Abstract
In 1999, researchers and policy makers recognized the challenge of creating an integrated patient-centered cancer care process across the many types of care from risk assessment through end of life. More than a decade later, there has been limited progress towards that goal even though the standard reductionist approach to health services and medical research has resulted in major advances in tests, procedures, and individualized patient approaches to care. In this commentary, we propose that considering an entire care process within its multilevel context may increase progress towards an integrated experience and improvements in the quality of care. As an illustrative case, we describe the multilevel context of care delivery for the process of follow-up to an abnormal screening mammogram. By taking a multilevel perspective on this process, we identify a rich set of options for intervening and improving follow-up to abnormalities and therefore outcomes of screening. We propose that taking this multilevel perspective when designing interventions may improve the quality of cancer care in an effective and sustainable way.
Introduction
Delivery of cancer care in the United States is a complex process in need of improvement (1). In 1999, the Institute of Medicine characterized care as a provider-centered, poorly coordinated, and inefficient process that serves patient populations unequally (1). Historically, efforts to improve care have emphasized the reductionist approach of developing focused advances in single tests, treatment techniques, and well-defined isolated steps in care (2). For example, research in the last decade has improved diagnostic tests and treatments for breast cancer (3, 4). There has also been a reduction in mortality from cancer procedures like surgery because they've been performed in high, rather than low, volume centers (5). While these types of changes have contributed to a reduction in mortality for some cancers in the last decade, the sum of the improvement in these steps falls short of being an integrated and supportive process for cancer patients, and a recent review reported little progress in the cancer care process since the 1999 IOM report (1,5,6). To address this problem we suggest an alternative to the reductionist view; a multilevel perspective on an entire process of care. After reviewing the multilevel context of care delivery in the United States, and considering the example of the process of following up after an abnormal mammogram, we conclude with a discussion of potential interventions at three levels of the context of care that all could affect follow-up to abnormal screening.
The multilevel context of healthcare delivery and the process of follow-up to an abnormal mammogram
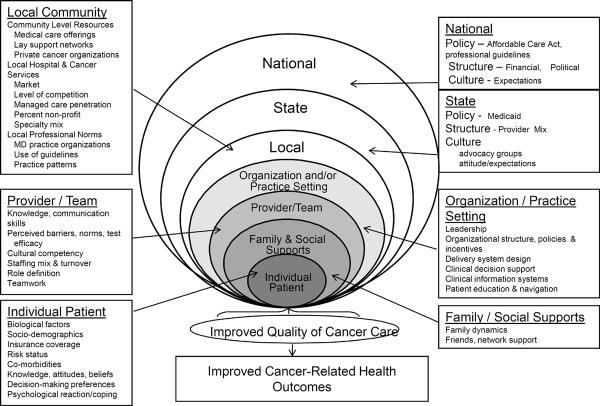
The multilevel perspective reflects consideration of the nested levels of influence upon care from individual patients and their families, to provider teams, organizations, communities, states, and the nation (Figure 1) (7). Factors at each of the levels shown in Figure 1 can affect the follow-up process and therefore are a useful focus when considering intervention strategies (8).
Figure 1.
