Abstract
Respiratory control entails coordinated activities of peripheral chemoreceptors (mainly the carotid bodies) and central chemosensors within the brain stem respiratory network. Candidates for central chemoreceptors include Phox2b-containing neurons of the retrotrapezoid nucleus, serotonergic neurons of the medullary raphé, and/or multiple sites within the brain stem. Extensive interconnections among respiratory-related nuclei enable central chemosensitive relay. Both peripheral and central respiratory centers are not mature at birth, but undergo considerable development during the first two postnatal weeks in rats. A critical period of respiratory development (~P12–13 in the rat) exists when abrupt neurochemical, metabolic, ventilatory, and electrophysiological changes occur. Environmental perturbations, including hypoxia, intermittent hypoxia, hypercapnia, and hyperoxia alter the development of the respiratory system. Carotid body denervation during the first two postnatal weeks in the rat profoundly affects the development and functions of central respiratory-related nuclei. Such denervation delays and prolongs the critical period, but does not eliminate it, suggesting that the critical period may be intrinsically and genetically determined.
Keywords: Carotid body, medullary raphé nuclei, nucleus tractus solitarius, pre-Bötzinger complex, retrotrapezoid nucleus/parafacial respiratory group, ventrolateral medulla
1. Introduction
In mammals, respiration commences before birth, undergoes drastic changes during early postnatal life, and continues throughout the lifespan of the animal. The intake of oxygen ultimately leads to the coupling of oxidative phosphorylation and electron transport in the mitochondria for the generation of ATP, the currency for vital cellular functions. Carbon dioxide is disposed of by the respiratory system. This simple yet vital function necessitates the exquisite orchestration of numerous players at multiple levels in mammals, such as the upper airway and bronchopulmonary structures, carotid bodies, cranial nerves and nuclei, spinal cord, brain stem neural network, as well as the musculoskeletal and cardiovascular systems. Each of these components is under the influence of a multitude of neurochemicals, including neurohormones, neurotransmitters, and neuromodulators. The control of these neurochemicals, in turn, is regulated by cellular functions, feedback loops, transcription, and translation.
The respiratory system is not mature at birth, but undergoes significant development postnatally. The exact sequence of development for each of the component parts is not well understood and is likely to differ among species. This review concentrates on peripheral and central chemoreceptors/chemosensors, and how their interactions influence the development of respiratory control. Chemoreceptors in the respiratory system, by definition, transduce the gaseous and pH signals from the blood into neural signals. Chemosensors, as referred to in this review, include both chemoreceptors and cells downstream of the receptors that are also sensitive to the chemical signal. Chemosensors are vital for the proper functioning of the respiratory system under normal conditions, but they are even more critical when the mixture of component gases is outside of the normal range, as their signals elicit responses that ultimately determine survival or non-survival. The sensitivity of chemoreceptors undergoes adjustments during normal development, but when exposed to abnormal gas mixtures for extended periods of time, their properties and influence on respiration will likewise be modified. This review touches mainly upon some aspects of normal development as well as responses to specific experimental manipulations during the first 3 postnatal weeks in rats. Greater emphasis is paid to the critical period of postnatal respiratory development (around P12–13 in the rat), when sudden neurochemical, metabolic, ventilatory, and electrophysiological changes occur. The existence and details of interactions between peripheral and central chemoreceptors remain poorly understood. The goal of the review is not to provide definitive answers, but rather, serve as a springboard for new ideas, debate, suggestions, and future experimentations. For more in-depth coverage on specific topics, readers are referred to several excellent reviews in recent years on peripheral and central chemoreception (Fong, 2010; Gauda et al., 2009; Nurse, 2010; Prabhakar, 2011), including a Special Issue on Central Chemoreception (Nattie and Forster, 2010).
2. Peripheral chemoreceptors: the carotid body
The glomus cells (chief cells or type I cells) of the carotid body constitute the major peripheral chemoreceptors. They sense and respond to decreased partial pressure of O2, increased partial pressure of CO2, and decreased pH in the abundance of arterial blood flowing through them (Gonzalez et al., 1994). These cells synapse with chemoafferent fibers that form the sinus branch of the glossopharyngeal nerve (whose cell bodies occupy the petrosal ganglion), which projects centrally to various nuclei in the brain stem (Finley and Katz, 1992). There are reciprocal synapses between the glossopharyngeal afferent fibers and glomus cells, as well as reciprocal synapses between the glomus cells (Nurse, 2010).
The exact mechanism of hypoxia-induced chemotransduction within the carotid body is not understood, but it involves an increase in [Ca2+]i and Ca2+-dependent release of neurotransmitters from glomus cells as well as inhibition of specific O2-sensitive K+ channels (TASK and BKCa channels) (Biscoe et al., 1989; Gonzalez et al., 2009; Peers et al., 2010). Neurochemicals found within the rat carotid body include ATP, acetylcholine (ACh), γ-aminobutyric acid (GABA), serotonin (5-hydroxytryptamine or 5-HT), adenosine, dopamine, norepinephrine, histamine, and neuropeptide Y (Gauda et al., 2004; Katz et al., 1993; Nurse, 2010; Oomori et al., 2002). The first eight neurochemicals are reportedly contained in and released by glomus cells (Nurse, 2010; Verna et al., 1993). ATP, acting on the ionotropic P2X2/3 receptors of the carotid sinus nerve afferent terminals, mediates fast-acting chemoexcitation conducted centrally (Prasad et al., 2001; Zhang et al., 2000). In addition, three endogenous gaseous messengers have been proposed. Nitric oxide released by the glossopharyngeal efferent fibers upon ATP-activation of their P2X receptors hyperpolarizes glomus cells (Campanucci et al., 2006; Prabhakar, 1999). Carbon monoxide also inhibits carotid body activity, whereas hydrogen sulfide reportedly enhances the organ’s sensory response to hypoxia (Prabhakar and Semenza, 2012). The development of this critical peripheral chemoreceptor, the carotid body, is intimately associated with the maturation of the respiratory system (Carroll and Kim, 2005; Donnelly, 2005).
Another peripheral chemosensor that plays a less important role but may take over some of the functions after carotid body denervation is the aortic body (Loeschcke, 1982; Serra et al., 2002). This structure and other possible peripheral chemosensitive sites will not be covered in this review.
3. Central chemoreceptor candidates
Unlike the definitive identification of peripheral chemoreceptors, that of central chemoreceptors has been under investigation for the past few decades but remains largely unresolved. The existence of special, central chemoreceptors for pH and/or PCO2 was postulated by von Euler and Söderberg in decerebrate cats (Von Euler and Soderberg, 1952). Loeschcke and his colleagues have localized chemoreceptors in the cat to the ventral surface of the medulla, where a rostral and a caudal chemosensitive area plus an intermediate area were identified (Loeschcke, 1982; Loeschcke et al., 1963). They also found that extracellular pH, which is dependent on tissue PCO2 and HCO3− concentrations in tissues, was the main chemical signal that determines the ventilatory response. These ground-breaking studies led to the discovery of a number of chemosenstive sites in the brain stem. Currently, there are three schools of thought regarding central chemoreceptor candidates: those favoring neurons in the retrotrapezoid nucleus (RTN) (Guyenet and Mulkey, 2010), medullary raphé neurons (Hodges and Richerson, 2010), or multiple chemoreceptor sites (Nattie, 2000).
