) and the PV (0.0058 dF/F per spike) interneurons. This analysis indicates that even if single-pyramidal neuron stimulation caused spiking in the PV interneurons, it was much rarer than in the SOM interneurons. To directly assess the sensitivity of our method for detecting functional connections to each cell type, we also performed a bootstrap analysis based on the cell-attached recordings (Figure S4C). We found that for a reliable connection in which a presynaptic burst evokes a single postsynaptic AP in most of the trials, <200 stimulation trials are sufficient to detect the connection in all the pyramidal, SOM, and PV neurons we have recorded from. Given the mean number of stimulation trials in our experiment was 370 ± 61 (SD), the lack of detectable ΔdF/Fpost-pre for the PV interneurons suggests that single-cell stimulation led to <0.55 additional AP per trial. This analysis thus provided a quantitative upper bound for the spike burst-to-spike transmission from pyramidal to PV neurons.
Correlation with local network activity
Since the balance between excitatory and inhibitory activities is a prominent feature of the neocortical network [26, 27], we wondered how the calcium fluctuations of the inhibitory interneurons are related to the local network activity. To generate a proxy for the local network activity, we inferred (see Experimental Procedures) and summed the spikes from every cell within the field of view excluding the identified interneurons (Figure 5A). Interestingly, the active and quiescent periods of the network well matched the calcium fluctuations observed in the PV interneuron (Figure 5B).
Figure 5. Calcium transients of PV interneurons are highly correlated with the local network activity.
(A) OGB-1 fluorescence (black) from four example cells within the same field of view of a PV interneuron during single-pyramidal neuron stimulation. The spike rate of each cell was inferred (gray) using a fast non-negative deconvolution algorithm (see Supplemental Experimental Procedures). (B) Network activity (gray trace) was defined as the sum of inferred spikes of all cells within the field of view except the stimulated pyramidal neuron and the identified PV or SOM interneuron (n = 34 for the example shown, including the 4 cells in (A)). Light gray bars, durations of current steps. Green trace, time-lapse fluorescence of a PV interneuron within the same field of view. (C, D) Correlation coefficients between the fluorescence of PV (green, n = 17) or SOM (red, n = 20) interneurons and activity of the network (C) or the patched neuron (D), during either control or stimulation trials. Solid red dots, functionally connected SOM interneurons (classified based on ΔdF/Fpost-pre, see Experimental Procedures).
To quantify the influences of the stimulated single pyramidal neuron versus the network activity, we calculated their correlation coefficients with the fluorescence fluctuation of each interneuron during the control (I = 0) and stimulation (I = 800 pA) periods (Figure 5C and 5D). We found a clear dissociation between the SOM and PV interneurons. With network activity, the PV interneurons showed higher correlation than SOM interneurons during both control (p = 7 × 10−9, two-sample t-test) and stimulation (p = 1 × 10−8) periods (Figure 5C). The correlation was also higher for the PV interneurons than for the population of imaged cells as a whole (p = 3 × 10−9 for stimulation and 8 × 10−12 for control, two-sample t-test), the majority of which should be pyramidal neurons. With the stimulated single pyramidal neuron, on the other hand, the SOM interneurons showed higher correlation than the PV interneurons during stimulation (p = 0.01, all SOM; p = 5 × 10−4, connected SOM, two-sample t-test), while the PV interneurons correlated better during control (p = 2 × 10−4) (Figure 5D), presumably because the spontaneous rather than the evoked spiking of the patched neuron was correlated with the network activity. This result on spontaneous activity complements the previous finding that during visual stimulation, the broadly tuned responses of GABAergic interneurons may be approximated as the sum of the sharply tuned responses of neighboring excitatory neurons [16, 28]. The low correlation between SOM interneurons and network activity is consistent with the smaller slow membrane potential oscillations in SOM neurons than in excitatory and fast-spiking neurons [29]. Together, these results show that the PV rather than the SOM subtype of interneurons tracks the summed activity of the local cortical network.
