Abstract
Treatment of localized aggressive periodontitis (LAP) may include systemic antibiotics, yet it is unclear at what stage of treatment planning antibiotics are most effective.
Aim
This retrospective analysis compared immediate vs. delayed antibiotic therapy on clinical parameters and gingival crevicular fluid (GCF) inflammatory mediators.
Material and Methods
At baseline, 3 and 6 months after treatment, clinical parameters [probing depth (PD), attachment level (CAL), bleeding on probing (BoP), and plaque] and GCF were collected from LAP participants, who received a 7-day antibiotic regimen immediately (ImA) or 3 months following (DelA) mechanical therapy.
Results
While both groups presented significant CAL reductions at 6 months, only ImA resulted in a reduction in mean PD at both 3 and 6 months, along with reductions in CAL and BoP at 3 months following therapy. In addition, GCF mediators were higher in DelA group at 3 months post-mechanical treatment, but were significantly reduced 6 months following antibiotic therapy.
Conclusions
ImA and DelA regimens were both effective in improving CAL by 6 months post-therapy. However, ImA allowed for better improvement in overall clinical parameters early in the course of treatment, concomitant with lower levels of inflammatory mediators within the GCF.
Keywords: systemic antibiotics, timing, aggressive periodontitis, inflammatory mediators, therapy
Introduction
Mechanical removal of bacteria by scaling and root planning (SRP) is the gold standard of periodontal therapy as it often provides satisfactory response for most cases of chronic periodontitis (Badersten et al., 1981a, Badersten et al., 1984a, Cobb, 1996, Kaldahl et al., 1988, Rosenberg et al., 1993). However, certain periodontal conditions, such as aggressive periodontitis, may benefit from adjunctive use of systemic antibiotics (Slots, 2004, Haffajee et al., 2006, Haffajee, 2006, Haffajee et al., 2003, Heitz-Mayfield, 2009, Herrera et al., 2008). Aggressive periodontitis patients often benefit more from the use of adjunctive systemic antibiotics when compared to SRP alone (Haffajee et al., 2003, Guerrero et al., 2005, Mestnik et al., 2010, Slots and Ting, 2002, Sgolastra et al., 2011, Aimetti et al., 2012). It has been suggested this is a result of elevated proportions of Aggregatibacter actinomycetemcomitans (Aa) present in this disease (Zambon et al., 1983, Slots et al., 1980, Mandell and Socransky, 1981). Because Aa is located at all mucosal surfaces in the oral cavity (Mombelli et al., 1994) and is able to invade soft tissues (Blix et al., 1992, Christersson et al., 1985, Sreenivasan et al., 1993) and extra oral sites (Rahamat-Langendoen et al., 2011, Castillo et al., 2011), it may recolonize the pocket rapidly after mechanical root debridement (van Winkelhoff et al., 1989, van Winkelhoff et al., 1992b, Slots and Rosling, 1983), making adjunctive antibiotic therapy a necessity to remove this organism from the periodontal lesion.
The combination of metronidazole and amoxicillin has been shown to be effective in preventing Aa re-colonization following mechanical therapy (Winkel et al., 1998, Winkel et al., 2001, Pavicić et al., 1994), and in the treatment of aggressive periodontitis patients (Xajigeorgiou et al., 2006, Guerrero et al., 2005, Buchmann et al., 2002, Kaner et al., 2007, Machtei and Younis, 2008, Sgolastra et al., 2011, Aimetti et al., 2012). Similarly, it has been demonstrated that amoxicillin and metronidazole administered immediately after initial SRP results in greater pocket depth reduction and attachment level gain than later administration during supportive periodontal therapy for generalized aggressive periodontitis (Kaner et al., 2007, Griffiths et al., 2011).
Despite numerous research studies on the use of antibiotics during periodontal therapy, it is still unclear at what point antibiotics should be prescribed during therapy for patients with aggressive periodontitis (Shaddox and Walker, 2009), particularly in localized disease, given its low prevalence. In addition, due to its rapid progressive nature, timing of antibiotic application becomes an essential part of clinical care for this disease. Therefore, the purpose of this retrospective analysis was to compare the clinical and inflammatory response of a systemic antibiotic regimen prescribed either immediately or 3 months following mechanical therapy in the treatment of localized aggressive periodontitis.
Materials and Methods
Participant Cohort
This was a retrospective analysis of a data subset from an ongoing clinical trial (Clinical Trial registration: #NCT01330719 at clinicaltrials.gov). While the main objective of the registered clinical trial is not to evaluate timing of antibiotic administration during therapy, differences in treatment responses were noted in the first subset of patients treated: the first 15 patients enrolled received antibiotics at the first follow up appointment, 3 months after mechanical therapy, while the following 17 patients enrolled received antibiotics immediately after mechanical therapy, at the first visit. Thus, the retrospective analysis of antibiotic administration timing between these patients is presented here. All data and samples collected were obtained under Institutional Review Board (IRB) informed consent (provided by the parents, in case of minors) at the University of Florida, Gainesville, FL. Inclusion criteria: 7–21 years old, African-American, diagnosed with localized aggressive periodontitis (LAP), defined by ≥2 teeth (incisor and or first molar, and no more than two teeth other than first molars and incisors) (Armitage, 1999) with pocket depth (PD) ≥5mm with bleeding on probing (BoP), clinical attachment level (CAL) ≥2mm and radiographic bone loss. Exclusion criteria: systemic diseases or conditions that influence the progression and/or clinical characteristics of periodontal disease; antibiotics within past 3 months; allergy to penicillin; use of medications that could influence the characteristics of periodontal disease; smokers or pregnant/lactating women. Complete medical and dental histories along with periodontal clinical parameters were collected including: PD, CAL, BoP, visible plaque, and radiographic examination of the compromised teeth. CAL was calculated from the cemento-enamel junction (CEJ) to the base of the pocket. If CEJ was subgingival, gingival margin was recorded as a negative value. If a restoration was present apical to the CEJ, CAL was calculated from the restoration margin to the bottom of the pocket. All measurements were performed by a calibrated examiner (IM) using a periodontal probe (UNC-15, Hu-Friedy, Chicago, IL) at six sites per tooth and recorded using a computer software program (Florida Probe, version 9.12.26, Gainesville, Fl). Calibration of examiner was obtained prior to the study when 80% of full-mouth duplicate measures of probing depth and CAL were within 1mm in 10 patients with moderate-severe chronic periodontitis.