The RTN was initially described as a thin sheet of neurons ventral to the facial nucleus in the ventrolateral medulla that projected to the dorsal and ventral respiratory groups and were suspected to contribute to respiratory chemoreception (Smith et al., 1989). Since then, those RTN neurons that are chemosensitive in the rat have been extensively characterized to be noncholinergic nonaminergic glutamate- and substance P-containing as well as Phox2b-expressing neurons (more recently called ccRTN for chemical-coded RTN neurons); they have intrinsic CO2 and pH sensitivity even in the absence of the carotid body; they are activated by CO2 in vivo and in vitro; and they correspond spatially to neonatal parafacial respiratory group (pFRG) neurons that reportedly mediate respiratory rhythmogenesis at that age (Guyenet and Mulkey, 2010; Lazarenko et al., 2009; Li et al., 1999; Onimaru et al., 2008). The fact that congenital central hypoventilation syndrome (CCHS), in which central chemosensitivity is essentially absent, is caused by a mutation of the PHOX2B gene with triplet polyalanine expansion strongly supports the role of RTN as central chemoreceptors (Amiel et al., 2003; Guyenet, 2008). However, many other brain regions are apparently involved as well in this disease (Patwari et al., 2010). Significantly, transgenic mice with a +7 alanine expansion of the 20-residue polyalanine tract of Phox2b gene exhibited irregular breathing, a lack of response to hypercapnia, and died soon after birth from central apnea (Dubreuil et al., 2008). Remarkably, only RTN/pFRG neurons were selectively lost in these mutants, suggesting that these neurons play an important role in respiration and in mediating central chemoreception (Dubreuil et al., 2008). RTN neurons are necessary for the hypercapnic response of the respiratory network during the perinatal period and under anesthesia, but the extent to which they sense changes in blood pH and contribute to the pH-dependent regulation of respiration in conscious adult rats is less clear. Other cell types (such as glial cells and vascular cells) have not yet been ruled out as possible chemoreceptors (Guyenet et al., 2010).
Medullary raphé neurons are located in the raphé magnus (RM), raphé obscurus (ROb), and raphé pallidus (RP) and many of them are serotonergic. They were initially reported to respond to increased PCO2 or decreased pH with either augmented or reduced firing rate (Wang et al., 1998). Whereas all acidosis-stimulated neurons were immunoreactive for the serotonin synthesizing enzyme tryptophan hydroxylase (TPH), not all acidosis-inhibited ones were (Wang et al., 2001b). The close proximity of these neurons to large medullary arteries makes them ideal candidates for sensing arterial blood CO2 (Bradley et al., 2002). Besides 5-HT, these neurons also contain thyrotropin-releasing hormone and substance P (Wang et al., 2001a; Wang et al., 2001b). The release of these three neurochemicals is thought to play an important role in the control of pH homeostasis and in stimulating the network of respiratory and autonomic neurons spread over numerous sites (Richerson, 2004). One of these sites is actually the RTN, where serotonin and pH have an additive effect on the discharge rate of neurons; however, serotonin reportedly does not affect the pH sensitivity of these neurons (Mulkey et al., 2007). In conscious adult rats exposed to increasing concentrations of CO2 from baseline to 20% for 5 min, a subset of large serotonergic neurons in the ventrolateral periaqueductal gray and ventrolateral part of the dorsal raphé nucleus were labeled with the protooncogene c-fos, thus implicating them as central chemoreceptors in vivo (Johnson et al., 2005). Transgenic mice with conditional knockout of Lmx1b gene in Pet1-expressing 5-HT neurons showed an almost complete absence of central 5-HT neurons, yet they survived to adulthood (Zhao et al., 2006). However, when these adult mice were challenged with hypercapnia, their ventilatory response was reduced by 50%, even though their baseline ventilation and hypoxic ventilatory response were normal (Hodges et al., 2008). These findings support the role of at least a subset of 5-HT neurons in CO2 chemoreflex and in increasing the gain of the respiratory network sensitivity to hypercapnia (Hodges et al., 2008). Despite the compelling evidence, questions remain as to the exact percentage of serotonergic neurons that respond directly to acidification in vivo, whether true serotonergic central chemoreceptors are restricted to specific medullary midline raphé neurons, and whether the CO2 response is simply a reflection of synaptic inputs (Guyenet et al., 2010). Serotonergic neurons in the midbrain are reportedly also chemosensitive and may mediate non-respiratory responses to hypercapnia, such as arousal (Richerson, 2004).
The third school of thought is championed by Nattie’s group, who proposed a widespread distribution of central chemoreceptors (Nattie, 2000). Multiple sites are necessary in this model because they allow for regional diversity, imbalance, state-dependence, and an enhancement of the collective whole (Nattie and Li, 2009). RTN neurons were found to respond to focal acidification with increased ventilation only in wakefulness but not in sleep (Li et al., 1999), whereas medullary raphé neurons did so only in sleep and not in wakefulness (Nattie and Li, 2001), and neurons in the nucleus tractus solitarius (NTS) responded during both states (Nattie and Li, 2002). When the RTN and caudal raphé neurons were simultaneously inhibited in conscious rats, reductions in ventilation and the hypercapnic response were seen in both wakefulness and non-rapid eye movement sleep (Li et al., 2006). However, whereas RTN neurons responded to CO2 when raphé neurons were inhibited, the latter did not respond to CO2 when RTN neurons were inhibited, causing the authors to conclude that caudal raphé neurons provide a non-CO2-dependent tonic drive to respiration by potentiating the effect of RTN or other downstream sites, whereas rostral medullary raphé neurons do respond to increased CO2 (Li et al., 2006). Other central chemoreceptors proposed include the NTS (Nattie and Li, 2002), the pre-Bötzinger complex (PBC) (Solomon et al., 2000), noradrenergic neurons of the locus coeruleus (LC) (Gargaglioni et al., 2010), orexinergic neurons of the hypothalamus (Williams et al., 2007), the ventral medullary surface (VMS) caudal to the RTN, and the fastigial nucleus of the cerebellum (Mitchell et al., 1963; Nattie and Li, 2009).