Impact of single neuron burst on local circuit activity
Single-neuron stimulation in rodent neocortex can have a global impact in vivo [20–22], although the underlying circuit mechanisms are unknown. We assessed the circuit-level influence of single-neuron burst spiking by analyzing the entire population of imaged neurons. The 1189 imaged cells were ranked by the stimulation-induced fluorescence change, ΔdF/Fpost-pre. As described above, a small subset of the neurons consisting of SOM interneurons (red) and putative pyramidal neurons (black) had significant ΔdF/Fpost-pre, whereas the majority of the cells were insensitive to the single-neuron perturbation (PV, green; unidentified, gray) (Figure 6A). Interestingly, compared to the control (Figure 6B), the stimulation also caused larger negative ΔdF/Fpost-pre in some cells, thus extending both the positive and negative tails of the ΔdF/Fpost-pre distribution. However, the inhibitory effect was generally weaker than the excitatory effect and not statistically significant for any individual neuron based on our functional connection criterion. The overall rates of inferred spikes in all unidentified cells were not significantly different between the stimulation and control periods (Figure S5, p = 0.32, paired t-test), suggesting that the excitation of some neurons evoked by the bursts of the patched neuron was balanced by inhibition of other cells, presumably mediated by polysynaptic pathways involving inhibitory interneurons.
Figure 6. The influence of single-pyramidal neuron stimulation on the local cortical circuits.
(A) Trial-averaged ΔdF/Fpost-pre for all the cells imaged during stimulation trials. The cells were ranked by their (mean – 3 × SEM) value, which was used to identify functional connectivity (red, SOM interneuron; green, PV interneuron; black, connected, putative pyramidal neurons; gray, unconnected unidentified neurons). Error bar: ±3 × SEM. (B) The same set of cells ranked by their trial-averaged ΔdF/Fpost-pre during control trials.
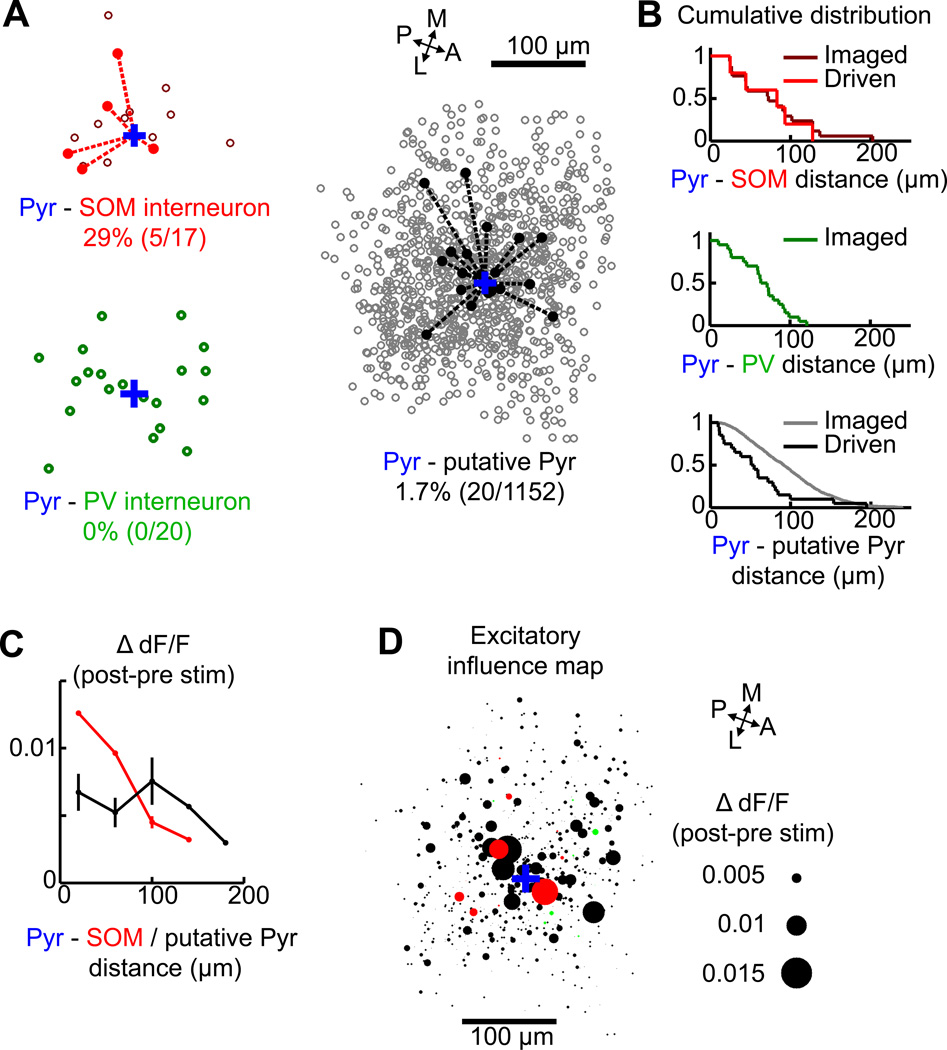
We constructed a spike-transmission map for each neuronal type based on the location of each imaged cell relative to the stimulated neuron (Figure 7A); solid symbols and dashed lines indicate neurons with significant trial-averaged calcium increases in response to the stimulation. The observed functional connections showed no clear anisotropy in the primary visual cortex (p = 0.51, SOM; p = 0.44, putative pyramidal neurons; Rayleigh