The Multilevel Context of Cancer Care
Adapted from Zapka (8)
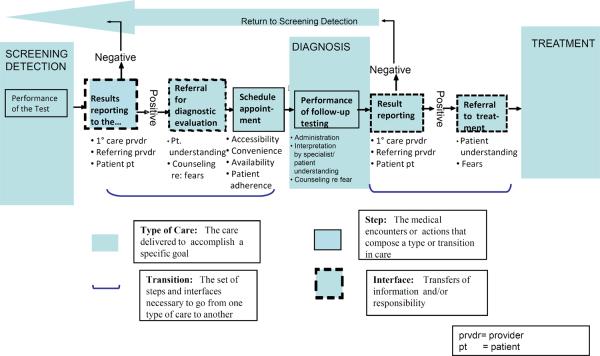
We selected the process of follow-up to abnormal mammograms as the illustrative example for this commentary because an abnormal mammogram is critical to the mortality reduction afforded by mammography and it is a point of vulnerability in the care process. Health care consists of multiple small steps, which are the circumscribed tasks that one individual undertakes, such as recommending a diagnostic mammogram or performing a biopsy. Steps are linked by communication among providers including physicians and other types of professional staff, patients, and their families. We call these points of communication the interfaces of care. At these interfaces people pass information and responsibility for what needs to happen next. Prevention, detection, and treatment are types of care along the care continuum that serve specific goals, but the transitions between them are also critical to success (9). The sum of all the steps, interfaces, types, and transitions in care across the cancer continuum is the overall process of cancer care (10). The follow-up of an abnormal screening mammogram is the transition from detection to diagnosis that we depict in Figure 2 (10,11).
Figure 2.
The Follow-up Process for an abnormal screening mammogram
Adapted from Zapka et al (8)
As noted earlier, factors in the multilevel context of care can be the target of an intervention. For example, at the individual level, an educational intervention of patients and primary care clinicians can address and improve their knowledge of screening and diagnostic testing. But other contextual levels also affect care. For example, whether the follow-up testing is done by the screening physician, how the care team tracks screening abnormalities and their resolution, the cultural competence of the providers, the design of the medical record, and the performance incentives adopted by the organization all will affect the behavior of the patient and providers and therefore the quality of care (Figure 1)(8). We recognize that major policy changes at the state and national levels can also affect care processes but we focus in this commentary on three levels (individual women, provider team, organization) for simplicity. Furthermore these are levels that health care leaders and providers can immediately and directly address to achieve improvements in the quality of care. Our focus on these three levels does not negate the importance of advocacy and interaction with people working at the state and national levels to improve cancer care delivery.
There are no nationally representative estimates of failure to follow-up an abnormal mammogram, but reported estimates in single populations and clinics vary from 9 to 50% (12–16). The variation in estimates is likely due to operational definitions of follow-up completion, as well as patient population and institutional differences (8, 12). The definitional challenge is illustrated in Figure 2 where follow-up could be defined as occurring when follow-up testing starts (administration) or is complete (after interpretation and reporting). For abnormal mammograms, follow-up completion could be as simple as additional imaging on the same day as the screening examination or as complex as additional imaging on one or more subsequent days followed by a biopsy some time later. Timeliness of follow-up has also been investigated. Furthermore, “follow-up” could also be concerned with whether the diagnostic evaluation is appropriate for the type of abnormality. Thus, some of the variation in follow-up is due to differences in definition and in the process of follow-up used by a facility where mammography occurs.
Differences in the study populations and/or practice organization included in the studies also affect follow-up estimates. Rosenberg and colleagues evaluated the time to follow-up initiation in 160 U.S. radiology facilities in the Breast Cancer Surveillance Consortium and found that the median time was 14 days, but that the proportion of women seen within this timeframe varied from 20% to more than 80% across the facilities (14). Richardson and colleagues found a median of 25 days to diagnosis among women with abnormal mammograms in the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) (16). Neither study was able to estimate with certainty what proportion of women was not seen at all, but their estimates varied between 27% and 7% (14, 16). Even in view of this variation in definition and study population, it seems clear that follow-up of abnormal breast screening is a significant problem that limits the potential morbidity and mortality reduction afforded by mammography screening.
Interventions to improve follow-up to abnormal mammogram
Intervention development and testing to improve follow-up to screening began in the mid-1980s. A review of reviews regarding follow-up of abnormal tests concluded that patient education, reminders to the physician, and organizational changes such as implementing automated reminders all positively influence the likelihood of follow-up (8). To date, most intervention strategies have focused on a single level intervention (e.g. the Individual women or provider, provider teams, or the organization). While many of these intervention studies showed positive effects, delayed and incomplete follow-up of abnormal mammograms has persisted.