At present, there is no consensus as to which site or sites represent(s) true central chemoreceptors. Definitive conclusions await in vivo documentation of the magnitude and percentage of response to alterations in blood pH or gases by local activation and inhibition of specific neurons (or other cell types) in the absence of any external connections in conscious, unanesthetized animals, and this may pose a technical challenge (Nattie and Li, 2009). In addition, the mechanism of chemotransduction needs to be elucidated. However, there is no doubt that there are many loci within the brain stem that are chemosensitive, either via direct chemoreception or via synaptic connections. For this reason, all discussions below will simply refer to the specific nuclei by name without labeling them as central chemoreceptors.
4. Interactions between peripheral and central mediators of chemosensation
4.1 Anatomical consideration
The carotid sinus nerve relays excitatory signals from the carotid body centrally to a number of brain stem nuclei in the dorsomedial medulla and the ventrolateral medulla (VLM) (Fig. 1). Specifically, it projects heavily and bilaterally to the commissural and medial nuclei of NTS (NTSCOM and NTSM), moderately and bilaterally to the intermediate, interstitial, and dorsolateral nuclei of NTS, ipsilaterally to the ventrolateral nucleus of NTS (NTSVL) and the caudal VLM in the region of nucleus retroambiguus, and sparsely to the dorsal motor nucleus of the vagus (DMNX) and area postrema (Finley and Katz, 1992). Thus, peripheral chemosensory afferents are able to interact with cardiorespiratory neurons throughout the NTS and in the VLM.
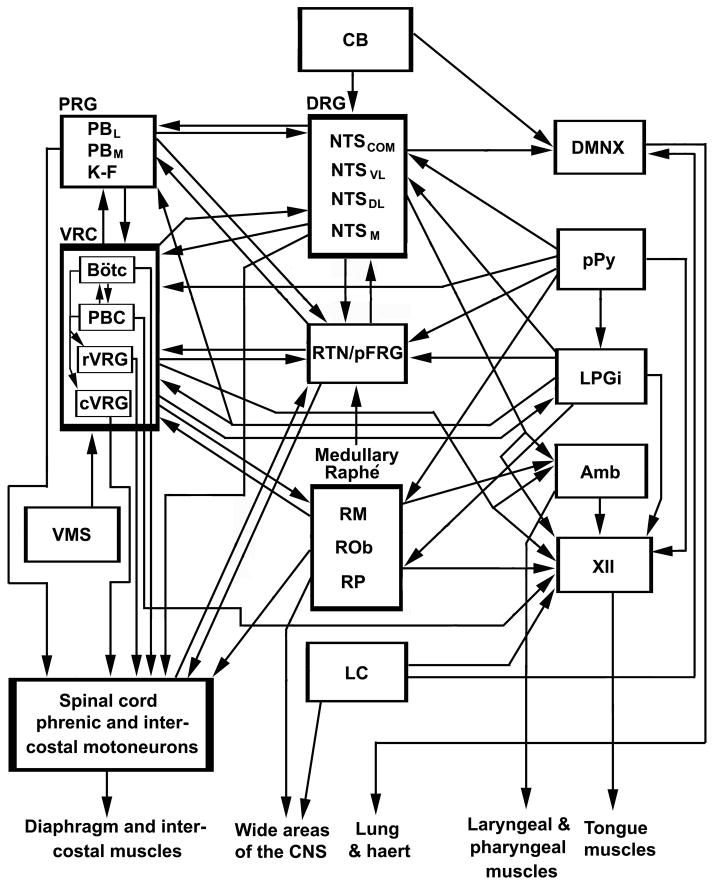
Fig. 1.
Schematic diagram of connections linking the peripheral chemoreceptor carotid body directly and indirectly to many mediators of central chemosensitivity within the respiratory network. All connections are based on published papers, but not all published reports are included. XII, hypoglossal nucleus; Amb, nucleus ambiguus; BötC, Bötzinger complex; CB, carotid body; cVRG, caudal ventral respiratory group; DMNX, dorsal motor nucleus of the vagus; DRG, dorsal respiratory group; K–F, Kölliker-Fuse nucleus; LC, locus coeruleus; LPGi, lateral paragigantocellular nucleus; NTS, nucleus tractus solitarius (including the commissural [NTSCOM], dorsolateral [NTSDL], medial (NTSM], and ventrolateral [NTSVL] subnuclei); PBC, pre-Bötzinger complex; PBL, PBM, lateral and medial parabrachial nuclei; pPy, parapyramidal region; PRG, pontine respiratory group; RM, raphé magnus, ROb, raphé obscurus, RP, raphé pallidus; RTN/pFRG, retrotrapezoid nucleus/parafacial respiratory group; VRG, ventral respiratory group (cVRG, rVRG, caudal and rostral VRG); VMS, ventral medullary surface; VRC, ventral respiratory column. The term “ventrolateral medulla or VLM” is not included here because it is loosely used by different investigators to mean one or more nuclear groups separately listed here.
The NTS, in turn, projects widely to three brain stem respiratory groups: the pontine respiratory group (PRG, made up of the parabrachial (PB) nuclei and the Kölliker-Fuse nucleus (K–F)), the dorsal respiratory group (DRG, composed of interconnected NTS subnuclei), and the ventral respiratory column (VRC). The VRC includes the Bötzinger complex (BötC), PBC, the rostroventral respiratory group (rVRG), the caudoventral respiratory group (cVRG), and may also include the retrofacial nucleus, nucleus ambiguus (Amb), and the RTN/pFRG) (Aicher et al., 1995; Cunningham and Sawchenko, 1989; Garcia et al., 2011; Herbert et al., 1990; Nunez-Abades et al., 1993; Ross et al., 1985; Smith et al., 2009; Takakura et al., 2006). The NTS also projects to DMNX, phrenic motoneurons, and intercostal motoneurons (Mtui et al., 1993; Ross et al., 1985) (Fig. 1). In this way, the original message from the peripheral chemoreceptors can theoretically reach wide areas of the brain stem, forming a chemosensory network that ultimately affects the respiratory output of the lungs, diaphragm, and intercostal muscles.
Interconnections also exist among the major central chemoreceptor candidates. Besides direct connections from the NTS to the RTN described above, the RTN also receives serotonergic input from the medullary raphé, but presumably by a pH-independent mechanism (Mulkey et al., 2007). Serotonergic neurons from RM, ROb, and RP also project to the rostral VRG (Ellenberger and Feldman, 1990; Holtman et al., 1990) and are reciprocally connected with the rostral ventrolateral medulla (Zagon, 1993), in addition to their projections to wide areas of the central nervous system. Thus, there are extensive interconnections among the DRG, VRG, and PRG. Figure 1 depicts known interconnections within the respiratory-related network. However, not all connections are included.
