Summary
Linoleic acid (LA) is the most abundant polyunsaturated fatty acid in human diets, a major component of human tissues, and the direct precursor to the bioactive oxidized LA metabolites (OXLAMs), 9- and 13 hydroxy-octadecadienoic acid (9- and 13-HODE) and 9- and 13-oxo-octadecadienoic acid (9- and 13-oxoODE). These four OXLAMs have been mechanistically linked to pathological conditions ranging from cardiovascular disease to chronic pain. Plasma OXLAMs, which are elevated in Alzheimer’s dementia and non-alcoholic steatohepatitis, have been proposed as biomarkers useful for indicating the presence and severity of both conditions. Because mammals lack the enzymatic machinery needed for de novo LA synthesis, the abundance of LA and OXLAMs in mammalian tissues may be modifiable via diet. To examine this issue in humans, we measured circulating LA and OXLAMs before and after a 12-week LA lowering dietary intervention in chronic headache patients. Lowering dietary LA significantly reduced the abundance of plasma OXLAMs, and reduced the LA content of multiple circulating lipid fractions that may serve as precursor pools for endogenous OXLAM synthesis. These results show that lowering dietary LA can reduce the synthesis and/or accumulation of oxidized LA derivatives that have been implicated in a variety of pathological conditions. Future studies evaluating the clinical implications of diet-induced OXLAM reductions are warranted.
Keywords: Linoleic acid, HODE, hydroxy-octadecadienoic acid, oxoODE, oxo-octadecadienoic acid, oxidation, OXLAM, PUFA, polyunsaturated fatty acid
Introduction
Oxidized linoleic acid metabolites (OXLAMs) are pleiotropic bioactive derivatives of linoleic acid (LA, 18:2n-6) that have been implicated in a variety of pathological conditions [1,2,3,4,5,6,7,8,9]. As a major component of oxidized low-density lipoprotein (LDL) [7,10,11] and atherosclerotic plaques [12,13], OXLAMs are reported to play a central role in foam cell formation and the pathogenesis of atherosclerosis [4,8,14]. OXLAMs also can act as endogenous TRPV1 receptor channel activators (i.e. endovanilloids)[1,2], facilitating peripheral and central pain sensitization. Circulating OXLAMs, which are elevated in Alzheimer’s dementia [15] and non-alcoholic steatohepatitis (NASH)[3], have been proposed as mechanism-based biomarkers useful for indicating the presence and severity of both conditions.
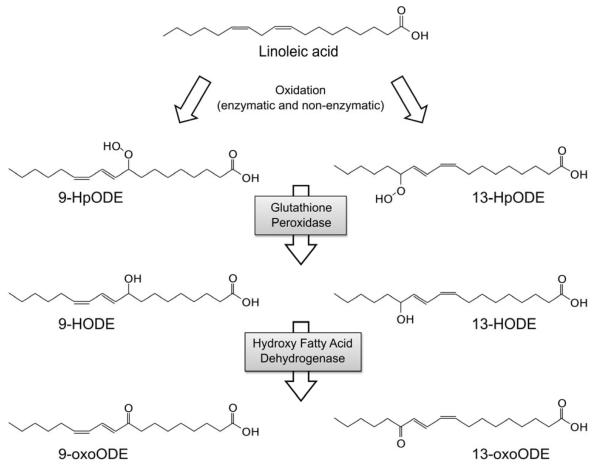
LA is the most abundant polyunsaturated fatty acid in human diets [16,17,18], a major component of human tissues, and the direct precursor to the OXLAMs 9- and 13- hydroxy-octadecadienoic acid (9- and 13-HODE) and 9- and 13-oxo-octadecadienoic acid (9- and 13-oxoODE)[19] (Fig.1). LA oxidation can proceed enzymatically via the actions of 12/15-lipoxygenase, cyclooxygenase or the cytochrome P450 enzyme family [19,20], or non-enzymatically via free radical-mediated oxidation [21].
Figure 1. Oxidative metabolism of linoleic acid.
LA can be enzymatically or non-enzymatically converted to 9- and 13-HpODE, with subsequent enzymatic conversion to hydroxy (9- and 13-HODE) and ketone (9- and 13-oxoODE) derivatives. The initial step (A) can be catalyzed by 12/15-lipoxygenase, cyclooxygenase, or cytochrome P450 enzymes. Abbreviations: LA: linoleic acid; LOX: lipoxygenase; COX: cyclooxygenase; HpODE; hydroperoxy-octadecadienoic acid; HODE: hydroxy-octadecadienoic acid; oxoODE: oxo-octadecadienoic acid.
Because humans cannot synthesize LA de novo [17,22,23], dietary LA is the sole source of LA in blood and other tissues. These LA stores in turn serve as precursor pools for endogenous OXLAM synthesis. Hence, diet-induced reductions in LA content may subsequently decrease the abundance of OXLAMs in vivo, with potential implications for conditions characterized by TRPV1 hyperactivity (e.g. chronic pain), as well as cardiovascular, hepatic and neurodegenerative diseases. To our knowledge, however, the relationship between dietary LA and plasma OXLAMs has not been reported in humans.
We hypothesized that lowering dietary LA would reduce plasma OXLAMs as well as their precursor LA in circulating lipid pools, in humans with chronic headaches.