test). For the SOM interneurons, the median distance from the stimulated pyramidal neuron to the driven SOM neuron was 83 µm (Figure 7B top plot, red line), comparable to the median distance for all the imaged SOM neurons (72 µm, dark red line). However, the response amplitude of the SOM interneurons decreased with the distance from the stimulated pyramidal neuron (Fig. 7C, red line; correlation coefficient = −0.96, p = 0.01). For the putative pyramidal neurons, the mean distance from the stimulated cell was 53 µm for the driven cells (Figure 7B bottom plot, black line), which is shorter than the median distance for all imaged pyramidal neurons 91 µm, gray), but the response amplitude showed no significant distance dependence (Fig. 7C, black line; correlation coefficient = −0.12, p = 0.60). To further visualize the spatial influence of single-neuron burst spiking on the local cortical circuits, we generated an ”excitatory map” using the diameter of each circle to represent the magnitude of ΔdF/Fpost-pre for all imaged neurons with positive responses (ΔdF/Fpost-pre > 0), regardless of whether the response of the cell was statistically significant (Figure 7D). Cells with large responses (ΔdF/Fpost-pre > 0.006) were all found within 100 µm of the patched cell.
Figure 7. Spatial maps of the functional connections.
(A) Spatial x–y distribution of imaged (open circles) and connected (filled circles and dotted lines) neurons with significant ΔdF/Fpost-pre for each cell type relative to the patched neuron (blue cross). A-P, anterior-posterior; M-L, medial-lateral. (B) Distributions of distance from the patched cell to each type of imaged and connected neurons. (C) Distance dependence of response amplitude for functionally connected SOM interneurons (red) and putative pyramidal neurons (black). (D) Excitatory influence map of the imaged neurons with ΔdF/Fpost-pre > 0 (red, SOM; green, PV; black, unidentified). Each circle represents a cell and the diameter represents the magnitude of ΔdF/Fpost-pre.
Discussion
Our results showed that spike transmission in cortical layer 2/3 can be measured with cellular resolution in vivo, therefore providing a new method for mapping functional connectivity. Using this approach, we provided a quantitative assessment of the effects of a spike burst in a single pyramidal neuron on the local cortical circuits. We found marked differences in the probability and temporal dynamics of the functional connections among different types of cortical neurons.