4.2 Physiological consideration
Early studies by Loeschcke and his group showed that carotid sinus nerve stimulation increased ventilation in adult cats, but stronger stimulation led to decreased respiratory frequency and reduced blood pressure (Loeschcke et al., 1963). There was evidence of a positive interaction between blood CO2 tension and impulses in the sinus nerve, but the intracranial interaction was thought to be distinct from and in addition to input from the carotid body. More recent studies in awake dogs found that selective stimulation of a single carotid body (after denervating the other one) with hypoxic, normocapnic perfusate significantly enhanced ventilatory responsiveness to central hypercapnia, whereas inhibition of the isolated carotid body with hyperoxic, hypocapnic perfusate markedly reduced central hypercapnic response (Blain et al., 2010). Thus, peripheral chemoafferents do play an important role in modifying central PCO2 and pH chemosensitivity, disputing the notion that they are separate entities. The question is: Where is the site of peripheral-central interaction?
The common site of interaction between carotid sinus nerve activity and central “acidsensitive receptors” was relegated to the ventral medullary surface (Loeschcke, 1982). Hypoxia and electrical stimulation of the carotid sinus nerve elicited Fos-like immunoreactivity in catecholaminergic and serotonergic neurons of the rat brain stem, including the VLM, LC, A5 noradrenergic cell groups in pons, RM, RP, VMS, and less so in the DMNX, as well as non-aminergic neurons in the PB, K–F, and elsewhere (Erickson and Millhorn, 1994). However, using Phox2b as a marker with the rationale that Phox2b mutation causes a disruption of central chemosensitivity (see section 3 above), a different view has been proposed (Stornetta et al., 2006). In this scheme, all Phox2b-labeled neurons in the brain stem form an “uninterrupted chain” that integrates peripheral and central chemoreception. It starts with carotid afferents projecting to Phox2b-immunoreactive (ir) neurons in the NTS, which, in turn, project to Phox2b-ir RTN neurons and more caudally to Phox-2b-ir neurons within the ventral respiratory column (VRC). Not included in this “chain” are the central pattern generator (CPG), the A5, A6, and A7 noradrenergic cell groups, and serotonergic neurons (Stornetta et al., 2006). Whether these two schemes are mutually exclusive, co-existent, or with one more dominant than the other, are at present unknown. Suffice it to say that there is cross-talk between peripheral and central chemosensitive areas and the two are likely to be interdependent (Smith et al., 2010).
5. Developmental considerations
5.1 At birth (P0 to P1)
Birth heralds in a sudden increase in arterial partial pressure of oxygen (PaO2) from ~25–30 Torr in utero to ~60 Torr at atmosphere (Brouillette and Waxman, 1997). This necessitates a transient suppression of the peripheral chemoreceptor organs, the carotid bodies (Mortola, 2001). Indeed, the carotid body is not mature at birth, and the carotid chemoreceptor sensitivity to O2 is not fully functional at birth (Sterni et al., 1999). Thus, if confronted with a hypoxic challenge at this time, the weak O2 sensitivity leads to a relatively weak ventilatory response (V̇E) (Liu et al., 2009). There is an interesting dichotomy at this age, for under normoxia, the animal’s ventilatory rate is relatively high to compensate for a poor efficiency in gas exchange due to limited alveolar surface area (Burri, 1974), but its metabolic rate (rate of oxygen consumption [V̇O2] and carbon dioxide production [V̇CO2]) is the lowest as compared to the remaining first 3 postnatal weeks (Liu et al., 2009). This is related to a weak or absent thermoregulatory response and moderate tissue growth at this time (Mortola, 1987). In response to hypoxia, the metabolic rate becomes relatively high. Thus, at P0, the ratios of V̇E/V̇O2 and V̇E/V̇CO2 are at their highest under normoxia, but are at their lowest under hypoxia when compared to normoxia (Liu et al., 2009).
The low metabolic rate at P0–P1 is also demonstrable at the cellular level by means of cytochrome oxidase, a metabolic marker of neuronal activity (Wong-Riley, 1989). In nine brain stem nuclei examined, including the PBC, Amb, NTSVL, DMNX, hypoglossal nucleus (XII), and the medial accessory olivary nucleus (IOma), the activity of this enzyme was at its lowest at P0–P1 as compared to the remaining first 3 postnatal weeks (Liu and Wong-Riley, 2002, 2003). The PBC is a presumed kernel of respiratory rhythmogenesis (Smith et al., 1991). The Amb, XII, and DMNX are involved in controlling upper airway muscles, tongue muscles, and targets of the vagus nerve, respectively, during respiration (Jordan, 2001; Lowe, 1980; St-John, 1998). The NTSVL, as does the DMNX, receives input from the carotid body and relays peripheral chemoreceptive information centrally, including the rostral ventral respiratory group (rVRG) (Finley and Katz, 1992; Holtman et al., 1990). The IOma projects mainly to the cerebellum, which plays a role in respiratory and cardiovascular control (Harper et al., 1998).
Thus, both the carotid body and central respiratory-related neurons are not mature at birth. Likewise, CO2 sensitivity (response to hypercapnia) is low at birth (Davis et al., 2006).
5.2 P2 to P7
At P2–P7 under normoxia, the metabolic rate has increased to meet the requirements of tissue growth and to compensate for greater heat diffusion and heat loss due to a greater surface area-to-body mass ratio and the absence of a thick insulation of fur (Liu et al., 2009; Mortola, 1984, 1987; Sant’Anna and Mortola, 2002). The lungs are also developing and improving on gas exchange (Blanco, 1995; Burri, 1974). The ventilatory rate under normoxia is reduced and V̇E/V̇O2 and V̇E/V̇CO2 ratios are reduced though stable as compared to values at P0–P1 (Liu et al., 2009), signifying a greater efficiency of gas convection (Bennett and Hicks, 2001). It also implies that the respiratory control network is now better able to modulate ventilation to match lung development and metabolic needs of the body. Thus, by grouping the entire first postnatal week as “newborn” or “neonate” (Bertin et al., 1993; Gozal et al., 2003; Mortola et al., 1986; Peyronnet et al., 2000), the difference between the first two days and the rest of the first week might have been overlooked.
5.2.1 Development of neurochemicals
In the brain stem, various neurochemicals undergo developmental changes during the first postnatal week. The expression of the excitatory neurotransmitter glutamate and its N-methyl-D-aspartate (NMDA) receptors increased between P2 and P7, but with a distinct though gentle fall at P3 or P4 in the PBC, NTSVL, Amb, IOma, XII, and DMNX (Liu and Wong-Riley, 2002, 2005). On the other hand, the expression of the inhibitory neurotransmitter GABA, GABAB receptors, and glycine receptors showed a rise at P3 or P4 before falling or remaining at a plateau for the rest of the first week in the same nuclei (Liu and Wong-Riley, 2002, 2005). Subunit 2 of the AMPA receptors (GluR2 or GluA2) exhibited a pattern just like those of the inhibitory neurochemicals, perhaps because it decreases the permeability of Ca2+ (Brorson et al., 1999) and reduces neuronal excitation. This transient and moderate imbalance in neurochemical expression was paralleled by a plateau or a slight fall in cytochrome oxidase activity at P3 or P4 in the same nuclei (Liu and Wong-Riley, 2002, 2003; Liu and Wong-Riley, 2001). P3 was the only time in the first postnatal week that the response in frequency to acute hypoxia measured plethysmographically was below the baseline after the first 30 seconds (P < 0.05) (Liu et al., 2006). At the cellular level within XII, the amplitude of miniature excitatory postsynaptic currents (mEPSCs) was significantly reduced at P3 (P < 0.05), the only time point in the first postnatal week when a day-to-day significance was found and contributed to a 30% reduction in the charge transfer of mEPSCs (calculated from the time integral of individual mEPSCs) (Gao et al., 2011). The charge transfer increased at P4 (P < 0.05) and peaked at P7. On the other hand, the frequency of inhibitory mIPSCs rose significantly from P2 to P3 (P < 0.001) in the 1st week, and this was correlated with an abrupt and significant rise in the amplitude of glycinergic mIPSCs at P3 (P < 0.05) (Gao et al., 2011). Thus, the neurochemical, metabolic, ventilatory, and electrophysiological changes around P3–P4 all point to a slight imbalance at this time.