We suggest that the challenge of follow-up to abnormal mammograms reflects the broader challenge of delivering high quality integrated patient-centered medical care in the US. Like the challenge in general, inadequate follow-up to an abnormal mammogram has been recognized for a long time. Furthermore, many evidence-based interventions have been developed to improve the quality of care. But many providers and organizations do not adopt those improvements in practice. Delayed or incomplete follow-up to mammography is therefore an interesting example for illustrating the challenge of improving the quality of cancer care within individual practices and organizations in the United States.
To address this challenge, several recent studies have investigated “navigation” interventions to improve follow-up to abnormal mammograms. Navigation to promote screening has been shown to be successful in low income and vulnerable populations, but testing of navigation via controlled trials to promote follow-up is a relatively recent phenomena (17). We identified 4 studies of follow-up to mammography and two that studied the follow-up of colorectal cancer screening tests (18, 19). While the components, methods, intensity, target population and setting of navigation vary across studies, all include verbal guidance regarding the follow-up process shown in Figure 2. In a 2006 observational study, Battaglia demonstrated that a navigator intervention appeared to be effective(20) and introduction of case managers in the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) shortened the time to follow-up among women seen through that program (21). Four recent randomized trials tested navigation interventions in mammography and confirmed its efficacy (22–25). All four trials showed significantly higher proportions of follow-up completion in the intervention compared to control arm (94–97% vs. 66–78%). One also showed a more rapid evaluation (mean of 25 vs 43 days, p=0.001)(26). All the randomized trials were conducted at the individual woman level in disadvantaged communities, among a variety of populations including Korean, African American, and Hispanic women. Bastani and colleagues considered the individual patient factors of beliefs and knowledge when designing an intervention using public health workers who telephoned women (27). Other navigation programs have addressed factors beyond beliefs and knowledge and have included, for example, the navigator attending appointments with the women (25). Another program involved having the radiologist staff schedule the follow-up appointment with the patient (26). None of the 4 studies, however, reported measures of effects of the navigator on other providers, or on system changes within the practice or organization.
Despite the research evidence for the success of navigation and a rising interest and support for its use across the continuum, it is available to a limited proportion of the population (17). We suggest that one reason for the lack of adoption is that navigation has unanticipated implications for providers and organizations. For example, navigators in the trials we identified had differing skill levels, as well as variable relationships and interactions with the provider team. They included peer counselors (23, 25), people with a bachelor's degree and 2 years of clinical experience (26), and social workers (24). Thinking about redefining roles and training the team how to incorporate this array of navigators into their care process are critical to subsequent adoption. Navigators must understand the tests and the meaning of the abnormality. They must interact with other providers to get the results of tests, understand what is next for the patient in the care process, and then either address a woman's barriers or literally guide her across the barriers to the next step in the process. In effect, they create another interface for communication and a new cost for delivering care. Given that reality, it is not surprising that there has been a long history of demonstration projects and tests of navigators but they have not been widely adopted (28,17). Furthermore navigators work on a case-by case basis to overcome the complexity and limitations of the follow-up process and our system of care, but do not change the underlying process (29). We suggest it is time to also consider interventions that directly address that system and the process of care.
Conceptualizing a new approach to intervention development
We advocate reconsideration of screening mammography follow-up from 3 perspectives: 1) the conceptualization of the problem as a multilevel issue, 2) identification of potentially modifiable factors at multiple levels of the context of care that may be subject to intervention, and 3) the need for interventions that explicitly consider and measure effects in the multilevel context of care. In the following sections, we discuss each of these three key issues in detail.