Materials and Methods
Patient characteristics
This study was approved by the Institutional Review Board of the University of North Carolina-Chapel Hill. All patients provided written informed consent prior to participation. The cohort consisted of 67 subjects meeting the International Classification of Headache Disorders diagnostic criteria for Chronic Daily Headache [24]. Fifty-six of 67 (84%) randomized subjects completed the 12-week intervention phase. The mean age at randomization was 41.3 (range 20-62; SD 11.5). Eighty-five percent of randomized subjects were female and 88% were Caucasian.
Study Design
The main study was designed to evaluate the effects of lowering dietary LA, with or without concurrent increases in n-3 PUFAs, on plasma and erythrocyte fatty acids (primary outcomes) and clinical headache characteristics (secondary outcomes)(21). These outcomes will be reported in another publication. However, after our trial was already in progress, OXLAMs were implicated in the etiology of physical pain (1-2), and proposed as mechanism-based biomarkers in NASH (3). Recognizing that our LA lowering interventions provided a unique opportunity to evaluate whether diet-induced OXLAM reductions are possible in humans, we developed an interdisciplinary collaboration to specifically evaluate whether lowering dietary LA reduces plasma OXLAMs as well as their precursor LA in circulating lipid pools.
The dietary methods and methods employed to evaluate LA intake have been previously published [24]. In brief, after a 4-week baseline phase participants were randomized to one of two dietary interventions, to be maintained for 12 consecutive weeks. Both interventions were designed to markedly reduce LA intake. Group 1 consumed average US amounts of n-3 fatty acids; Group 2 also increased intake of n-3 fatty acids as shown in Table 2. Our diet method integrated the following key elements: 1) provision of foods accounting for two-thirds of caloric intake; 2) bi-monthly diet counseling; 3) self-monitoring; and 4) an intervention-specific website. All study participants were provided with, and instructed to exclusively use low-LA oils and fat sources, including vegetable oils and salad dressings. Because most US packaged food items contain substantial amounts of added LA, participants were also provided with a variety of low-LA substitute foods, including crackers, tortillas, breads and popcorn. Research foods were procured and prepared by the UNC Nutrition Research and Metabolism Core of the Clinical and Translational Research Center. Nutrient intakes were assessed using six unannounced telephone-administered 24-hour recalls; three administered prior to the intervention, and three administered in the final four weeks of the twelve-week intervention. Nutrient values were estimated using Nutritional Data System for Research software in version 2009-2010, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN [25].
Table 2.
Diet-induced reductions in circulating LA and its OXLAM products
| Variable | Baseline (Median) |
12-week (Median) |
% Change (Median) |
P-value |
|---|---|---|---|---|
| Diet (% of energy) | ||||
|
| ||||
| Dietary LA | 6.74 (5.54, 8.11) | 2.42 (2.09, 3.06) | −64.1 | p<0.001 |
|
| ||||
| Plasma OXLAMs (nM) | ||||
|
| ||||
| 9-HODE | 266 (209, 365) | 227 (182, 274) | −14.7 | p = 0.001 |
| 13-HODE | 268 (229, 376) | 243 (205, 294) | −9.5 | p < 0.001 |
| Total HODEs | 538 (442, 756) | 474 (385, 564) | −12.0 | p < 0.001 |
| 9-oxoODE | 169 (140, 229) | 146 (116, 203) | −13.8 | p = 0.01 |
| 13-oxoODE | 265 (213, 351) | 204 (155, 273) | −23.2 | p < 0.001 |
| Total oxoODEs | 431 (353, 555) | 352 (265, 478) | −18.3 | p < 0.001 |
| Total OXLAMs | 979 (823, 1310) | 851 (693, 1007) | −13.1 | p < 0.001 |
|
| ||||
| Concentration of LA in plasma fatty acid pools (nmol/ml) | ||||
|
| ||||
| Total plasma LA | 3075 (2622, 3357) | 2615 (2249, 3038) | −14.9 | p < 0.001 |
| Phospholipid-LA | 813 (726, 941) | 675 (593, 795) | −17.0 | p < 0.001 |
| Triglyceride-LA | 567 (337, 749) | 395 (293, 548) | −30.3 | p < 0.001 |
| Cholesteryl ester-LA | 1592 (1429, 1816) | 1440 (1191, 1665) | −9.6 | p = 0.002 |
| Free fatty acid-LA | 58 (36, 81) | 53 (42, 69) | −9.4 | p = 0.35 |
|
| ||||
| Relative abundance of LA in circulating fatty acid pools (%Composition) | ||||
|
| ||||
| Phospholipid-LA | 22.5 (20.9, 24.9) | 20.0 (18.0, 21.7) | −11.2 | p < 0.001 |
| Triglyceride-LA | 20.6 (17.1, 24.1) | 17.3 (14.8, 19.6) | −16.0 | p < 0.001 |
| Cholesteryl ester-LA | 55.5 (52.6, 58.5) | 50.5 (46.7, 52.9) | −9.1 | p < 0.001 |
| Free fatty acid-LA | 16.0 (14.0, 18.0) | 15.2 (13.6, 16.4) | −5.2 | p < 0.001 |
| Erythrocyte-LA | 12.0 (11.0, 12.7) | 10.3 (9.3, 11.0) | −14.5 | p < 0.001 |
Pre-to-post comparisons of dietary LA (n=55), plasma OXLAMs (n=55), LA fractions (n=55), and erythrocytes (n=52) were analyzed with the Wilcoxon Signed-Rank test for matched pairs. Blood collection was unsuccessful for one subject. Erythrocytes were not collected on four subjects. Abbreviations: LA: linoleic acid; OXLAMs: oxidized linoleic acid metabolites; HODE: hydroxy-octadecadienoic acid; oxoODE: oxo-octadecadenoic acid.