The serotonergic system undergoes developmental adjustments as well. In the respiratory system, serotonin is involved in the modulation of respiratory rhythmogenesis (Bonham, 1995; Hilaire et al., 1997; Pena and Ramirez, 2002), respiratory motoneuron excitability (Hilaire and Duron, 1999); phrenic long-term facilitation (Baker-Herman and Mitchell, 2002; Liu et al., 2011), upper airway reflexes (Haxhiu et al., 1998), responses to hypoxic or hypercapnic challenges (Taylor et al., 2005; Tryba et al., 2006), enhancement of the excitability of hypoglossal motoneurons, especially during wakefulness (Horner, 1996; Zhan et al., 2002), and central chemosensitivity (Hodges and Richerson, 2008; Richerson, 2004). 5-HT’s action is mediated by a number of receptor subtypes (mainly 5-HT1A, 5-HT1B, and 5-HT2A), with a net excitatory tonic drive to maintain respiratory output during wakefulness (Hodges and Richerson, 2008; Richerson, 2004). The expressions of TPH, serotonin transporter (SERT), 5-HT1A, 5-HT1B, and 5-HT2A receptors monitored in a number of brain stem respiratory-related nuclei generally showed an initial lower value at P2–4 followed by a peak and a high plateau from P5 to ~P11 (Liu and Wong-Riley, 2008, 2010a, 2010b). Thus, other than the initial dip, the serotonergic system in the brain stem is functioning probably at its maximum during the first one and half postnatal weeks in rats.
5.2.2 Response to hypoxia
Hypoxia is known to cause a hypoxic ventilatory response (HVR) that is biphasic, with an initial increase in ventilation followed by a depression known as hypoxic ventilatory depression (HVD) or hypoxic ventilatory roll-off, which is more pronounced in developing animals (Mortola, 1984; Powell et al., 1998). The early excitatory phase is known to be mediated by the carotid body–NTS pathway and involves glutamate and its NMDA receptors (Kazemi and Hoop, 1991; Ohtake et al., 1998). However, other central pathways have also been considered (Mizusawa et al., 1994; Ohtake et al., 2000). HVD, on the other hand, is generally regarded as arising from a CNS mechanism (Vizek et al., 1987), although a peripheral mechanism involving arterial chemoreceptors has not been entirely excluded (Bissonnette, 2000). A number of neurotransmitters and neuromodulators have been implicated in HVD. These include GABA (Kazemi and Hoop, 1991; Richter et al., 1999), 5-HT (Di Pasquale et al., 1992; Richter et al., 1999), adenosine (Neylon and Marshall, 1991; Richter et al., 1999), and platelet-derived growth factor (PDGF-β) (Gozal et al., 2000; Simakajornboon and Kuptanon, 2005). Hypoxic hypometabolism may also contribute to HVD (Mortola, 1999). Moreover, nitric oxide may play a role in both excitatory and inhibitory components of the HVR in developing rats (Gozal et al., 1997).
Thus, HVR necessitates the interaction between peripheral and central components of the chemoresponsive pathway. Indeed, hypoxic stimulation induces the expression of c-fos that is rapid, transient, and polysynaptic within multiple nuclei in the brain stem, including NTS, VLM, RP, VMS, and area postrema (Erickson and Millhorn, 1991).
The response to acute hypoxia at P2–P7 is very different from that at P0–P1 in rats. The metabolic rate was very low under hypoxia as compared to normoxia (Liu et al., 2009; Saetta and Mortola, 1987). This is consistent with Mortola’s maxim that “the higher the normoxic V̇O2, the greater its decrease during hypoxia” (Mortola, 2004). This protective hypometabolic response, in conjunction with a relatively high ventilatory rate (Liu et al., 2006), high V̇E/V̇O2 and V̇E/V̇CO2 ratios (Liu et al., 2009), a maturing carotid body (Bamford et al., 1999; Hertzberg et al., 1990), and a maturing central respiratory network (Hilaire and Duron, 1999; Singer et al., 1998) enable the animals to meet an acute hypoxic challenge at this time. The chemosensitivity of the carotid body is reset toward the adult level during the first week after its initial silencing at birth, and is able to influence breathing even under normal conditions (Hertzberg et al., 1990). Increased chemosensitivity after the first day of life is also attributable to a decrease in the release of the inhibitory dopamine from carotid glomus cells (Hertzberg et al., 1990). The response of glomus cells to hypoxia, hypercapnia, or combined hypoxia and hypercapnia increases with age from P1 to P8, as does the response of carotid sinus nerve to hypoxia; however, the nerve response to hypercapnia reportedly does not change significantly, and its response to combined hypoxia and hypercapnia does not occur during the first postnatal week (Bamford et al., 1999). Thus, whereas the maturation of glomus cells corresponds and likely contributes to the maturation of the carotid chemoreceptor response to hypoxia, they do not appear to be involved in the multiplicative effect of hypoxia and hypercapnia (Bamford et al., 1999).
5.2.3. Response to chronic hypoxia
Chronic hypoxia from birth and during postnatal maturation is known to blunt the ventilatory response and carotid chemoreceptors’ response to hypoxia. The underlying mechanism has been traced to an impairment of glomus cells’ normal developmental increase in O2 sensitivity and an elimination of their normal developmental enhancement of hypoxia-induced increase in [Ca2+]i (Sterni et al., 1999). However, there was little effect on the cells’ response to increased K+ and no alteration of their response to CO2.