Periodontal Treatment
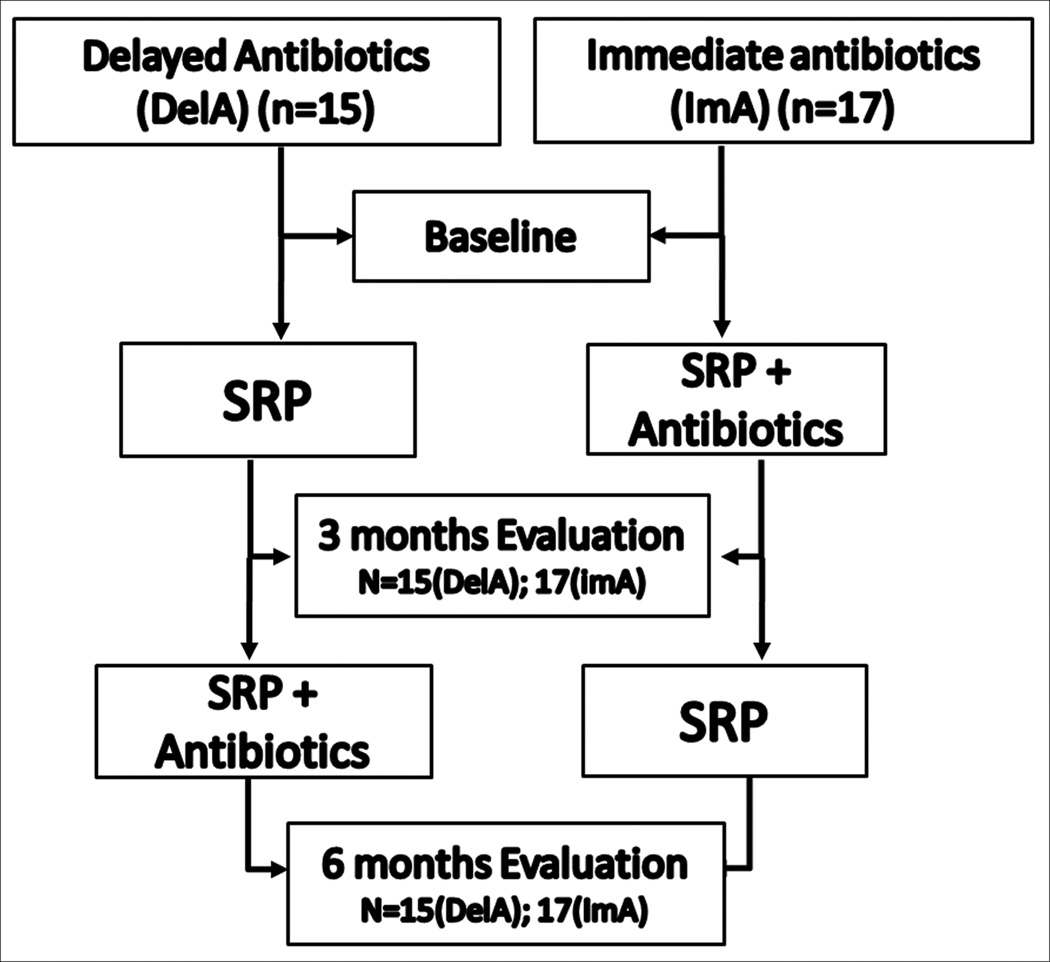
Treatment was provided by two experienced periodontists (IA or LMS), different than the examiner (IM). Both clinicians were trained to use the same instruments and sequence. Both treated a similar number of patients in each group and used their own clinical judgment regarding time of instrumentation necessary for each case. Although the examiner did not know the details of the course of treatment of each subject evaluated, no intentional masking of the treatments was performed since this was not the initial intent of this study. At the initial visit, 3 and 6 month follow-up appointments, all participants received full mouth debridement with an ultrasonic device (Cavitron Jet Plus, Dentsply, York, PA) and scaling and root planing (SRP) of deeper sites under anesthesia, as required. Oral hygiene instructions (OHI) and tools, including an electric toothbrush (Vitality, Oral B, Belmont, CA), were also provided. Participants were placed on a systemic antibiotic regimen of 500mg of amoxicillin and 250mg of metronidazole three times per day (t.i.d.) for 7 days. Participants and their parents were provided verbal and written instructions on medication administration, side effects and a medication log. Compliance was monitored by the return of remaining medication at each visit. The first 15 patients were started on the antibiotic regimen at the 3 month follow-up appointment (DelA), while the following 17 patients began the antibiotic regimen immediately after the initial mechanical therapy, at the first visit (ImA) (Fig. 1).