Conceptualization of the problem
Delayed or incomplete follow-up after an abnormal mammogram needs to be considered as a challenge to the care process rather than a problem of an individual woman's beliefs and knowledge. As described here and elsewhere, the follow-up process involves multiple steps where providers and their patients must take specific action(s) (Figure 2) (8). A woman will successfully complete the follow-up process only when all relevant individuals and institutions do their part. The roles of the provider team, the capabilities of the information system, and the relationships between organizations involved in screening and diagnosis all affect how the individuals behave in the process. Such a view means considering the problem as a system challenge rather than a problem at the level of an individual woman. Furthermore, intervention efforts focusing on a single woman who may have 1 or 2 abnormal mammograms in her lifetime doesn't reach many people. A multi-level intervention simultaneously targeted to women, physicians, and the organization has the potential to reach thousands of women with abnormal mammograms within a single health care setting.
Recognition of factors at multiple levels of the context of care that affect steps and interfaces of care
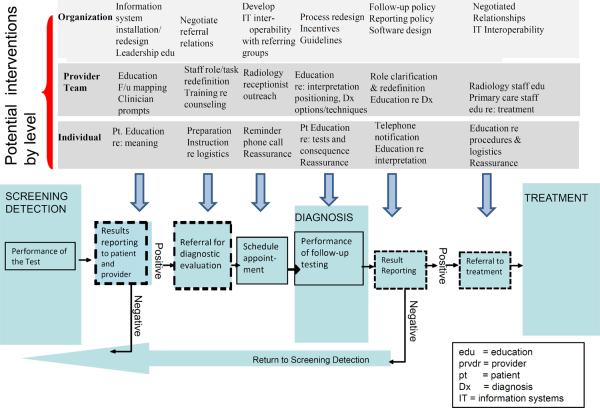
Figure 3 shows three levels (individual woman, provider team, and organization) of the ecological model across the three types of care (screening detection, diagnosis, and treatment) and details steps and interfaces that could be affected. The challenge is to consider how factors in the multilevel context affect the steps and interfaces in the process of follow-up to an abnormal test. It is the steps and interfaces that must be affected to change the follow-up process.
Figure 3.
Potential Interventions in selected levels of the Multilevel Context of Cancer Care
Adapted from Zapka et al (8)
We focus on follow-up to mammography and show where interventions might affect some potential steps and interfaces in Figure 3. But one could also consider follow-up to a positive fecal occult blood test, or abnormal Papanicolau test. That consideration would involve different steps and interfaces, and as a result offer different opportunities for improving the process of care. Developing a multilevel intervention to improve the follow-up of abnormal screening tests first requires clarifying the process of care and then considering the factors that might affect specific steps and interfaces that are critical to successful follow-up care.
Research at the individual woman level has identified socioeconomic factors, insurance coverage, beliefs and knowledge that may be associated with failure to complete follow-up (8,13,30,26). But women must know they have an abnormality before any of their beliefs and fears can be addressed. Problems with women not getting their screening test results were the motivation for the Mammography Quality Standards Act requirement that women be notified of their results by the interpreting radiologist (31). Once patients are informed of an abnormality, then they must communicate with their provider team to identify the next steps. Figure 3 shows specific points in the follow-up process where providers can address knowledge and fears that affect the woman's likelihood of follow-up (13). There has been a generation of work to improve understanding women's knowledge and beliefs about breast cancer, and most of the navigation studies included consideration of these issues, but the work by Bastani et al demonstrates that while it may be necessary to address these beliefs, it is not sufficient (27).
Identifying the person who will assess and address a woman's knowledge and beliefs for each step in the follow-up process is a starting point for the provider level intervention, but other activities must also be investigated to assure follow-up. Figure 3 notes that the provider team can map the follow-up process and identify the specific steps and interfaces in their system. Coaching providers to evaluate the steps and interfaces of their care process is one potential intervention for identifying where care delivery may be vulnerable to losing results, failing to notify women, or failing to arrange the referral, and track the results. Once the steps are clear then role redefinition offers an opportunity to identify who is responsible for contacting women at each point along the pathway, and how information and communications systems will be used. The physician, licensed practical nurse, receptionist, or an administrative person could be assigned responsibility for specific steps in the process and use an information system to assure everyone knows what the other has done (32,33). Whoever is assigned the work must have the appropriate knowledge and skill, so there is a training component for providers and other personnel that also could be part of an intervention. The members of the provider team could use mail, phone, internet portals, email or in-person visits to communicate the next steps in the evaluation to the woman; they could schedule the follow-up appointment and communicate with the other providers performing the evaluation. While workloads in primary care are extraordinary, organizing work and clarifying roles to address work functions and distributing responsibilities across members of the provider team could offer relief for everyone, and assurance that the work gets done.