5.2.4 Response to hyperoxia
In contrast to hypoxia, which activates the carotid body and thus the central respiratory network, hyperoxia during the first 2 postnatal weeks, when the carotid body is maturing, blunts the chemosensitivity of the carotid body and impairs the ventilatory response to acute hypoxic challenge in the young as well as the adult (Bavis et al., 2003; Donnelly et al., 2009). As little as 3 to 5 days of hyperoxia significantly reduced presynaptic calcium response and catecholamine release from glomus cells, and it decreased postsynaptic afferent nerve excitability as well as its conduction velocity (Donnelly et al., 2009). Hyperoxia caused a progressive decrease in ventilation by ~19% one day after birth to ~29% seven days after birth (Hertzberg et al., 1990), strongly indicating an increasing influence from the carotid body. Hyperoxia from 24 to 36 h before birth to postnatal day 3 (P3) significantly reduced the protein levels of BDNF and the transcript levels of a neuropeptide Vgf, a modest reduction in the expression of receptors for glial-derived neurotrophic factor (GDNF) Ret, but an upregulation of cerebellum 1 gene Cbln1 (Dmitrieff et al., 2011). Centrally, there appears to be a window of susceptibility to hyperoxia during the first two postnatal weeks in rats, as chronic hyperoxia (60% O2) at this time attenuated the HVR in the adult, but it did not affect the hypercapnic response (Bavis et al., 2011a; Ling et al., 1997). The blunting effect persisted for months even after the animals had returned to normoxia. As the single-unit chemoafferent nerve response and glomus cell calcium response fully recovered after 7 – 8 days in normoxia, the long-lasting suppression in HVR was not likely to be due to decreased O2 sensitivity of individual chemoreceptor cells, but rather, to a permanent reduction in carotid body size and degeneration of chemoafferent neurons (Bavis et al., 2011b). Hyperoxia decreased BDNF expression after only 7 days and tyrosine hydroxylase levels after 14 days in the NTS (Chavez-Valdez et al., 2012). One day of exposure to hyperoxia (at P7) actually augmented HVR of neonatal rats, and this effect was not dependent on reactive oxygen species, such as superoxide anion (Roeser et al., 2011). The augmentation probably reflects an increase in chemoafferent nerves’ response and glomus cell Ca2+ response to acute hypoxia (Donnelly et al., 2009). However, hyperoxia for 3 – 5 days (starting at P7) suppressed the response to hypoxia, indicating a time-dependent change in the HVR induced by postnatal hyperoxia (Donnelly et al., 2009).
Chronic hyperoxia (60% O2) for the first month in rats led to marked hypoplasia of the carotid body, a significant loss of unmyelinated axons in the carotid sinus nerve, a loss of dopaminergic neurons in the petrosal ganglia, and a life-long impairment of carotid chemoreceptor function (Erickson et al., 1998; Fuller et al., 2002).
5.2.5 Response to hypercapnia
Hypercapnic stimulation of the carotid body with 100% CO2 saturated in saline elicited in adult rats an increase in mean arterial pressure, respiratory rate, tidal volume, and minute ventilation thought to be mediated by NTScom neurons (Vardhan et al., 1993), which, together with other NTS subnuclei, projects to the ventral respiratory group (Smith et al., 1989). Both carotid and intracranial chemoreceptors are deemed critical to a normal ventilatory CO2-H+ chemosensitivity; however, the carotid exerts a greater influence at low levels of hypercapnia, whereas central chemoreceptors are more dominant at high levels (Forster et al., 2008). Carotid chemoreceptors are also critical in maintaining a stable and normal eupneic PaCO2 (Forster et al., 2008).
The hypometabolic response to hypoxia at P2 to P7 is not replicated in hypercapnia. In studying twelve species from four mammalian orders, Mortola and Lanthier found that 5% CO2 increased ventilation in all newborn species without changes in their metabolic rate and no change in their body temperature (Mortola and Lanthier, 1996). In the rat, the hypercapnic response (to 5% CO2 for 10 min) tended to decrease during the first postnatal week, but increased on P10 due to augmented tidal volume; and c-fos mRNA expression increased in putative chemosensitive areas, such as the caudal NTS and the VLM, but not in the LC nor the majority of midline raphé neurons (Wickstrom et al., 2002). With 10% CO2 exposure for 1 h, c-fos protein was found in the VLM, the lateral paragigantocellular (LPGi) and gigantocellular nuclei, the medullary midline complex, as well as in the ROb and RP (Belegu et al., 1999). The number of activated neurons was higher at P5 than P40, suggesting that central chemosensitive neurons are “well developed” at P5 (Belegu et al., 1999). However, this development is by no means complete during the first week. In fact, the initial higher response was followed by a fall around P8 (Putnam et al., 2005), and full maturation of the hypercapnic response is probably not reached until after the end of the 2nd postnatal week in rats (Davis et al., 2006). This likely reflects a period of adjustment needed for cellular and synaptic maturation in the chemosensitive network.
5.2.6 Carotid body denervation
As the carotid body is a major peripheral chemoreceptor and its afferents provide a tonic excitatory input to medullary neurons under normal conditions (Finley and Katz, 1992; Hodges et al., 2005) as well as a potent signal during acute and chronic hypoxia (Forster et al., 2000), it is postulated to play a role in the development of brain stem respiratory nuclei. Denervation of the carotid body at 2–3 and 7–8 days of age in the rat led to significantly higher mortality than in sham-operated animals (Serra et al., 2001). Furthermore, carotid body denervation between P2 and P7 led to a significant loss in body weight and a reduction in somal size of the PBC, suggesting that P2–P7 is a sensitive window for somal size development in the PBC modifiable by peripheral chemoafferent input (Liu et al., 2003). Denervation also induced a significant decrease in cytochrome oxidase activity of the PBC as well as a distinct delay and prolongation of the maturational process, especially when denervation was done at P3 (Liu et al., 2003) as well as during the critical period (see section 5.4.4). Thus, the carotid body influences the development of the PBC and perhaps other brain stem respiratory-related nuclei.
It is clear that environmental perturbations can lead to a variety of responses that differ depending on the species, age, gender, mode and duration of disturbance, state of wakefulness or sleep, and techniques used.
5.3 Second and third postnatal weeks excluding the critical period
With the exception of the critical period around P12–P13 to be discussed in section 5.4, the 2nd and 3rd postnatal weeks in the rat are characterized by a relatively mature carotid body and a still maturing brain stem respiratory network. Responses of glomus cells to hypoxia or hypercapnia have plateaued after P8, and the carotid sinus nerve’s response to hypoxia has also matured by P8; however, the sinus nerve’s response to hypercapnia does not change with age and its response to combined hypoxia and hypercapnia does not appear until 16 – 21 days of age in the rat, indicating a non-glomus cell mechanism (Bamford et al., 1999). Likewise, after an initial period of adjustment between P8 and P10, rats have acquired a more mature pattern of ventilation and metabolism (except for the critical period), with stable V̇O2 and V̇CO2 to body weight ratio and relatively stable V̇E/V̇O2 and V̇E/V̇CO2 ratios both in normoxia and acute hypoxia (Liu et al., 2009). Under normoxia, breathing frequency (f) increased with age to peak at P13 and then declined gradually; both tidal volume (VT) and minute ventilation (V̇E) significantly increased in the 2nd week, followed by a progressive increase in VT and a relative plateau in V̇E (Liu et al., 2006). However, when adjusted to body weight, both VT and V̇E showed minor fluctuations with a distinct peak at P13, after which VT remained stable whereas V̇E exhibited a gradual decline until P21, most likely reflecting the fall in f after P13 (Liu et al., 2006). Thus, under normoxia, ventilation is maturing during the 2nd and 3rd postnatal weeks.