Figure 1. Flowchart of Treatment Approaches.
At the initial visit and the 3 and 6 months follow-up appointments, participants received full mouth debridement and SRP of deeper sites under anesthesia, Oral hygiene instructions (OHI) and tools were provided at all treatment visits. Participants were placed on a systemic antibiotic regimen of 500mg of amoxicillin and 250mg of metronidazole t.i.d for 7 days. 17 patients began the antibiotic regimen immediately following the initial mechanical therapy (ImA), while 15 patients were started on the antibiotic regimen at the 3 month follow-up appointment (DelA).
Gingival Crevicular Fluid (GCF) Sampling
GCF samples were collected from a periodontally diseased site [PD≥5mm and CAL≥2mm with BoP] at baseline, 3 and 6 months following mechanical therapy. The site for GCF collection was isolated with cotton rolls, gently air-dried and supragingival plaque was removed. A collection strip (PerioPaper GCF collection strips, Oraflow Inc, Plainview, NY) was inserted 1–2mm into the pocket for ~10sec. The volume of GCF was measured (Periotron 8000, Oraflow Inc, Plainview, NY) in order to obtain a specific range of protein content compatible with soluble mediator analysis (data not shown). Samples were eluted from the strip into 300µl of phosphate buffered saline (PBS) by centrifugation and stored at −80°C until the assays were performed.
Inflammatory Mediator Analysis
Fluorescence detection kits (Milliplex® 14-plex cyto/chemokine detection kits, Millipore, St. Charles, MO) were used to detect and quantify 14 cyto/chemokines [eotaxin, interleukin(IL)-1-beta (IL1β), IL2, IL6, IL7, IL8, IL10, IL12(p40), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFNγ), monocyte chemotactic protein-1 (MCP1), macrophage inflammatory protein 1 alpha (MIP1α), and tumor necrosis factor alpha (TNFα)]. Fifty µl of eluates and 25µl of a cyto/chemokine capture bead cocktail were placed in a 96-well filter plate and incubated overnight at 4°C. After which, 25µl of a biotin labeled anti-cytokine cocktail and 75µl of assay buffer were incubated for 1.5hrs. This was followed by incubation with 25µl of SAV-PE for 30mins and 25µl of stop reagent for 5min. Incubations were performed in the dark while shaking gently. Data were acquired using instrumentation (Luminex®100™, Millipore, St. Charles, MO) and analyzed using software (Milliplex Analyst®, Viagene Tech, Carlisle, MA), standard curves and five-parameter logistics. Values were normalized to total protein content (BCA Protein Assay, Pierce Thermo Scientific, Rockford, IL).
Statistical analysis
Means were calculated for all clinical parameters for both groups at each time point. CAL was considered the primary outcome variable and PD was considered the secondary variable. Within group differences were evaluated by One–way ANOVA with Tukey’s multiple comparison test. Between group differences were evaluated by unpaired T-test or Mann-Whitney tests. Differences between groups after antibiotics administration were also compared (3 months after antibiotic administration). P-value for soluble mediators analysis was adjusted with Bonferroni’s correction (p<0.004). Due to its retrospective nature, a sample size was not calculated for this study. However, pos-hoc power analysis performed indicated that the group numbers presented here (n=15 and n=17) provide a power of 85% to detect differences in CAL/PD of 1mm between groups for affected sites (PD>4mm with BoP and CAL>2mm) with a standard deviation of up to 0.9mm.
Results
All participants were recruited from Leon County Health Department, Tallahassee, Florida, from February 2007 to November 2009. Following the collection of baseline clinical parameters, thirty-two participants diagnosed with local aggressive periodontal disease (LAP), underwent conventional mechanical therapy. Cohort demographics are summarized in Table 1. All participants completed the antibiotic regimen (ImA (n=17) and DelA (N=15)). A total of four patients (n=2/group) received a modified dosage of amoxicillin (250mg chewable tablets, tid, 7 days) according to their weight. All participants were compliant with medications and follow-up appointments. No side effects were reported by any of the patients after the antibiotic regimen. In addition, there were no differences in the clinical parameters collected at baseline when comparing the ImA and DelA treatment groups (Table 2). In both treatment groups, all clinical parameters were collected 3 and 6 months following initial mechanical therapy.
Table 1.
Cohort Demographics.
| DelA (n=15) | ImA (n=17) | p value* | |
|---|---|---|---|
| Age (years) | 14.13 ± 1.04 | 13.71 ± 1.10 | 0.7822 |
| Females (%) | 80 | 64.7 | 0.353 |
| African-Americans (%) | 100 | 100 | 1 |
Mean age values followed by standard deviation. DelA=delayed antibiotics (administered at 3 months post SRP-baseline); ImA=immediate antibiotics (administered concomitantly with SRP at baseline).
denotes differences between groups by T-test and Mann-Whitney.
Table 2.