It might also be possible for the referring physicians and radiology departments to share concerns and adopt procedures satisfactory and safe for all. Radiologists have legal responsibilities as well as financial incentives to notify women of their mammography results. Who is responsible for assuring the follow-up occurs is less clear. The referring provider, the woman, and the radiologist all must communicate to assure the woman acts on the notification. Consideration of the provider level means defining and redefining provider roles but it also opens possibilities for testing ways to distribute functions, negotiate relationships, and clarify responsibilities among primary care and radiology providers as well as women.
There are issues that need to be addressed at the organizational level as well (Figure 3). Organizations vary tremendously in their structure; from small groups of primary and specialty care providers taking only privately insured people, to large integrated health plans, or Federally Qualified Health centers serving the uninsured. An established referral pattern, information systems that exchanged information on referrals, or clear commercial relationships would facilitate the follow-up process and be affected by the type of organizations involved. Organizations could be encouraged to negotiate regarding the logistics of referrals and who provides the functions that successfully ensures a woman traverses the follow-up evaluation. To some extent the Accountable Care Organizations called for in the Affordable Care Act are a mechanism to incent the discussions among organizations that address some of the issues of relationships and referrals (34). The Affordable Care Act incents the adoption of electronic medical records so organizations could establish software that tracks women and facilitates communication among all involved. Administrators in organizations could establish a culture of rapid evaluation and support teams in accomplishing changes needed to achieve the goal of completing follow-up. The discussions and cultural change needed to achieve that goal are all organizational functions and need to be considered with intervention strategies at other levels that depend upon such change (35).
Multilevel interventions involve changes at specific levels but they also depend upon interaction between levels. Reminders and prompts mean someone must assume responsibility for developing the system and financing the reminder and how it is delivered. If the reminder depends upon an information system, then it must be a system that can perform the appropriate function, and works within the organization's information technology environment (32,33). At least one study shows that an information system with incorrect data will stimulate the wrong behavior (33). Whether the reminder is being sent by a computer, a designated provider team member, or administrative personnel in the organization, the communication is an interaction. That interaction involves resources the organization must supply to the provider team who in turn must communicate with the woman.
Testing of Multilevel Interventions
Ultimately, the efficacy and effectiveness of any proposed multilevel intervention must be tested. While it is possible to continue testing interventions that focus exclusively at the individual patient or provider level, the point of this commentary is that such a reductionist approach does not encourage investigation of the intervention's consequences at other levels of the context in which it is being implemented. The Institute of Medicine identified the need to conceive of behavior as a multilevel problem in its 2001 report on “Health & Behavior: the Interplay of Biological, Behavioral, and Societal Influences” (36). Meissner et al called for multilevel interventions in 2004, and the National Cancer Institute supported a national meeting on the topic in March 2011(2,37,38). Conference participants discussed 12 papers that are published in “Multilevel Interventions in Health Care Across the Cancer Continuum” (2, 39). Creating and testing multilevel interventions is not simple but the time has come to begin the hard work of doing it (2, 35, 40,41,42).
Considerations for multilevel interventions include the following: 1) identifying the appropriate levels for intervening and conceiving of the interactions between the levels, and the mechanism of the intervention effect (2, 35, 43); 2) measuring the intervention effects at each level and on the overall quality of care delivered (1, 44); 3) timing the measurements or obtaining serial measurements in order to capture the effect; 4) evaluating the overall outcome of all the improvements (45, 46); and 5) estimating the size and scope of such work and the need for appropriate collaborations, method development, and budgeting (40). Systems science, mathematical modeling, and conceiving of interventions that include explicit interactions are all now possible (35,47). Considering these models, and taking multilevel factors into consideration leads to complexity, but there is relatively little choice but to proceed.