5.3.1 Development of neurochemicals
The 2nd and 3rd postnatal weeks (other than the critical period) mark a time when the expression of glutamate increases with age and NMDA receptor subunit 1 either plateaued or increased with age, but that of GABA and GABAB receptors declined with age; on the other hand, the expression of glycine receptors generally increased with age, and that of GluR2 either increased, plateaued, or declined with age (Liu and Wong-Riley, 2002, 2005). These correlate well with a general increase in cytochrome oxidase activity with age in the same brain stem nuclei (Liu and Wong-Riley, 2002, 2003). The serotonergic system, however, underwent a major transition between the 2nd and 3rd postnatal weeks. With a few minor exceptions, the expressions of TPH, SERT, 5-HT1A, 5-HT1B, and to a lesser extent 5-HT2A receptors in a number of respiratory-related nuclei switched from a relatively high plateau sustained until P11 to a low level at and after P12 (Liu and Wong-Riley, 2010a, 2010b). This implies that the relatively strong serotonergic influence on the respiratory network during the first one and half postnatal weeks gives way to a possibly more stabilized state of homeostatic modulation after the critical period.
5.3.2 Response to hypoxia
In response to acute hypoxia, other than the unique reaction during the critical period (see section 5.4), f responses were generally above the baseline (normoxia) before P12, but fell below the baseline at P12 and after, whereas VT and V̇E were both above the baseline (except for P13) and steadily increased until P21 (Liu et al., 2006). Clearly, the system is maturing to cope with acute hypoxia during the 2nd and 3rd postnatal weeks, which makes the uncharacteristic response during the critical period even more intriguing. The ability to respond adequately to hypoxia after the critical period is due mainly to increasing VT, as f tends to decrease with age (at least up to P21, the oldest age examined) (Liu et al., 2006).
5.3.3. Response to hypercapnia
As discussed above, the response to hypercapnia does not mature fully until after the 2nd postnatal week. This was verified at the cellular level when medullary raphé neurons in brain slices were challenged with respiratory acidosis. More neurons responded with increased firing rate if they were from rats older than P12 than younger ones (Wang and Richerson, 1999). Rats subjected to perinatal hypercapnia (5% CO2) 1 to 3 days before birth through the first 2 postnatal weeks exhibited a rapid, shallow breathing pattern for only 2 weeks after return to normocapnia, and when challenged with acute hypercapnia immediately after perinatal hypercapnia, their response was reduced again for only 2 weeks before returning to normal levels thereafter (Bavis et al., 2005). Such hypercapnia-induced, transient plasticity appears to be shared by both developing and adult animals (Bavis et al., 2005).
5.3.4 Response to intermittent hypoxia
Acute intermittent hypoxia (AIH) in adult rats induced long-lasting increases in phrenic nerve output known as phrenic long-term facilitation (pLTF), a condition not seen with continuous (9 or 20 min) hypoxia (Baker and Mitchell, 2000). AIH also led to LTF in P10 but not P1 or P4 rats (Julien et al., 2008). This respiratory plasticity is thought to be mediated by a serotonin-dependent increase in brain-derived neurotrophic factor (BDNF) in spinal segments containing phrenic motoneurons (Baker-Herman et al., 2004). Severe AIH, on the other hand, shifted pLTF from a serotonin-dependent to an adenosine-dependent form of phrenic motor facilitation (pMF) (Nichols et al., 2012).
Chronic intermittent hypoxia (CIH) in the adult resulted in sensitization of the hypoxic sensory response and the induction of sensory long-term facilitation (LTF) in the carotid body, whereas CIH in the neonate led to only the sensitization of the hypoxic sensory response without inducing sensory LTF (Prabhakar et al., 2007). Significantly, CIH during the first 10 postnatal days enhanced the hypoxic ventilatory response only in male but not in female rat pups, and it reduced the ventilatory LTF in P10 rats, leading to higher apnea frequency during recovery (Julien et al., 2008). Altered responses to CIH can be reversed if induced in the adult but not in the neonate, and neonatal carotid bodies (1–10 days) are more sensitive to CIH than the adult ones (Pawar et al., 2008). Increased sensory response and sensory LTF may be beneficial initially, but chronically they may lead to disordered breathing, increased sympathetic tone, and systemic hypertension (Prabhakar et al., 2007).
5.4 Critical period of postnatal respiratory development in the rat (around P12–P13)
What makes the end of the 2nd postnatal week a unique period of respiratory development in the rat? This is a time when concurrent and sudden changes occur at the neurochemical, metabolic, ventilatory, and electrophysiological levels.
5.4.1 Neurochemical changes during the critical period
Neurochemically, the expressions of excitatory neurochemicals (glutamate and NMDA receptor subunits NR1 and NR2A) fell precipitously, whereas those of inhibitory neurochemicals (GABA, GABAB receptors, and glycine receptors) rose significantly in the PBC, NTSVL, Amb, XII, and DMNX, but not in the non-respiratory cuneate nucleus, at P12 (Liu and Wong-Riley, 2002, 2005, 2010c). This indicates a period of suppressed excitation and heightened inhibition. The significant reduction in NR2A, with the fastest rise and shortest decay time (Chen et al., 1999; Erreger et al., 2005) at P12 may considerably attenuate excitatory neurotransmission in the respiratory nuclei during the critical period. In the PBC and NTSVL, it will reduce respiratory drive, especially in response to insults such as hypoxia. In the Amb and XII, it will attenuate airway patency during respiration. The significant reduction of NR2C (with a decay time faster than that of NR2D) in the PBC at P12 may also contribute to decreased respiratory drive at this time. The concomitant decrease in the expressions of glutamate and glutamate receptor subunits NR1 and NR2A at P12 in multiple brain stem respiratory nuclei most likely contributes to a transient attenuation of glutamatergic transmission via NRs within the respiratory network during the critical period. On the other hand, the transient but significant rise in the expressions of GABA, GABAB, and glycine receptors in the PBC, NTSVL, Amb, XII, IOma, and DMNX at P12 (Liu and Wong-Riley, 2002, 2005) indicates a transient dominance of inhibitory neurotransmission at this time.