Driving cortical circuits with single-neuron stimulation
Single-cell stimulation in vivo is sufficient to evoke whisker movements [21], drive sensory perception [20], and modify the global brain state [22]. However, the immediate consequence of such stimulation within the local cortical circuits was poorly understood. In this study, we have found that driving a single pyramidal neuron causes spiking activity in a subset of other cortical neurons within ~100 µm of the stimulated cell. In layers 2/3 of the mouse primary visual cortex, there are ~ 15 × 104 neurons per mm3 [30], of which ~ 80% are excitatory neurons. Among the inhibitory neurons in this cortical region, 8 to 24% are SOM interneurons [31, 32]. Based on these cell density estimates and the probability of functional connectivity measured in our study (Figure 7A), we estimated that within 100 µm of the stimulated cell, there are ~ 600 neurons, of which the single-neuron burst spiking recruits ~14 pyramidal neurons and 3 – 9 SOM interneurons. The actual number of activated neurons may be larger, because a stringent statistical criterion was used in this study to test for functional connectivity (see Experimental Procedures; Figure 6 and S4C), and not all SOM interneurons express GFP in the transgenic GIN mice [24].
Although in principle the activation of some of the cortical neurons could be mediated by polysynaptic pathways rather than direct monosynaptic connections, we believe that the polysynaptic effects are unlikely to have a major contribution. Because the number of activated pyramidal neurons is quite small, and for each of these activated neurons the additional spiking caused by the stimulation is much less than the high-frequency bursts evoked in the patched neuron, the number of polysynaptic, second-order spikes should be much smaller than the number of first-order spikes in the monosynaptically connected cells. For the activated SOM interneurons, their response showed longer latencies (Figure 3C). Although it is possible that the cessation of SOM interneuron activity could evoke rebound spiking in pyramidal neurons [33], we have not observed such long-latency responses due to single-cell stimulation. Spiking of the SOM interneurons, however, may inhibit PV interneurons and contribute to their lack of response to the spike bursts of the patched pyramidal neuron.
The axons of SOM interneurons arborize extensively in layer 1, which contains long-range intracortical connections. The high pyramidal-SOM functional connectivity observed in this study may thus play important roles in the dynamic coupling between cortical areas and in modifying the global brain state in response to single neuron burst spiking [22]. Note that in this study, the mouse cortex was in stable UP/DOWN (slow-wave oscillation) state throughout each experiment, and we did not observe any brain state switch following single-pyramidal neuron stimulation (based on EEG recorded in frontal brain areas, data not shown). In addition to differences in species and stimulation protocol, the most probable reason is the use of chlorprothixene during anesthesia (see Experimental Procedures), which enhanced the stability of recording [34] but minimized the occurrence of persistent-UP (desynchronized) brain state.
Spike transmission among excitatory and inhibitory neurons
Several features of the spike transmission patterns that we have observed in vivo are consistent with the known dynamic properties of the synapses. For the PV interneurons, we found no detectable response to single pyramidal neuron stimulation but a high correlation with the local network activity. Although spiking of a single layer 2/3 pyramidal neuron can induce a large excitatory postsynaptic potential (EPSP) in fast-spiking interneurons [12], this synapse is strongly depressing, so the EPSP amplitudes in a high-frequency train become progressively smaller [15]. Therefore, one would expect synchronous spiking from multiple neurons to be more effective than sequential spikes from the same neuron in driving the PV interneuron. Indeed, a recent study showed that synchronous spiking in a group of ~100 layer 2/3 excitatory neurons can effectively drive nearby fast-spiking cells in vivo [35]. Functionally, our finding supports the notion that PV interneurons closely track and balance the overall level of excitation in the local cortical network [26, 27]. It is also possible that some of the correlated activity between PV interneurons and the local network results from common inputs, for example from layer 5 where cortical slow-wave oscillations originate [36].
The SOM Martinotti interneurons receive facilitating excitatory inputs from the pyramidal neurons [15, 29]. Such facilitation can greatly enhance the EPSPs evoked by consecutive spikes of the same presynaptic neuron [13, 14], which could explain why the SOM interneurons were reliably driven by single-neuron spike bursts in vivo. The delayed activation and the threshold of ~ 5 APs observed for the pyramidal-SOM connections (Figure 3C and 3D) are also consistent with synaptic facilitation. Thus, together with the data from PV interneurons, our results support distinct mechanisms for activating the two inhibitory circuits: whereas the SOM interneurons are integrators of single-pyramidal neuron burst spiking, the PV interneurons are coincidence detectors of local network activity [4, 37]. This functional dichotomy may be a critical factor governing how spikes from cortical pyramidal neurons are selectively routed to different inhibitory circuits in vivo.