Changes in Pocket Depth and Clinical Attachment Level.
| Parameters | Groups | baseline | 3 months | 6 months |
|---|---|---|---|---|
| PD | Del A | 5.85±0.15 | 5.38±1.50 | 4.73±2.24 |
| ImA | 5.67± 0.16 | 4.30±1.31*# | 3.81±1.18*# | |
| %(N) PD>4mm | Del A | 12.47±2.10 (19.7±11.7) |
10.27±1.96 (16.8±13.1) |
4.67±0.99* (7.8±6.6) |
| ImA | 12.88±2.48 (19.5±15.1) |
6.56±1.62*# (11.1±11.7) |
5.47±1.66* (9.4±11.1) |
|
| CAL | Del A | 3.64±0.26 | 3.06±0.35 | 1.94±0.38* |
| ImA | 3.91±0.39 | 1.97±0.34*# | 1.83±0.37* | |
| %(N) CAL>2mm | Del A | 9.14±1.56 (14.5±8.9) |
7.12±1.18 (12.0±8.1) |
2.69±0.69* (4.5±4.8) |
| ImA | 10.71±2.30 (15.9±13.6) |
3.88±1.06* (6.5±7.4) |
3.66±1.42* (6.2±9.2) |
|
| BoP | Del A | 13.68±2.21 | 10.14±1.92 | 11.40±1.91 |
| ImA | 20.12±2.59 | 10.65±1.40* | 9.67±1.92* | |
| Plaque | Del A | 58.86±3.89 | 41.55±6.00*# | 21.65±3.06* |
| ImA | 51.65±7.03 | 26.44±4.37* | 27.32±4.71* |
Mean values followed by standard deviation. DelA=delayed antibiotics (administered at 3 months post SRP-baseline); ImA=immediate antibiotics (administered concomitantly with SRP at baseline). PD= pocket depth for diseased sites (PD>4mm+BoP+CAL≥2mm), CAL=clinical attachment level for diseased sites, %(N)= percent of sites (number of sites); BoP=bleeding on probing.
p<0.05 from baseline by ANOVA with Tuckey’s multiple comparisons;
p<0.05 between groups by unpaired T-test and Mann-Whitney test.
While the ImA group showed significant reductions in mean PD at 3 and 6 months post-therapy, (1.37 ±1.06 and 1.86 ± 0.93 respectively; p<0.0001), no significant reduction in mean PD at either 3 or 6 months (0.44± 0.49mm and 0.65 ± 1.80mm respectively; p>0.05) was observed in the DelA group (Table 2). In addition, the percentage of sites with a PD >4mm was reduced in both ImA and DelA groups 6 months post-therapy, although a significant reduction in PD 3 months following treatment was observed only in the ImA group (Table 2). Accordingly, the ImA regimen resulted in greater PD reduction at both 3 and 6 months post-therapy when compared to the DelA antibiotic regimen (Table 2). Similarly, while a reduction in mean CAL was observed at both 3 and 6 months following therapy in the ImA group (2.10 ± 0.8mm and 2.08 ± 1.83mm, respectively, p=0.0002) a significant reduction was observed only at 6 months in the DelA group (1.70±1.40mm, p=0.0030) (Table 2). A decrease in the percentage of sites with CAL>2mm at both 3 and 6 months was also observed in the ImA group, while this decrease was observed only at 6 months in the DelA group (Table 2). The ImA regimen resulted in greater CAL reduction at 3 months when compared to the DelA antibiotic regimen, while equivalent CAL reductions were observed at 6 months, regardless of antibiotic regimen (Table 2). Interestingly, when comparing PD reductions and CAL gain three months post-antibiotic therapy (3 months after antibiotics in DelA vs 3 months after antibiotics in ImA group), no differences were observed between groups (p>0.05). This indicated the antibiotic regimen was effective in reducing clinical parameters of disease once applied, regardless of when in the treatment course this occurred.
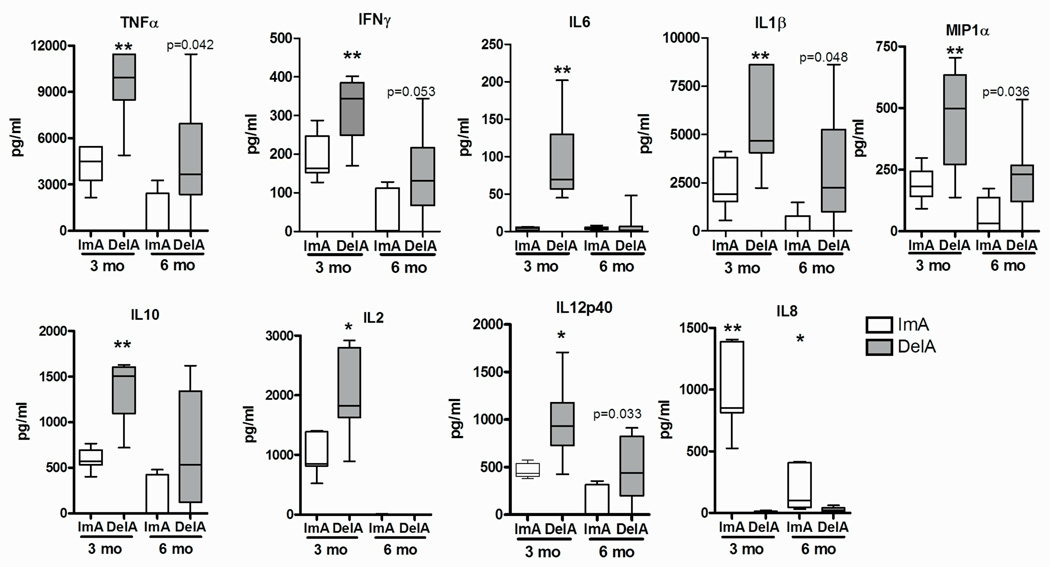
While plaque index was decreased 3 and 6 months post-therapy in both treatment groups (Table 2), only ImA group showed a significant decrease in the percentage of sites with BoP at 3 and 6 months post-therapy (Table 2). Although BoP has been utilized as a clinical marker of inflammation, the levels of inflammatory mediators in the GCF were quantified to more specifically evaluate the affect of the ImA vs. DelA regimens on local inflammation. While baseline values for all inflammatory markers were similar for both groups (data not shown, p>0.05) and both regimens resulted in reductions of most inflammatory markers at 3 months and 6 months following therapy, a more significant reduction in the pro-inflammatory mediators TNFα, IFNγ, IL6, IL1α, MIP1α, IL10, IL2, and IL12(p40) was observed in GCF from the ImA treatment group at 3 months. While a more pronounced reduction in the expression of soluble mediators within the ImA group remained at 6 months, only a trend of significance was observed in between the two regimens. Interestingly, IL8 was elevated in the ImA group compared to DelA group at both time points (Fig. 2). While IL7 and MCP1 were not detected, no additional significance was observed for the remaining markers.