Sample collection
Sample preparation and analyses were performed by investigators who were blinded to study protocol and clinical data. Fasting blood was drawn at baseline and again after 12-weeks of exposure to the dietary interventions. Whole blood was collected into ethylenediaminetetraacetic acid (EDTA) tubes. Samples were immediately centrifuged at 2960 rpm for 15 min at room temperature. Plasma and erythrocyte aliquots were prepared and immediately transferred to a − 80°C freezer until analysis. Analyses of OXLAMs, plasma lipid fractions, and erythrocyte fatty acids were performed at the Cleveland Clinic Department of Cell Biology, National Institute on Aging Brain Physiology and Metabolism Section, and National Institute on Alcohol Abuse and Alcoholism Laboratory of Membrane Biochemistry and Biophysics, respectively.
OXLAM analysis
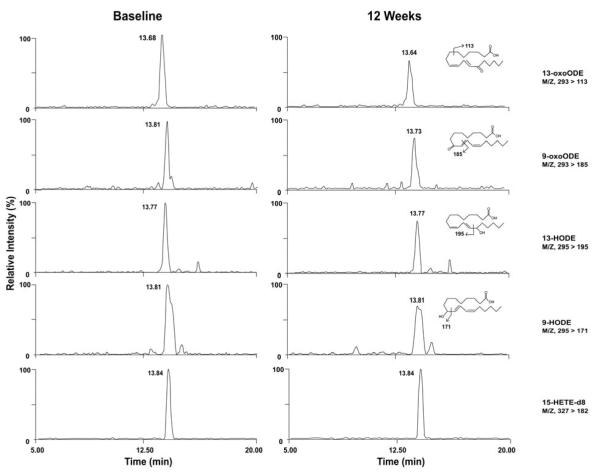
Plasma OXLAMs were analyzed as previously described [3]. Briefly, plasma (50 μl), internal standard [15(S)-HETE-d8, 10μL of 1000 ng/ml] and sodium hydroxide were added to the glass test tubes, overlaid with argon, and sealed. Lipids were hydrolyzed at 60°C under argon atmosphere for 2 h and then the released fatty acids were extracted into the hexane layer twice by liquid/liquid extraction using hexane and hexane/isopropanol with 4M acetic acid. With each extraction, tubes were capped under argon. The combined hexane layers were dried under nitrogen gas and then re-suspended in 200 μl 85% methanol/water (v/v). Fatty acid oxidation products (free plus esterified) in plasma were quantified using liquid chromatography online electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS) (Fig. 2) [3]. Briefly, 40 μl of lipid extract was injected onto an HPLC (Waters 2690 Separations Module, Franklin, MA) system, and the oxidized fatty acids and their precursors were separated through a C18 column (Phenomenex, ODS, 2 × 150 mm, 5 μm, Rancho Palos Verdes, CA). The oxidized fatty acids and their precursors were quantified on a triple quadrupole mass spectrometer (Quattro Ultima, Micromass, Manchester, UK) using ESI in negative ion mode and multiple reaction monitoring (MRM) using characteristic parent → daughter ion transitions for the specific molecular species monitored. The lipid peroxidation products analyzed included structurally specific species 9- and 13-HODE, 9- and 13-oxoODE, and their precursor LA. 15-HETE-d8 (Cayman Chemical, Ann Arbor, MI) was used as internal standard for calibration of oxidized fatty acids in plasma, as previously described [3].
Figure 2. Detection and quantification of OXLAM profile by ESI/LC/MS/MS.
Individual isomers of HODEs and oxoODEs formed by LA oxidation were quantified with a single injection. Lipid extracts were resolved by HPLC and monitored online by ESI/LC/MS/MS [3]. Abbreviations: HODE: hydroxy-octadecadenoic acid; oxoODE, oxo-octadecadenoic acid; 15-HETE-d8, internal standard.
Fatty acid analysis
Plasma Fractions
Total lipids were extracted from 200 μl of plasma in 3 ml of 2:1 chloroform/methanol following the addition of unesterified heptadecaenoic acid (17:0) as an internal standard (0.14 nmol/μl) for unesterified fatty acids. KCl (0.5M, 0.75 ml) was then added to separate the aqueous phase. The bottom chloroform layer was separated and re-extracted with 2 ml chloroform. The pooled extracts were dried down and separated into neutral lipid subclasses using thin layer chromatography on silica gel-60 plates (EM Separation Technologies, Gibbstown, NJ, USA). The lipid subclasses (total phospholipid, triacylglycerol, cholesteryl ester, unesterified fatty acid) were separated using the solvent system: heptane: diethylether: glacial acetic acid (60:40:3, v/v/v) [26]. Authentic standards of the lipid classes were run on separate lanes on the plates to identify lipid bands under ultraviolet light, after spraying with 0.03% 6-p-toluidine-2-naphthalene sulfonic acid in 50 mM Tris-HCl buffer (pH 7.4) (w/v). The bands were scraped into test tubes and methylated with 1% H2SO4-methanol for 3 h at 70°C [27]. Before methylation, di-17:0 PC was added to each tube as an internal standard for phospholipids, triglycerides and cholesteryl esters. The prepared fatty acid methyl esters (FAME) were analyzed using a gas-chromatography system (6890N, Agilent Technologies, Palo Alto, CA, USA) equipped with an SPTM-2330 fused silica capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) (Supelco, Bellefonte, PA, USA) and a flame ionization detector. Fatty acid concentrations were calculated by proportional comparison of peak areas of samples to the area of the 17:0 internal standard.