A small subset of putative pyramidal neurons was also activated by single-pyramidal neuron stimulation. The activation of these neurons was somewhat surprising given that most of the connections between pyramidal neurons are quite weak [9]. However, the neocortex in vivo operates in a high background activity regime that could boost spike transmission. Moreover, although most pyramidal-pyramidal connections are weak, previous studies in slices have demonstrated rare connections with unusually large EPSPs [9, 10]. The spike transmission pathways we have observed in vivo may largely reflect these rare connections, possibly representing the essential building blocks for the fine-scale excitatory subnetworks in the neocortex [38].
Mapping functional connectivity in vivo
Our results have demonstrated the feasibility of mapping cell-type specific spike transmission in vivo by advancing an optical probing technique previously used in vitro [39]. Such functional connectivity maps depend on a combination of anatomical connectivity, functional synaptic properties, and the context of network activity. Compared to cross-correlation analysis of extracellularly recorded spike trains, which has been used to reveal putative monosynaptic connections in vivo [8, 40], our technique allows unequivocal cell type identification and a direct test of the causal relationship between each neuronal pair. Compared to high-resolution wiring diagram reconstruction [1, 2], our approach enables the search for not only the rules governing functional connections but also their modulation by experience and behavior.
Experimental Procedures
C57BL/6, GIN, PV-Cre × tdTomato, or CaMKIIα × tdTomato mice (postnatal day (P)60–120) were anaesthetized with urethane (0.75–1.25 g/kg) and chlorprothixene (5 mg/kg). A 1.5 mm-diameter craniotomy was made above the primary visual cortex for in vivo two-photon imaging. Layer 2/3 cortical neurons were labelled with Oregon Green 488 BAPTA-1 AM (OGB-1) via bolus loading. In transgenic mice, somatostatin (SOM) or parvalbumin (PV) interneurons were identified using different combinations of excitation and emission wavelengths. Under visual guidance, we targeted a neuron (150 – 300 µm deep) near an OGB-1-loaded interneuron with a patch electrode containing internal solution and Alex 594. Whole-cell recording and stimulation were done in current-clamp mode. During a stimulation episode (~2.5 min), 135 current steps (800 pA for 100 ms/step, 1 s inter-step interval) were injected, while intracellular membrane potential and 2400 time-lapse image frames of OGB-1 fluorescence were simultaneously acquired. During a control episode, the same protocol was used but no current was injected. Control and stimulation episodes were alternated. Fractional changes in fluorescence (dF/F) were trial-averaged by aligning to the onsets of the current steps. The effect of single-neuron stimulation on neighbouring neurons was quantified by calculating ΔdF/Fpost-pre, the difference in mean dF/F within 200 ms windows before and after stimulation. A cell was identified as connected when mean ΔdF/Fpost-pre > 3 × s.e.m.
Detailed procedures are included in Supplemental Experimental Procedures.
Supplementary Material
Highlights.
-
-
Functional connectivity between cortical neurons was mapped in vivo
-
-
Single-neuron burst spiking drives subset of SOM and pyramidal neurons to fire
-
-
PV interneurons are less sensitive to single-pyramidal neuron stimulation
-
-
Activities of PV interneurons and the local network are highly correlated
Acknowledgements
We thank Wenzhi Sun and Chengyu Li for help with patch clamping; Joshua Vogelstein for the spike inference code; and Seunghee Lee and Henry Alitto for discussions. This work was supported by a Croucher Foundation Fellowship to A.C.K., NIH R01 EY018861 and NSF 22250400-42533 to Y.D..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental Information includes Supplemental Experimental Procedures, and five figures.