Figure 2. ImA antibiotic regimen more rapidly and robustly decreases GCF inflammatory mediators.
GCF collected 3 and 6 months following initial mechanical therapy in participants whom received an antibiotic regimen immediately following initial therapy (ImA) or at the 3 month follow-up treatment visit (DelA) were qualitatively and quantitatively analyzed for 14-cytokine and chemokines. **p<0.001 and *p<0.004 between groups. p values >0.05 trending towards significance are indicated
Discussion
The present study shows that administration of an antibiotic regimen either immediately following mechanical therapy or 3 months after initial mechanical treatment resulted in reductions in pocket depth (PD) and clinical attachment (CAL) levels 6 months post-intial therapy. Moreover, the use of antibiotics immediately after mechanical therapy provided not only a more rapid decrease in PD, but also a more robust reduction in this clinical parameter. Interestingly, the delayed implementation of the antibiotic regimen still allowed for significant and equivalent reduction in CAL and inflammation levels (BoP and GCF markers) by 6 months post-initial therapy. When comparing clinical parameters 3 months following antibiotic treatment, no difference could be observed between groups, indicating that the antibiotic regimen is effective in reducing clinical parameters of disease once it is applied. The difference here lies in the time it takes for the major benefits to occur (total duration of treatment). The early prescription of antibiotics seems to result in a more rapid achievement and maintenance of the major clinical benefits. This is highlighted by the improvement in clinical parameters observed 3 months following immediate antibiotic intake. It is important to note the 3 month timepoint comparison is evaluating the effects of antibiotics versus non-antibiotics, since the delayed group (DelA) had yet to receive the antibiotic regimen. The favorable results with the use of antibiotics at this time point for the immediate antibiotic group (ImA) compared to the DelA group has also been reported by other investigators, using the same antibiotic combination (Sgolastra et al., 2011, Mestnik et al., 2010, Aimetti et al., 2012).
A previous study (Kaner et al., 2007) also observed advantages of immediate administration of the same regimen of antibiotics used in the current study, although for a slightly longer period of time, 10 days compared to 7 days used in the present study. Interestingly, these investigators also observed improved short-term (3 months) PD and CAL responses in the immediate antibiotic group. Furthermore, they reported additional benefits after delayed administration of antibiotics, which is also in agreement with the present findings. Although in both these studies, the two antibiotic regimens provided similar end results at 6 months, immediate antibiotic usage provided beneficial clinical responses earlier in the treatment, which persisted at 6 months. Since aggressive periodontitis is a relatively fast progressing disease, characterized by a hyper-inflammatory response (Shaddox et al., 2010), earlier reduction of bacterial insult and inflammatory processes may be beneficial to hamper the disease progression.
A possible explanation for the greater benefits of the immediate application of antibiotics in aggressive periodontitis could be the fact that the combination of amoxicillin and metronidazole may provide immediate reduction of putative periodontopathogens such as A.actinomycetemcomitans from within tissues, whereas SRP may have limited ability to do so, as reported in previous studies (Xajigeorgiou et al., 2006, van Winkelhoff et al., 1992a, Renvert et al., 1990). This inability to remove important bacteria from within tissues could possibly enable these “invading” organisms to re-colonize the pocket and maintain a continuous insult to the host. In addition, antibiotics will also help control re-colonization of the lesion with commensal/non-pathogens which under normal circumstances would not induce robust immune response, but could potentially aggravate an ongoing pathogenic inflammatory response. Antibiotics allow for not only clearance of pathogens, but also provide time for the host’s inflammatory recovery before bacterial re-colonization occurs. Persistent bleeding and higher levels of inflammatory markers after SRP presented by the delayed antibiotic group in the present study could have been a result of this continuous “induction” of the host’s inflammatory process by the present bacteria. Once antibiotics were applied in the immediate group, an initial significant reduction of clinical parameters and inflammatory markers was observed and maintained for 6 months. On the other hand, the delayed group only obtained mild reductions in inflammatory markers and clinical parameters initially after SRP, but once antibiotics were administered, additional reductions were observed. One exception was IL8, which was increased in the ImA group at both time points. This chemokine is known to induce chemotaxis for innate immune cells, i.e. neutrophils. In addition, IL8 also plays an important role in the process of wound healing, which could explain its higher levels in the early antibiotic usage group. This early and more robust reduction in pro-inflammatory response, coupled with an elevated anti-inflammatory response, could have contributed to early improvement in clinical parameters observed in the immediate antibiotic group. Similar results in local inflammatory markers after treatment with antibiotics have been reported previously (de Lima Oliveira et al., 2012). Thus, the immediate application of antibiotics may be more beneficial for the treatment of this type of aggressive inflammatory process.