Conclusion
Historically, cancer research has focused on valid intervention tests of specific technologies and therapies that are then poorly or slowly adopted because contextual factors provide barriers. We suggest that it is time to consider the multilevel context from the outset of intervention development and that such consideration may contribute to improvements in the quality of cancer care that are both effective and sustainable.
Reference List
- (1).Institute of Medicine, Commission on Life Sciences National Research Council . Ensuring quality cancer care. National Academy Press; Washington, DC: 1999. [Google Scholar]
- (2).Taplin SH, Anhang Price R, Edwards HM, Foster MK, Breslau ES, Chollette V, et al. Introduction to the journal supplement: understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;(44):2–10. doi: 10.1093/jncimonographs/lgs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw. 2009;7(2):193–201. doi: 10.6004/jnccn.2009.0013. [DOI] [PubMed] [Google Scholar]
- (4).Barni S, Petrelli F, Cabiddu M, Cazzaniga ME, Cremonesi M. From the trastuzumab era to new target therapies: beyond revolution. Ann Oncol. 2007;18(Suppl 6):vi1–vi4. doi: 10.1093/annonc/mdm214. [DOI] [PubMed] [Google Scholar]
- (5).Spinks T, Albright HW, Feeley TW, Walters R, Burke TW, Aloia T, et al. Ensuring quality cancer care: A follow-up review of the Institute of Medicine's 10 recommendations for improving the quality of cancer care in America. Cancer. 2012 May 15;118(10):2571–82. doi: 10.1002/cncr.26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wagner EH, Aiello Bowles EJ, Greene SM, Tuzzio L, Wiese CJ, Kirlin B, et al. The quality of cancer patient experience: perspectives of patients, family members, providers and experts. Qual Saf Health Care. 2010;19(6):484–9. doi: 10.1136/qshc.2010.042374. [DOI] [PubMed] [Google Scholar]
- (7).Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12(1):4–13. [PubMed] [Google Scholar]
- (8).Zapka J, Taplin SH, Price RA, Cranos C, Yabroff R. Factors in quality care--the case of follow-up to abnormal cancer screening tests--problems in the steps and interfaces of care. J Natl Cancer Inst Monogr. 2010;2010(40):58–71. doi: 10.1093/jncimonographs/lgq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–41. doi: 10.1001/jama.297.8.831. [DOI] [PubMed] [Google Scholar]
- (10).Taplin SH, Rodgers AB. Toward improving the quality of cancer care: addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;2010(40):3–10. doi: 10.1093/jncimonographs/lgq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Anhang Price R, Zapka J, Edwards H, Taplin SH. Organizational factors and the cancer screeningprocess. J Natl Cancer Inst Monogr. 2010;2010(40):38–57. doi: 10.1093/jncimonographs/lgq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wujcik D, Fair AM. Barriers to diagnostic resolution after abnormal mammography: a review of the literature. Cancer Nurs. 2008;31(5):E16–E30. doi: 10.1097/01.NCC.0000305764.96732.45. [DOI] [PubMed] [Google Scholar]
- (13).Chen ET, Eder M, Elder NC, Hickner J. Crossing the finish line: follow-up of abnormal test results in a multisite community health center. J Natl Med Assoc. 2010;102(8):720–5. doi: 10.1016/s0027-9684(15)30658-1. [DOI] [PubMed] [Google Scholar]
- (14).Rosenberg RD, Haneuse SJ, Geller BM, Buist DS, Miglioretti DL, Brenner RJ, et al. Timeliness of follow-up after abnormal screening mammogram: variability of facilities. Radiology. 2011;261(2):404–13. doi: 10.1148/radiol.11102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Yabroff KR, Breen N, Vernon SW, Meissner HI, Freedman AN, Ballard-Barbash R. What factor are associated with diagnostic follow-up after abnormal mammograms? Findings from a U.S. National Survey. Cancer Epidemiol Biomarkers Prev. 2004;13(5):723–32. [PubMed] [Google Scholar]