References
- 1.Bock DD, Lee W-CA, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, Reid RC. Network anatomy and in vivo physiology of visual cortical neurons. Nature. 2011;471:177–182. doi: 10.1038/nature09802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818. [DOI] [PubMed] [Google Scholar]
- 3.Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann KK, Young JAT, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- 5.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 6.Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- 8.Fujisawa S, Amarasingham A, Harrison MT, Buzsaki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nature Neuroscience. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefort S, Tomm C, Sarria JCF, Petersen CCH. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61:301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Song S, Sjostrom PJ, Reigl M, Nelson S, Chklovskii DB. Highly nonrandom features of synaptic connectivity in local cortical circuits. Plos Biology. 2005;3:507–519. doi: 10.1371/journal.pbio.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473:87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001;292:2295–2299. doi: 10.1126/science.1061395. [DOI] [PubMed] [Google Scholar]
- 13.Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nature Neuroscience. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nature Neuroscience. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- 16.Hofer SB, Ko H, Pichler B, Vogelstein J, Ros H, Zeng HK, Lein E, Lesica NA, Mrsic-Flogel TD. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nature Neuroscience. 2011;14:1045–1052. doi: 10.1038/nn.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haider B, McCormick DA. Rapid neocortical dynamics: cellular and network mechanisms. Neuron. 2009;62:171–189. doi: 10.1016/j.neuron.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Kock CPJ, Sakmann B. High frequency action potential bursts (>= 100 Hz) in L2/3 and L5B thick tufted neurons in anaesthetized and awake rat primary somatosensory cortex. Journal of Physiology-London. 2008;586:3353–3364. doi: 10.1113/jphysiol.2008.155580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisman JE. Bursts as a unit of neural information: Making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- 20.Houweling AR, Brecht M. Behavioural report of single neuron stimulation in somatosensory cortex. Nature. 2008;451:65–68. doi: 10.1038/nature06447. [DOI] [PubMed] [Google Scholar]
- 21.Brecht M, Schneider M, Sakmann B, Margrie TW. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004;427:704–710. doi: 10.1038/nature02266. [DOI] [PubMed] [Google Scholar]
- 22.Li CYT, Poo MM, Dan Y. Burst spiking of a single cortical neuron modifies global brain state. Science. 2009;324:643–646. doi: 10.1126/science.1169957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 24.Ma YY, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J. Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J. Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O/'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 2010;67:858–871. doi: 10.1016/j.neuron.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CCH. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nature Neuroscience. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- 30.Schuz A, Palm G. Density of neurons and synapses in the cerebral cortex of the mouse. J. Comp. Neurol. 1989;286:442–455. doi: 10.1002/cne.902860404. [DOI] [PubMed] [Google Scholar]
- 31.Xu XM, Roby KD, Callaway EM. Immunochemical Characterization of Inhibitory Mouse Cortical Neurons: Three Chemically Distinct Classes of Inhibitory Cells. J. Comp. Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonchar Y, Wang Q, Burkhalter AH. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Frontiers in Neuroanatomy. 2007;2 doi: 10.3389/neuro.05.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berger TK, Silberberg G, Perin R, Markram H. Brief bursts self-inhibit and correlate the pyramidal network. Plos Biology. 2010;8 doi: 10.1371/journal.pbio.1000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J. Neurosci. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mateo C, Avermann M, Gentet LJ, Zhang F, Deisseroth K, Petersen CCH. In Vivo Optogenetic Stimulation of Neocortical Excitatory Neurons Drives Brain-State-Dependent Inhibition. Curr. Biol. 2011;21:1593–1602. doi: 10.1016/j.cub.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature Neuroscience. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 37.Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura Y, Dantzker JLM, Callaway EM. Excitatory cortical neurons form fine-scale functional networks. Nature. 2005;433:868–873. doi: 10.1038/nature03252. [DOI] [PubMed] [Google Scholar]
- 39.Peterlin ZA, Kozloski J, Mao BQ, Tsiola A, Yuste R. Optical probing of neuronal circuits with calcium indicators. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3619–3624. doi: 10.1073/pnas.97.7.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.London M, Roth A, Beeren L, Hausser M, Latham PE. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature. 2010;466:123–127. doi: 10.1038/nature09086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.