Erythrocytes
Erythrocytes were obtained from EDTA blood samples after the plasma and buffy coat were removed. Following Bligh/Dyer extraction [28], erythrocyte aliquots were heated at 100°C for one hour with methanol containing 14% boron trifluoride to generate FAME. These were then extracted into hexane and analyzed with a GC/FID gas chromatograph (Agilent 6890) equipped with a 30-m DBFFAP capillary column. Fatty acids were identified through comparison with a standard fatty acid methyl ester mixture (GLC-462). Values are expressed as percentages of total red blood cell fatty acids.
Data Analysis
Non-parametric analyses were employed due to the presence of non-normal distributions. Pre-to-post intervention comparisons were tested with the Wilcoxon Signed-Rank test for matched pairs. A Mann Whitney U test was used for between group comparisons. P values less than 0.05 were considered significant. In addition, in an exploratory manner, Spearman’s coefficients were used to evaluate potential correlations between circulating LA pools and OXLAMs.
Results
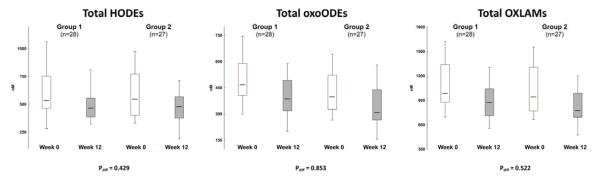
Lowering dietary LA from 6.7 to 2.4% of calories for 12-weeks in 55 patients with chronic headaches significantly reduced the abundance of the four measured OXLAMs (9- and 13-HODEs, 9- and 13-oxoODEs), as well as their precursor LA in circulating lipid pools (Table 2). There were no significant differences in OXLAM reductions between the two low-LA intervention groups (Fig. 3), one with and one without added dietary n-3 fatty acids (Table 1), indicating that the observed reductions were primarily due to the decrease in dietary LA. The combined results of the two LA-lowering intervention groups are presented in Table 2.
Figure 3. Comparable OXLAM reductions in the two LA lowering intervention groups.
There were no between-group differences in the median reductions in HODEs, oxo-ODEs or total OXLAMs, indicating that the observed changes were primarily due to the decrease in dietary LA rather than the n-3 fatty acids provided only to Group 2. Data for the combined groups are presented in subsequent tables and figures. The box-whisker plot is represented with the lower boundary of the box indicating the 25th percentile, the line within the box indicating the median value, and the upper boundary indicating the 75th percentile. The whiskers extend to the non-outlier range. P values represent between group comparisons using a Mann Whitney U test. Abbreviations: HODE: hydroxy-octadecadienoic acid; oxo-ODE: oxo-octadecadienoic acid; OXLAM: oxidized linoleic acid metabolite.
Table 1.
Median fatty acid contents of the LA lowering dietary interventions
| Diet Group | Calories | Fat | SFA | PUFA | n-6 LA | n-3 ALA | n-3 EPA + DHA |
|---|---|---|---|---|---|---|---|
| Pre-intervention Phase | |||||||
| Combined (n=55) | 1861 | 33.4 | 10.5 | 7.6 | 6.7 | 0.7 | 46 mg |
| Intervention Phase | |||||||
| Combined (n=55) | 1742 | 30.7 | 13.2 | 4.4 | 2.4 | 1.1 | 305 mg |
| Group 1 (n=28) | 1859 | 30.4 | 14.0 | 3.5 | 2.4 | 0.7 | 76 mg |
| Group 2a (n=27) | 1596 | 30.7 | 12.9 | 6.0 | 2.5 | 1.6 | 1,482 mg |
Fatty acid intake is expressed as a percentage of daily food energy (en%), except for n-3 EPA+DHA, which is expressed in mg. Based on six 24-hour dietary recalls administered on non-consecutive days. One of the 56 randomized subjects did not complete 24-hour recalls.
Indicates that dietary n-3 ALA, EPA and DHA were increased in Group 2 only. Abbreviations: Fat: total fat; SFA: total saturated fat; PUFA, total polyunsaturated fat; LA: linoleic acid; ALA: alpha-linolenic acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid.
Distinctions between OXLAM species and their precursor pools
Median reductions in the LA content of the four plasma fractions were significantly different using Friedman ANOVA (p=0.02), with the most pronounced changes in the phospholipid (PL) and triglyceride (TG) pools (Table 2). Unlike LA, the overall amount of total fatty acids did not change in any of these plasma lipid fractions (Table 3). Although reductions in oxoODEs tended to be more pronounced than HODEs (-18.3% vs. -12.0%), this difference was not statistically significant (p=0.45).
Table 3.
No changes in the total fatty acid content of plasma lipid classes
| Plasma Lipid Classes (nmol/ml; n=55) |
Baseline (Median) |
12-week (Median) |
% Change (Median) |
P-value (Median) |
|---|---|---|---|---|
| Total fatty acids | 9512 (7784, 11586) | 9552 (7978, 11394) | +0.4 | 0.89 |
| Total PL | 3663 (3159, 4101) | 3622 (3204, 3971) | −1.1 | 0.43 |
| Total TG | 2519 (1679, 3563) | 2479 (1692, 3422) | −1.6 | 0.67 |
| Total CE | 2827 (2498, 3285) | 2995 (2596, 3433) | +6.0 | 0.46 |
| Total FFA | 342 (235, 441) | 362 (275, 446) | +6.0 | 0.63 |
Pre-to-post comparisons of total plasma fatty acids in plasma lipid classes were analyzed with the Wilcoxon Signed-Rank test for matched pairs. Abbreviations: PL: phospholipid; TG: triglyceride; CE: cholesteryl ester; FFA: free fatty acids.