- (16).Richardson LC, Royalty J, Howe W, Helsel W, Kammerer W, Benard VB. Timeliness of breast cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program, 1996-2005. Am J Public Health. 2010;100(9):1769–76. doi: 10.2105/AJPH.2009.160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin. 2011;61(4):237–49. doi: 10.3322/caac.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Lasser KE, Murillo J, Lisboa S, Casimir AN, Valley-Shah L, Emmons KM, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med. 2011;171(10):906–12. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- (19).Percac-Lima S, Grant RW, Green AR, Ashburner JM, Gamba G, Oo S, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24(2):211–7. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Battaglia TA, Roloff K, Posner MA, Freund KM. Improving follow-up to abnormal breast cancer screening in an urban population. A patient navigation intervention. Cancer. 2007;109(2 Suppl):359–67. doi: 10.1002/cncr.22354. [DOI] [PubMed] [Google Scholar]
- (21).Lobb R, Allen JD, Emmons KM, Ayanian JZ. Timely care after an abnormal mammogram among low-income women in a public breast cancer screening program. Arch Intern Med. 2010;170(6):521–8. doi: 10.1001/archinternmed.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Ferrante JM, Gonzalez EC, Pal N, Roetzheim RG. Effects of physician supply on early detection of breast cancer. J Am Board Fam Pract. 2000;13(6):408–14. doi: 10.3122/15572625-13-6-408. [DOI] [PubMed] [Google Scholar]
- (23).Maxwell AE, Bastani R, Vida P, Warda US. Results of a randomized trial to increase breast and cervical cancer screening among Filipino American women. Prev Med. 2003;37(2):102–9. doi: 10.1016/s0091-7435(03)00088-4. [DOI] [PubMed] [Google Scholar]
- (24).Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2007;44(1):26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- (25).Crump SR, Shipp MP, McCray GG, Morris SJ, Okoli JA, Caplan LS, et al. Abnormal mammogram follow-up: do community lay health advocates make a difference? Health Promot Pract. 2008;9(2):140–8. doi: 10.1177/1524839907312806. [DOI] [PubMed] [Google Scholar]
- (26).Ferrante JM, Chen PH, Kim S. The effect of patient navigation on time to diagnosis, anxiety, and satisfaction in urban minority women with abnormal mammograms: a randomized controlled trial. J Urban Health. 2008;85(1):114–24. doi: 10.1007/s11524-007-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Bastani R, Mojica CM, Berman BA, Ganz PA. Low-income women with abnormal breast findings: results of a randomized trial to increase rates of diagnostic resolution. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1927–36. doi: 10.1158/1055-9965.EPI-09-0481. [DOI] [PubMed] [Google Scholar]
- (28).Whitley E, Valverde P, Wells K, Williams L, Teschner T, Shih YC. Establishing common cost measures to evaluate the economic value of patient navigation programs. Cancer. 2011;117(15 Suppl):3618–25. doi: 10.1002/cncr.26268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Robinson-White S, Conroy B, Slavish KH, Rosenzweig M. Patient navigation in breast cancer: a systematic review. Cancer Nurs. 2010;33(2):127–40. doi: 10.1097/NCC.0b013e3181c40401. [DOI] [PubMed] [Google Scholar]
- (30).Bastani R, Yabroff KR, Myers RE, Glenn B. Interventions to improve follow-up of abnormal findings in cancer screening. Cancer. 2004;101(5 Suppl):1188–200. doi: 10.1002/cncr.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Mammography Quality Standards Act. 2012 http://www.fda.gov/radiation-emittingproducts/mammographyqualitystandardsactandprogram/default.htm; Consulted 7/3/2012.