Different antibiotic regimens have been studied in periodontal disease therapy, including aggressive periodontitis (Haffajee et al., 2003). There seems to be no consensus on which antibiotic or specific dosages is most effective. The current study employed the combination of amoxicillin 500mg and metronidazole 250mg because it had been studied previously, where effective results in the treatment of both chronic and aggressive periodontitis were observed (Winkel et al., 2001, van Winkelhoff et al., 1992b, van Winkelhoff et al., 1989, Kaner et al., 2007, Aimetti et al., 2012). Since the cohort of the current study consisted of young participants, the lower dose of metronidazole (250mg) was utilized, where no side effects have been reported as of yet. This antibiotic choice was also effective in reducing mean PD at 3 and 6 months post-therapy in the ImA group (1.37 ±1.06 and 1.86 ± 0.93, respectively), which is similar to what has been reported by Kaner et al 2007 (mean PD reduction of 1.85 and 1.95 at 3 and 6 months, respectively), using a similar antibiotic regimen. The magnitude of gain in the current study was slightly smaller compared to that reported by Kaner et al. This could be attributed to the slightly longer antibiotic regimen used by Kaner et al (10 days versus 7 days on the present study) along with slightly different diagnosis and cohort age (generalized aggressive and mean age 34 versus localized and mean age 13 in the present study).
Some limitations are associated with the present study. For instance, this was a retrospective evaluation and not a randomized trial. While retrospective analyses are helpful in understanding treatment effects, randomized controlled trials need to be conducted to confirm the benefits of immediate antibiotic administration. The present study was conducted within the same clinic and within a homogeneous population with very similar diseased characteristics. In addition, examinations and treatments of both groups were performed by the same group of calibrated investigators, which is clearly an advantage on the conduct of a retrospective study. Although both therapists were experienced and calibrated to the treatments and both treated patients in both experimental groups, one may argue that their individual clinical judgment used to determine extent of therapy could have been a possible source of bias. Previous studies, however, have shown similar clinical results when comparing non-surgical treatment by different experienced operators (Badersten et al., 1981b, Badersten et al., 1984b). An additional limitation includes the inability to evaluate the specific response for deep and moderate pockets since LAP disease is localized to a limited number of teeth. Finally, this study is only powered to detect differences of 1mm or greater between groups, which is considered a clinically significant difference. However, potential smaller differences remaining between groups at 6 months could have been missed in the present investigation. Thus, longer follow-up with a greater number of subjects is still warranted to confirm the present findings.
Within the limitations of the present study, we conclude that although both treatment regimens result in satisfactory clinical results at 6 months, immediate systemic antibiotic application seems to be more advantageous in the treatment of localized aggressive periodontitis, as it results in an earlier greater improvement in clinical parameters and local inflammatory response when compared to delayed antibiotic use.
Clinical relevance.
Scientific rationale: It is unclear at what stage the clinician should prescribe antibiotics during treatment of aggressive periodontitis. Principal findings: Antibiotics administration immediately after or 3 months following mechanical therapy was similarly effective in reducing overall clinical parameters by 6 months post-therapy. However, immediate application provides better improvement in clinical parameters by 3 months post-therapy, which is concomitant with decreased levels of local inflammatory mediators. Practical implications: Antibiotics should be prescribed earlier rather than later to achieve more rapid improvement of aggressive periodontitis.
Acknowledgments
Support provided by NIH/NIDCR R01DE019456
Footnotes
No conflicts of interest reported by any of the authors.
References
- Aimetti M, Romano F, Guzzi N, Carnevale G. Full-mouth disinfection and systemic antimicrobial therapy in generalized aggressive periodontitis: a randomized, placebo-controlled trial. Journal of Clinical Periodontology. 2012;39:284–294. doi: 10.1111/j.1600-051X.2011.01795.x. [DOI] [PubMed] [Google Scholar]
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Annals of Periodontology. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Badersten A, Nilveus R, Egelberg J. Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis. Journal of Clinical Periodontology. 1981a;8:57–72. doi: 10.1111/j.1600-051x.1981.tb02024.x. [DOI] [PubMed] [Google Scholar]
- Badersten A, Nilveus R, Egelberg J. Effect of nonsurgical periodontal therapy. II. Severely advanced periodontitis. Journal of Clinical Periodontology. 1984a;11:63–76. doi: 10.1111/j.1600-051x.1984.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Badersten A, Nilveus R, Egelberg J. Effect of nonsurgical periodontal therapy. II. Severely advanced periodontitis. Journal of Clinical Periodontology. 1984b;11:63–76. doi: 10.1111/j.1600-051x.1984.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Badersten A, Nilvéus R, Egelberg J. Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis. Journal of Clinical Periodontology. 1981b;8:57–72. doi: 10.1111/j.1600-051x.1981.tb02024.x. [DOI] [PubMed] [Google Scholar]