Correlations between circulating LA pools and OXLAMs
Circulating OXLAMs correlated with the LA content of several OXLAM precursor pools at baseline, and after the 12-week dietary intervention. At baseline, total OXLAMs correlated with total plasma LA (Spearman rho(r)=0.48, p<0.01), as well as the LA content of erythrocytes (r=0.28, p=0.04). Of the plasma lipid fractions, the strongest OXLAM correlations were present in the phospholipid-LA (PL-LA) fraction (r=0.47, p<0.01), however baseline TG-LA (r=0.28, p=0.04) and CE-LA (r=0.33, p=0.01) were also significantly correlated with OXLAMs. After the 12-week intervention, total OXLAMs remained correlated with total plasma LA (r=0.40, p<0.01), PL-LA (r=0.50, p<0.01), TG-LA (r=0.37, p<0.01), and erythrocyte LA (r=0.42, p<0.01), but were no longer correlated with CE-LA (r=0.15, p=0.27). OXLAMs were not correlated with FFA-LA before (r=0.01, p=0.93) or after the dietary intervention (0.04, p=0.80).
Discussion
We report for the first time that lowering dietary LA reduces OXLAMs in humans, here among subjects with chronic headaches. This link between dietary LA and OXLAMs may have important implications for pathological conditions linked to increased activity or abundance of OXLAMs (e.g. chronic pain, Alzheimer’s dementia, cardiovascular disease, NASH) [1,2,3,15]. To our knowledge, this is the first demonstration that changes in dietary LA can alter the abundance of plasma OXLAMs in humans, or that lowering dietary LA reduces the abundance of OXLAMs in any tissue in a human or animal model. Our findings are consistent with a report [29] that a 4-fold increase in dietary LA (from corn oil) produced a 5-fold increase in the 9- and 13-HODE content of mammary tissue in female mice. Collectively, these observations indicate that dietary LA may hold proximal control over the production and/or accumulation of OXLAMs in certain tissues.
We also found that lowering dietary LA reduced the abundance of LA in several circulating lipid fractions that may serve as precursor pools for endogenous OXLAM synthesis. Importantly, the concentration of total fatty acids did not change in PL, TG, CE or FFA (Table 3), indicating that observed reductions in LA and OXLAMs were unlikely to be secondary to a general reduction in plasma lipoproteins. LA reductions were most pronounced in the PL and TG fractions, indicating that these esterified fractions may be particularly responsive to dietary modification. However, since the relative LA abundance was reduced in all measured pools, and the LA content of other potential OXLAM sources (e.g. liver, adipose) was not analyzed, it is not possible to attribute the observed OXLAM reductions to any specific plasma lipid pool. Future studies using LA-tracers [30] and/or tissue procurement for OXLAM analysis could help clarify the most relevant in vivo OXLAM precursor pool(s).
The robust correlations observed between circulating OXLAMs and LA in several LA pools both at baseline and after the 12-week intervention are consistent with the hypothesis that multiple LA pools contribute to in vivo OXLAM formation. The 12-week LA lowering intervention may not have been of sufficient duration to establish a new steady state of CE-LA in circulation. Consistent with this, there was a robust correlation between OXLAMs and CE-LA at baseline was no longer present following the 12-week intervention. Importantly, the CE pool contains about half of all circulating LA. Longer trials with serial analyses of LA and OXLAMs may therefore help characterize the temporal relations between diet-induced alterations in each circulating LA pool and OXLAMs.
Potential implications of dietary modulation of OXLAMs
OXLAMs are among the most abundant oxidized fatty acid derivatives in human plasma, with >50-fold higher concentrations than the more extensively studied prostanoid and isoprostane derivatives of arachidonic acid [15,31]. Unlike prostanoids however, OXLAMs are readily incorporated into, and released from, esterified lipid pools including PL [32,33], TG [34] and CE [11]. In rodents, plasma OXLAMs are largely esterified within lipoproteins [10,35]. As a major component of oxidized LDL [7] as well as the foam cells and migrating vascular smooth muscle cells found in atherosclerotic lesions [12,13], OXLAMs have been implicated in cardiovascular disease (CVD) pathogenesis [4,8,9]. Therefore, the diet-induced reductions in circulating OXLAMs demonstrated here may have clinical relevance for CVD risk reduction.
If comparable reductions in the abundance of OXLAMs are achievable in other tissues, dietary LA lowering may have diverse physiological and clinical consequences. Since LA is the parent compound for OXLAM synthesis, diet-induced changes in tissue LA may induce corresponding alterations in local OXLAM concentrations. Human and animal trials have established that modifications in dietary LA alter the LA content of numerous tissue types [36,37,38]. Sensitivities to alterations in dietary LA are likely to be tissue-specific [39,40,41], with marked diet-induced alterations in the LA content of peripheral nerves [39], small intestine [41], liver [39], testes [39], adipose tissue [37,39], and skeletal muscle [39],and more modest changes in myelin and brain [39,40,42]. Remarkably, the abundance of LA in rat sciatic nerve increased linearly from 3 to 15% of total fatty acids when dietary LA was increased from 2 to 12% of energy [39]. In rodents, circulating LA readily crosses the blood brain barrier [42], where the majority is quickly oxidized to unknown products. It is presently unknown what proportion of these oxidized LA products are 9- and 13-HODEs and oxoODEs.