- (32).Taplin SH, Rollason D, Camp A, diDonato K, Maggenheimer E. Imagining an electronic medical record for turning cancer screening knowledge into practice. Am J Prev Med. 2010;38(1):89–97. doi: 10.1016/j.amepre.2009.09.037. [DOI] [PubMed] [Google Scholar]
- (33).Singh H, Wilson L, Petersen LA, Sawhney MK, Reis B, Espadas D, et al. Improving follow-up of abnormal cancer screens using electronic health records: trust but verify test result communication. BMC Med Inform Decis Mak. 2009;9:49. doi: 10.1186/1472-6947-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Public Law 111-148: Patient Protection and Affordable Care Act. 2010.
- (35).Weiner B, Lewis M, Clauser S, Stitzenberg K. In search of synergy: strategies for combining interventions at multiple levels. Natl Cancer Inst Monogr. 2012;2012(44):34–41. doi: 10.1093/jncimonographs/lgs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).National Research Council . Health & behavior: the interplay of biological, behavioral, and societal influences. The National Academies Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- (37).Meissner HI, Smith RA, Rimer BK, Wilson KM, Rakowski W, Vernon SW, et al. Promoting cancer screening: Learning from experience. Cancer. 2004;101(5 Suppl):1107–17. doi: 10.1002/cncr.20507. [DOI] [PubMed] [Google Scholar]
- (38).National Cancer Institute Multilevel Interventions in Health Care: Building the Foundation for Future Research. ( http://cancercontrol.cancer.gov/mli/meetingInfo.htm) (Consulted 7/3/ 2012)
- (39).Edwards HM, Taplin SH, Chollette V, Clauser SB, Prabhu Das IP, Kaluzny AD. Summary of the multilevel interventions in health care conference. J Natl Cancer Inst Monogr. 2012;2012(44):123–6. doi: 10.1093/jncimonographs/lgs018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Clauser S, Taplin S, Foster M, Fagan P, Kaluzny AD. Multilevel intervention research: lessons learned and pathways forward. J Natl Cancer Inst Monogr. 2012;44:127–33. doi: 10.1093/jncimonographs/lgs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Stange K, Breslau E, Dietrich A, Glasgow R. State-of-the-art and future directions in multilevel interventions across the cancer control continuum. J Natl Cancer Inst Monogr. 2012;2012(44):20–31. doi: 10.1093/jncimonographs/lgs006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health. 2007;28:413–33. doi: 10.1146/annurev.publhealth.28.021406.144145. [DOI] [PubMed] [Google Scholar]
- (43).Zapka J, Taplin SH, Ganz P, Grunfeld E, Sterba K. Multilevel factors affecting quality: examples from the cancer care continuum. J Natl Cancer Inst Monogr. 2012;44:11–9. doi: 10.1093/jncimonographs/lgs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Charns MP, Foster MK, Alligood EC, Benzer JK, Burgess JF, Jr, Li D, et al. Multilevel interventions: measurement and measures. J Natl Cancer Inst Monogr. 2012;44:67–77. doi: 10.1093/jncimonographs/lgs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Cleary P, Gross C, Zaslavsky A, Taplin S. Multilevel interventions: study design and analysis issues. J Natl Cancer Inst Monogr. 2012;44:49–55. doi: 10.1093/jncimonographs/lgs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Murray DM, Pennell M, Rhoda D, Hade EM, Paskett ED. Designing studies that would address the multilayered nature of health care. J Natl Cancer Inst Monogr. 2010;2010(40):90–6. doi: 10.1093/jncimonographs/lgq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Morrissey J, Hassmiller-Lich K, Anhang Price R, Mandelblatt J. Computational modeling and multilevel cancer control interventions. J Natl Cancer Inst Monogr. 2012;44:56–66. doi: 10.1093/jncimonographs/lgs014. [DOI] [PMC free article] [PubMed] [Google Scholar]