- Blix IJ, Hars R, Preus HR, Helgeland K. Entrance of Actinobacillus actinomycetemcomitans into HEp-2 cells in vitro. Journal of Periodontology. 1992;63:723–728. doi: 10.1902/jop.1992.63.9.723. [DOI] [PubMed] [Google Scholar]
- Buchmann R, Nunn ME, Van Dyke TE, Lange DE. Aggressive periodontitis: 5-year follow-up of treatment. Journal of Periodontology. 2002;73:675–683. doi: 10.1902/jop.2002.73.6.675. [DOI] [PubMed] [Google Scholar]
- Castillo DM, Sanchez-Beltran MC, Castellanos JE, Sanz I, Mayorga-Fayad I, Sanz M, Lafaurie GI. Detection of specific periodontal microorganisms from bacteraemia samples after periodontal therapy using molecular-based diagnostics. Journal of Clinical Periodontology. 2011;38:418–427. doi: 10.1111/j.1600-051X.2011.01717.x. [DOI] [PubMed] [Google Scholar]
- Christersson LA, Slots J, Rosling BG, Genco RJ. Microbiological and clinical effects of surgical treatment of localized juvenile periodontitis. Journal of Clinical Periodontology. 1985;12:465–476. doi: 10.1111/j.1600-051x.1985.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Cobb CM. Non-surgical pocket therapy: mechanical. Ann Periodontol. 1996;1:443–490. doi: 10.1902/annals.1996.1.1.443. [DOI] [PubMed] [Google Scholar]
- de Lima Oliveira AP, de Faveri M, Gursky LC, Mestnik MJ, Feres M, Haffajee AD, Socransky SS, Teles RP. Effects of periodontal therapy on GCF cytokines in generalized aggressive periodontitis subjects. Journal of Clinical Periodontology. 2012;39:295–302. doi: 10.1111/j.1600-051X.2011.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths GS, Ayob R, Guerrero A, Nibali L, Suvan J, Moles DR, Tonetti MS. Amoxicillin and metronidazole as an adjunctive treatment in generalized aggressive periodontitis at initial therapy or re-treatment: a randomized controlled clinical trial. Journal of Clinical Periodontology. 2011;38:43–49. doi: 10.1111/j.1600-051X.2010.01632.x. [DOI] [PubMed] [Google Scholar]
- Guerrero A, Griffiths GS, Nibali L, Suvan J, Moles DR, Laurell L, Tonetti MS. Adjunctive benefits of systemic amoxicillin and metronidazole in non-surgical treatment of generalized aggressive periodontitis: a randomized placebo-controlled clinical trial. Journal of Clinical Periodontology. 2005;32:1096–1107. doi: 10.1111/j.1600-051X.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- Haffajee AD. Systemic antibiotics: to use or not to use in the treatment of periodontal infections. That is the question. Journal of Clinical Periodontology. 2006;33:359–361. doi: 10.1111/j.1600-051X.2006.00916.x. [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. A systematic review. Annals of Periodontology. 2003;8:115–181. doi: 10.1902/annals.2003.8.1.115. [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Teles RP, Socransky SS. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontology 2000. 2006;42:219–258. doi: 10.1111/j.1600-0757.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield LJ. Systemic antibiotics in periodontal therapy. Australian Dental Journal. 2009;54(Suppl 1):S96–101. doi: 10.1111/j.1834-7819.2009.01147.x. [DOI] [PubMed] [Google Scholar]
- Herrera D, Alonso B, Leon R, Roldan S, Sanz M. Antimicrobial therapy in periodontitis: the use of systemic antimicrobials against the subgingival biofilm. Journal of Clinical Periodontology. 2008;35:45–66. doi: 10.1111/j.1600-051X.2008.01260.x. [DOI] [PubMed] [Google Scholar]
- Kaldahl WB, Kalkwarf KL, Patil KD, Dyer JK, Bates RE., Jr Evaluation of four modalities of periodontal therapy. Mean probing depth, probing attachment level and recession changes. Journal of Periodontology. 1988;59:783–793. doi: 10.1902/jop.1988.59.12.783. [DOI] [PubMed] [Google Scholar]
- Kaner D, Christan C, Dietrich T, Bernimoulin JP, Kleber BM, Friedmann A. Timing affects the clinical outcome of adjunctive systemic antibiotic therapy for generalized aggressive periodontitis. Journal of Periodontology. 2007;78:1201–1208. doi: 10.1902/jop.2007.060437. [DOI] [PubMed] [Google Scholar]
- Machtei EE, Younis MN. The use of 2 antibiotic regimens in aggressive periodontitis: comparison of changes in clinical parameters and gingival crevicular fluid biomarkers. Q. uintessence International. 2008;39:811–819. [PubMed] [Google Scholar]
- Mandell RL, Socransky SS. A selective medium for Actinobacillus actinomycetemcomitans and the incidence of the organism in juvenile periodontitis. Journal of Periodontology. 1981;52:593–598. doi: 10.1902/jop.1981.52.10.593. [DOI] [PubMed] [Google Scholar]
- Mestnik MJ, Feres M, Figueiredo LC, Duarte PM, Lira EA, Faveri M. Short-term benefits of the adjunctive use of metronidazole plus amoxicillin in the microbial profile and in the clinical parameters of subjects with generalized aggressive periodontitis. Journal of Clinical Periodontology. 2010;37:353–365. doi: 10.1111/j.1600-051X.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- Mombelli A, Gmür R, Gobbi C, Lang NP. Actinobacillus actinomycetemcomitans in adult periodontitis. I. Topographic distribution before and after treatment. Journal of Periodontology. 1994;65:820–826. doi: 10.1902/jop.1994.65.9.820. [DOI] [PubMed] [Google Scholar]