Diet-induced modifications in the OXLAM content of neuronal tissues such as sciatic nerve and brain may have important implications for conditions characterized by TRPV1 hyperactivity. OXLAM-mediated TRPV1 activation in rodents induced hyperalgesia and allodynia [1,2], which was reversed with OXLAM specific immunological neutralization [2], indicating that OXLAM-mediated signaling contributes to physical pain. Notably, pathologies characterized by TRPV1 hyperactivity are common in tissues that readily accumulate LA (e.g. fibromyalgia [43], irritable bowel syndrome [44,45], sciatica [46]).While it is not yet known whether lowering dietary LA reduces neuronal and glial OXLAM synthesis and accumulation, such a reduction would be expected to attenuate TRPV1 activation[47], and ameliorate conditions characterized by TRPV1 hyperactivity including physical pain. As TRPV1 is widely distributed in peripheral and central nervous system tissues[48], the ability to modulate endovanilloid tone through diet could have broad implications.
In the United States, per capita dietary LA tripled from about 2% of energy (en%) to 7 en% during the 20th century [49], which coincided with a marked increase in adipose tissue LA [50,51,52]. Because human adipose tissue LA has a half-life of 1-2 years [36,53,54], the gradual mobilization of LA from adipose stores into circulation is expected to lengthen the time to steady state when lowering dietary LA. Hence, more robust OXLAM reductions may be achievable by extending the duration of LA lowering dietary interventions beyond 12-weeks. However, the chronic pain population studied here may have been especially compliant due to the potential for pain relief. Therefore, the magnitude of OXLAM reductions observed in this 12-week trial may not be readily achievable in other populations.
In conclusion, dietary LA lowering reduces circulating oxidized LA derivatives that have been implicated in a variety of pathological conditions. Future studies evaluating the metabolic and clinical implications of diet-induced OXLAM reductions are warranted.
Acknowledgements
The authors would like to thank the following individuals for their research assistance: David Barrow for expertise with specimen collection, processing and management; Olafur Palsson, Beth Fowler, Carol Carr, Regina McCoy, and Tim McCaskill for design and functionality of the study website; Meg Mangan for 24-hour recall data collection and management; Jim Loewke for erythrocyte fatty acid analysis; Duk Hyun for nutrient composition analyses; and Chanee Lynch, Becky Coble and Angela Johnston for general research assistance.
Funding: The authors gratefully acknowledge funding support for this trial from the Mayday Fund (primary source); the National Institute on Alcohol Abuse and Alcoholism, NIH; the UNC Research Fellowship in Complementary and Alternative Medicine (grant T32-AT003378, NCCAM, NIH); the North Carolina Clinical and Translational Sciences Institute (grant UL1RR025747, NCRR, NIH); the UNC Nutrition Obesity Research Center, CHAI Core (grant DK056350, NIDDK, NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Mayday Fund or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. The Journal of clinical investigation. 2010;120:1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Journal of lipid research. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- [5].Aisen PS, Haines KA, Given W, Abramson SB, Pras M, Serhan C, Hamberg M, Samuelsson B, Weissmann G. Circulating hydroxy fatty acids in familial Mediterranean fever. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:1232–1236. doi: 10.1073/pnas.82.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Engels F, van Houwelingen AH, Buckley TL, van de Velde MJ, Henricks PA, Nijkamp FP. Airway hyperresponsiveness induced by 13-hydroxyoctadecadienoic acid (13-HODE) is mediated by sensory neuropeptides. Advances in prostaglandin, thromboxane, and leukotriene research. 1995;23:361–363. [PubMed] [Google Scholar]
- [7].Spiteller G. Linoleic acid peroxidation--the dominant lipid peroxidation process in low density lipoprotein--and its relationship to chronic diseases. Chemistry and physics of lipids. 1998;95:105–162. doi: 10.1016/s0009-3084(98)00091-7. [DOI] [PubMed] [Google Scholar]
- [8].Jira W, Spiteller G, Carson W, Schramm A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chemistry and physics of lipids. 1998;91:1–11. doi: 10.1016/s0009-3084(97)00095-9. [DOI] [PubMed] [Google Scholar]
- [9].Ku G, Thomas CE, Akeson AL, Jackson RL. Induction of interleukin 1 beta expression from human peripheral blood monocyte-derived macrophages by 9-hydroxyoctadecadienoic acid. The Journal of biological chemistry. 1992;267:14183–14188. [PubMed] [Google Scholar]
- [10].Barlic J, Murphy PM. An oxidized lipid-peroxisome proliferator-activated receptor gamma-chemokine pathway in the regulation of macrophage-vascular smooth muscle cell adhesion. Trends in cardiovascular medicine. 2007;17:269–274. doi: 10.1016/j.tcm.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Belkner J, Wiesner R, Rathman J, Barnett J, Sigal E, Kuhn H. Oxygenation of lipoproteins by mammalian lipoxygenases. European journal of biochemistry / FEBS. 1993;213:251–261. doi: 10.1111/j.1432-1033.1993.tb17755.x. [DOI] [PubMed] [Google Scholar]