- Pavicić MJ, van Winkelhoff AJ, Douqué NH, Steures RW, de Graaff J. Microbiological and clinical effects of metronidazole and amoxicillin in Actinobacillus actinomycetemcomitans-associated periodontitis. A 2-year evaluation. Journal of Clinical Periodontology. 1994;21:107–112. doi: 10.1111/j.1600-051x.1994.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Rahamat-Langendoen JC, van Vonderen MG, Engstrom LJ, Manson WL, van Winkelhoff AJ, Mooi-Kokenberg EA. Brain abscess associated with Aggregatibacter actinomycetemcomitans: case report and review of literature. Journal of Clinical Periodontology. 2011;38:702–706. doi: 10.1111/j.1600-051X.2011.01737.x. [DOI] [PubMed] [Google Scholar]
- Renvert S, Wikstrom M, Dahlen G, Slots J, Egelberg J. Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pockets. Journal of Clinical Periodontology. 1990;17:345–350. doi: 10.1111/j.1600-051x.1990.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg ES, Torosian JP, Hammond BF, Cutler SA. Routine anaerobic bacterial culture and systemic antibiotic usage in the treatment of adult periodontitis: a 6-year longitudinal study. International Journal of Periodontics & Restorative Dentistry. 1993;13:213–243. [PubMed] [Google Scholar]
- Sgolastra F, Petrucci A, Gatto R, Monaco A. Effectiveness of Systemic Amoxicillin/Metronidazole as an Adjunctive Therapy to Full-Mouth Scaling and Root Planing in the Treatment of Aggressive Periodontitis: A Systematic Review and Meta-Analysis. Journal of Periodontology. 2011 doi: 10.1902/jop.2011.110432. [DOI] [PubMed] [Google Scholar]
- Shaddox L, Wiedey J, Bimstein E, Magnuson I, Clare-Salzler M, Aukhil I, Wallet SM. Hyper-responsive phenotype in localized aggressive periodontitis. Journal of Dental Research. 2010;89:143–148. doi: 10.1177/0022034509353397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaddox LM, Walker C. Microbial testing in periodontics: value, limitations and future directions. Periodontology 2000. 2009;50:25–38. doi: 10.1111/j.1600-0757.2008.00285.x. [DOI] [PubMed] [Google Scholar]
- Slots J. Systemic antibiotics in periodontics. Journal of Periodontology. 2004;75:1553–1565. doi: 10.1902/jop.2004.75.11.1553. [DOI] [PubMed] [Google Scholar]
- Slots J, Reynolds HS, Genco RJ. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infection & Immunity. 1980;29:1013–1020. doi: 10.1128/iai.29.3.1013-1020.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J, Rosling BG. Suppression of the periodontopathic microflora in localized juvenile periodontitis by systemic tetracycline. Journal of Clinical Periodontology. 1983;10:465–486. doi: 10.1111/j.1600-051x.1983.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Slots J, Ting M. Systemic antibiotics in the treatment of periodontal disease. Periodontology 2000. 2002;28:106–176. doi: 10.1034/j.1600-0757.2002.280106.x. [DOI] [PubMed] [Google Scholar]
- Sreenivasan PK, Meyer DH, Fives-Taylor PM. Factors influencing the growth and viability of Actinobacillus actinomycetemcomitans. Oral Microbioogyl & Immunology. 1993;8:361–369. doi: 10.1111/j.1399-302x.1993.tb00612.x. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff AJ, Rodenburg JP, Goené RJ, Abbas F, Winkel EG, de Graaff J. Metronidazole plus amoxycillin in the treatment of Actinobacillus actinomycetemcomitans associated periodontitis. Journal of Clinical Periodontology. 1989;16:128–131. doi: 10.1111/j.1600-051x.1989.tb01626.x. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff AJ, Tijhof CJ, de Graaff J. Microbiological and clinical results of metronidazole plus amoxicillin therapy in Actinobacillus actinomycetemcomitans-associated periodontitis. Journal of Periodontology. 1992a;63:52–57. doi: 10.1902/jop.1992.63.1.52. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff AJ, Tijhof CJ, de Graaff J. Microbiological and clinical results of metronidazole plus amoxicillin therapy in Actinobacillus actinomycetemcomitans-associated periodontitis. Journal of Periodontology. 1992b;63:52–57. doi: 10.1902/jop.1992.63.1.52. [DOI] [PubMed] [Google Scholar]
- Winkel EG, Van Winkelhoff AJ, Timmerman MF, Van der Velden U, Van der Weijden GA. Amoxicillin plus metronidazole in the treatment of adult periodontitis patients. A double-blind placebo-controlled study. Journal of Clinical Periodontology. 2001;28:296–305. doi: 10.1034/j.1600-051x.2001.028004296.x. [DOI] [PubMed] [Google Scholar]
- Winkel EG, van Winkelhoff AJ, van der Velden U. Additional clinical and microbiological effects of amoxicillin and metronidazole after initial periodontal therapy. Journal of Clinical Periodontology. 1998;25:857–864. doi: 10.1111/j.1600-051x.1998.tb02382.x. [DOI] [PubMed] [Google Scholar]
- Xajigeorgiou C, Sakellari D, Slini T, Baka A, Konstantinidis A. Clinical and microbiological effects of different antimicrobials on generalized aggressive periodontitis. Journal of Clinical Periodontology. 2006;33:254–264. doi: 10.1111/j.1600-051X.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- Zambon JJ, Christersson LA, Slots J. Actinobacillus actinomycetemcomitans in human periodontal disease. Prevalence in patient groups and distribution of biotypes and serotypes within families. Journal of Periodontology. 1983;54:707–711. doi: 10.1902/jop.1983.54.12.707. [DOI] [PubMed] [Google Scholar]