- [12].Brooks CJ, Harland WA, Steel G, Gilbert JD. Lipids of human atheroma: isolation of hydroxyoctade cadienoic acids from advanced aortal lesions. Biochimica et biophysica acta. 1970;202:563–566. doi: 10.1016/0005-2760(70)90131-1. [DOI] [PubMed] [Google Scholar]
- [13].Shibata N, Toi S, Shibata T, Uchida K, Itabe H, Sawada T, Kawamata T, Okada Y, Uchiyama S, Kobayashi M. Immunohistochemical detection of 13(R)-hydroxyoctadecadienoic acid in atherosclerotic plaques of human carotid arteries using a novel specific antibody. Acta histochemica et cytochemica. 2009;42:197–203. doi: 10.1267/ahc.09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kuhn H, Heydeck D, Hugou I, Gniwotta C. In vivo action of 15-lipoxygenase in early stages of human atherogenesis. The Journal of clinical investigation. 1997;99:888–893. doi: 10.1172/JCI119253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yoshida Y, Yoshikawa A, Kinumi T, Ogawa Y, Saito Y, Ohara K, Yamamoto H, Imai Y, Niki E. Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer’s disease patients and their potential as biomarkers. Neurobiology of aging. 2009;30:174–185. doi: 10.1016/j.neurobiolaging.2007.06.012. [DOI] [PubMed] [Google Scholar]
- [16].U.S. Department of Agriculture. Agricultural Research Service . What We Eat in America. NHANES 2007-2008; 2010. Nutrient Intakes from Food. Amounts Consumed per Individual, by Gender and Age. [Google Scholar]
- [17].Cunnane SC, Guesnet P. Linoleic acid recommendations--A house of cards. Prostaglandins Leukot Essent Fatty Acids. 2011;85:399–402. doi: 10.1016/j.plefa.2011.09.003. [DOI] [PubMed] [Google Scholar]
- [18].Whelan J. The health implications of changing linoleic acid intakes. Prostaglandins Leukot Essent Fatty Acids. 2008;79:165–167. doi: 10.1016/j.plefa.2008.09.013. [DOI] [PubMed] [Google Scholar]
- [19].Reinaud O, Delaforge M, Boucher JL, Rocchiccioli F, Mansuy D. Oxidative metabolism of linoleic acid by human leukocytes. Biochemical and biophysical research communications. 1989;161:883–891. doi: 10.1016/0006-291x(89)92682-x. [DOI] [PubMed] [Google Scholar]
- [20].Engels F, Willems H, Nijkamp FP. Cyclooxygenase-catalyzed formation of 9-hydroxylinoleic acid by guinea pig alveolar macrophages under non-stimulated conditions. FEBS Lett. 1986;209:249–253. doi: 10.1016/0014-5793(86)81121-8. [DOI] [PubMed] [Google Scholar]
- [21].Liu W, Yin H, Akazawa YO, Yoshida Y, Niki E, Porter NA. Ex vivo oxidation in tissue and plasma assays of hydroxyoctadecadienoates: Z,E/E,E stereoisomer ratios. Chemical research in toxicology. 2010;23:986–995. doi: 10.1021/tx1000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Holman RT, Caster WO, Wiese HF. The Essential Fatty Acid Requirement of Infants and the Assessment of Their Dietary Intake of Linoleate by Serum Fatty Acid Analysis. The American journal of clinical nutrition. 1964;14:70–75. doi: 10.1093/ajcn/14.2.70. [DOI] [PubMed] [Google Scholar]
- [23].Guesnet P, Lallemand SM, Alessandri JM, Jouin M, Cunnane SC. alpha-Linolenate reduces the dietary requirement for linoleate in the growing rat. Prostaglandins Leukot Essent Fatty Acids. 2011;85:353–360. doi: 10.1016/j.plefa.2011.08.003. [DOI] [PubMed] [Google Scholar]
- [24].Ramsden CE, Mann JD, Faurot KR, Lynch C, Imam ST, MacIntosh BA, Hibbeln JR, Loewke J, Smith S, Coble R, Suchindran C, Gaylord SA. Low omega-6 vs. low omega-6 plus high omega-3 dietary intervention for chronic daily headache: protocol for a randomized clinical trial. Trials. 2011;12:97. doi: 10.1186/1745-6215-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–1271. [PubMed] [Google Scholar]
- [26].Skipski VP, Good JJ, Barclay M, Reggio RB. Quantitative analysis of simple lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1968;152:10–19. doi: 10.1016/0005-2760(68)90003-9. [DOI] [PubMed] [Google Scholar]
- [27].DeMar JC, Jr., Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- [28].Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- [29].Johnson JA, Blackburn ML, Bull AW, Welsch CW, Watson JT. Separation and quantitation of linoleic acid oxidation products in mammary gland tissue from mice fed low- and high-fat diets. Lipids. 1997;32:369–375. doi: 10.1007/s11745-997-0047-7. [DOI] [PubMed] [Google Scholar]
- [30].Gao F, Kiesewetter D, Chang L, Rapoport SI, Igarashi M. Quantifying conversion of linoleic to arachidonic and other n-6 polyunsaturated fatty acids in unanesthetized rats. J Lipid Res. 2010;51:2940–2946. doi: 10.1194/jlr.M005595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [31].Kakutani S, Ishikura Y, Tateishi N, Horikawa C, Tokuda H, Kontani M, Kawashima H, Sakakibara Y, Kiso Y, Shibata H, Morita I. Supplementation of arachidonic acid-enriched oil increases arachidonic acid contents in plasma phospholipids, but does not increase their metabolites and clinical parameters in Japanese healthy elderly individuals: a randomized controlled study. Lipids in health and disease. 2011;10:241. doi: 10.1186/1476-511X-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cho Y, Ziboh VA. Incorporation of 13-hydroxyoctadecadienoic acid (13-HODE) into epidermal ceramides and phospholipids: phospholipase C-catalyzed release of novel 13-HODE-containing diacylglycerol. Journal of lipid research. 1994;35:255–262. [PubMed] [Google Scholar]
- [33].Milne GL, Seal JR, Havrilla CM, Wijtmans M, Porter NA. Identification and analysis of products formed from phospholipids in the free radical oxidation of human low density lipoproteins. Journal of lipid research. 2005;46:307–319. doi: 10.1194/jlr.M400311-JLR200. [DOI] [PubMed] [Google Scholar]
- [34].Haas TA, Bertomeu MC, Bastida E, Buchanan MR. Cyclic AMP regulation of endothelial cell triacylglycerol turnover, 13-hydroxyoctadecadienoic acid (13-HODE) synthesis and endothelial cell thrombogenicity. Biochimica et biophysica acta. 1990;1051:174–178. doi: 10.1016/0167-4889(90)90190-o. [DOI] [PubMed] [Google Scholar]
- [35].Shearer GC, Newman JW. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins, leukotrienes, and essential fatty acids. 2008;79:215–222. doi: 10.1016/j.plefa.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dayton S, Hashimoto S, Dixon W, Pearce ML. Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. Journal of lipid research. 1966;7:103–111. [PubMed] [Google Scholar]
- [37].Dayton S, Hashimoto S, Pearce ML. Influence of a diet high in unsaturated fat upon composition of arterial tissue and atheromata in man. Circulation. 1965;32:911–924. doi: 10.1161/01.cir.32.6.911. [DOI] [PubMed] [Google Scholar]
- [38].Adam O, Wolfram G, Zollner N. Influence of dietary linoleic acid intake with different fat intakes on arachidonic acid concentrations in plasma and platelet lipids and eicosanoid biosynthesis in female volunteers. Annals of nutrition & metabolism. 2003;47:31–36. doi: 10.1159/000068906. [DOI] [PubMed] [Google Scholar]
- [39].Bourre JM, Piciotti M, Dumont O, Pascal G, Durand G. Dietary linoleic acid and polyunsaturated fatty acids in rat brain and other organs. Minimal requirements of linoleic acid. Lipids. 1990;25:465–472. doi: 10.1007/BF02538090. [DOI] [PubMed] [Google Scholar]
- [40].Novak EM, Dyer RA, Innis SM. High dietary omega-6 fatty acids contribute to reduced docosahexaenoic acid in the developing brain and inhibit secondary neurite growth. Brain research. 2008;1237:136–145. doi: 10.1016/j.brainres.2008.07.107. [DOI] [PubMed] [Google Scholar]
- [41].Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, Hansen SH, Hansen HS. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochimica et biophysica acta. 2008;1781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- [42].DeMar JC, Jr., Lee HJ, Ma K, Chang L, Bell JM, Rapoport SI, Bazinet RP. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochimica et biophysica acta. 2006;1761:1050–1059. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- [43].Morris V, Cruwys S, Kidd B. Increased capsaicin-induced secondary hyperalgesia as a marker of abnormal sensory activity in patients with fibromyalgia. Neuroscience letters. 1998;250:205–207. doi: 10.1016/s0304-3940(98)00443-1. [DOI] [PubMed] [Google Scholar]
- [44].Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghoshs S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Akbar A, Yiangou Y, Facer P, Brydon WG, Walters JR, Anand P, Ghosh S. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut. 2010;59:767–774. doi: 10.1136/gut.2009.194449. [DOI] [PubMed] [Google Scholar]
- [46].Zeyzus JB, Thesis MS. TRPV1 mRNA is differentially expressed in different vertebral levels of rat dorsal root ganglia following sciatic nerve injury. Pittsburgh, PA: 2009. [Google Scholar]
- [47].Chen Y, Willcockson HH, Valtschanoff JG. Influence of the vanilloid receptor TRPV1 on the activation of spinal cord glia in mouse models of pain. Exp Neurol. 2009;220:383–390. doi: 10.1016/j.expneurol.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends in neurosciences. 2009;32:215–224. doi: 10.1016/j.tins.2008.12.006. [DOI] [PubMed] [Google Scholar]
- [49].Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. The American journal of clinical nutrition. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kingsbury KJ, Paul S, Crossley A, Morgan DM. The fatty acid composition of human depot fat. The Biochemical journal. 1961;78:541–550. doi: 10.1042/bj0780541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kingsbury KJ, Heyes TD, Morgan DM, Aylot C, Burton PA, Emmerson R, Robinson PJ. The effect of dietary changes on the fatty acid composition of normal human depot fat. The Biochemical journal. 1962;84:124–133. doi: 10.1042/bj0840124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. The American journal of clinical nutrition. 1998;67:25–30. doi: 10.1093/ajcn/67.1.25. [DOI] [PubMed] [Google Scholar]
- [53].Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Progress in lipid research. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- [54].Thomas EL, Frost G, Barnard ML, Bryant DJ, Taylor-Robinson SD, Simbrunner J, Coutts GA, Burl M, Bloom SR, Sales KD, Bell JD. An in vivo 13C magnetic resonance spectroscopic study of the relationship between diet and adipose tissue composition. Lipids. 1996;31:145–151. doi: 10.1007/BF02522613. [DOI] [PubMed] [Google Scholar]