Besides the inhibitory-excitatory imbalance, there is an apparent switch in the expression of GABAA receptor subunits from the neonatal α3 to the mature α1 form close to P12 in the PBC and NTS (Liu and Wong-Riley, 2004, 2006). Such a switch is likely to speed up the kinetics of GABAergic transmission by reducing its decay time (Bosman et al., 2002), thereby improving the efficiency of inhibition. An apparent developmental switch in NMDA receptor subunit expression from NR2D (GluN2D) to NR3B (GluN3B) also occurred possibly around P13 (Liu and Wong-Riley, 2010c). NR2D-containing receptors tend to have low conductance and the slowest kinetics (Wyllie et al., 1998), whereas NR3B-containing ones have faster kinetics and tend to decrease Ca2+ permeability of glutamate-induced current (Matsuda et al., 2002; Nishi et al., 2001). These subunit switches denote a period of synaptic remodeling and maturation that may entail some initial instability in neural transmission.
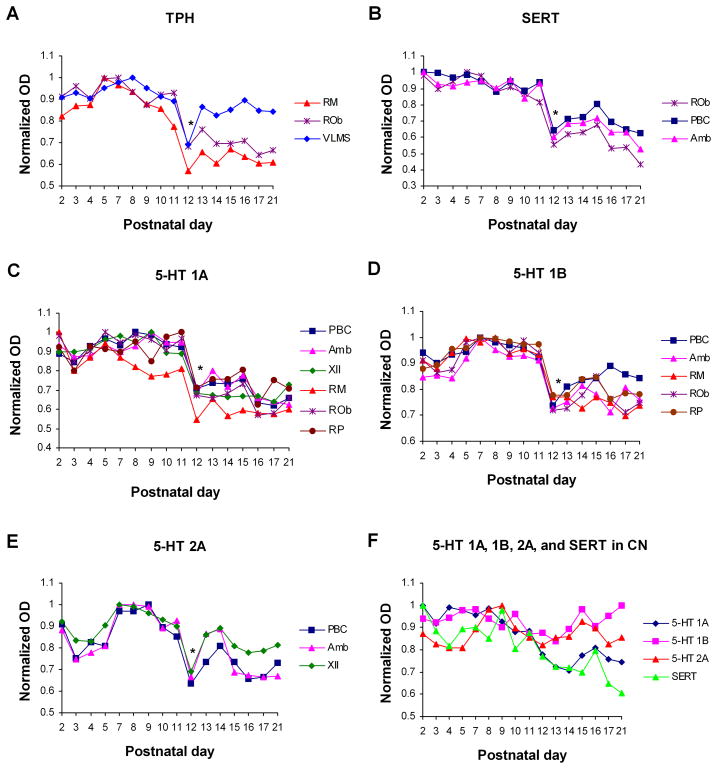
The serotonergic system also undergoes a dramatic adjustment during the critical period (Liu and Wong-Riley, 2008, 2010a, 2010b) (Fig. 2). The expression of TPH fell significantly at P12 in RM, ROb, and the ventrolateral medullary surface (VLMS) (P < 0.05), and fell without reaching significance (when compared with the immediately adjacent age group using the Tukey’s test) in the RP, LPGi and parapyramidal region (pPy). The expression of SERT plummeted at P12 in neurons of RM and ROb and in the neuropil of PBC, Amb, and RTN/pFRG, but at P10 in neurons of LPGi and pPy (P < 0.05). As for serotonergic receptors, the expression of 5-HT1A dropped precipitously at P12 from a high plateau in RM, ROb, RP, PBC, Amb, and XII (P < 0.05). The expression of 5-HT1B receptors also plunged at P12 from a high plateau in RM, ROb, RP, PBC, and Amb (P < 0.05). Likewise, the expression of 5-HT2A plummeted at P12 in the PBC, Amb, and XII (P < 0.05). The labeling of these receptors was also at its lowest at P12 in the RTN/pFRG, but because the fall was more gradual, it did not reach statistical significance. Remarkably, P12 was the only time point in the entire first 3 postnatal weeks when a statistically significant day-to-day difference was found (Liu and Wong-Riley, 2008, 2010a, 2010b). After P12, the levels of these serotonergic neurochemicals either remained low or rose again until P21. Thus, for some nuclei (RM, ROb, RP, PBC, Amb, and RTN/pFRG), P12 denotes the beginning of a down-regulation of TPH and SERT, suggesting that the initial surge of a primarily trophic effect of 5-HT has transitioned into a more stabilized state of homeostatic control. For the VLMS, P12 is the only time point in the first 3 postnatal weeks that the level of TPH is drastically reduced (Liu and Wong-Riley, 2010b). This region contains serotonergic neurons that allegedly contribute to central respiratory chemosensitivity (Richerson et al., 2005). LPGi is one of the most important sources of sympathetic excitatory drive from the medulla to the cardiovascular system (Lovick, 1987). Its extensive afferent and efferent connections (Van Bockstaele et al., 1989; Zec and Kinney, 2001), especially its projections to the ventral and dorsal respiratory groups as well as ROb and RP (Ellenberger and Feldman, 1990; Holtman et al., 1990; Zagon, 1993) indicate that it may integrate respiratory and cardiovascular rhythmic patterns (Gaytan et al., 1997). pPy is reportedly another candidate for central chemoreception (Ribas-Salgueiro et al., 2005; Richerson, 2004). It is also widely connected to medullary nuclei subserving cardiorespiratory and other autonomic functions, such as ROb, RP, NTS, and LPGi (Ribas-Salgueiro et al., 2005). Both LPGi and pPy also project to the RTN/pFRG (Cream et al., 2002), strongly suggesting that serotonergic neurons in LPGi and pPy exert global effect on the other brain stem respiratory nuclei. The down-regulation of SERT in these two regions two days prior to other respiratory-related regions (i.e., at P10 rather than P12) suggests that they may have a priming effect on the other nuclei (Liu and Wong-Riley, 2010b).
Fig. 2.
Optical densitometric measurements of immunoreaction product in individual neurons of various brain stem nuclei from P2 to P21. Data points are mean ± SEM. A. Tryptophan hydroxylase (TPH); B. serotonin transporter (SERT); C. 5-HT1A receptor; D. 5-HT1B receptor; E. 5-HT2A receptor; and F. 5-HT1A, 5-HT1B, and 5-HT2A receptors and SERT in the non-respiratory cuneate nucleus (CN) for comparison. The key for various nuclei is the same as in Fig. 1. Tukey’s tests comparing one age group with its immediately adjacent younger age group showed that P12 was the only time point in the first 3 postnatal weeks that a significant was found (*P < 0.05). (Modified from Liu and Wong-Riley, 2010a, b). For ease of comparison, the highest value of each graph in the original was adjusted to 1, and all of the other values were adjusted accordingly.
Overall, the precipitous fall in the expression of serotonergic neurochemicals within the narrow window of the critical period can adversely affect respiratory rhythmogenesis, inspiratory activity, gasping, and upper airway patency. The “net stimulatory effect” of 5-HT on respiratory output (Hodges and Richerson, 2008; Lindsay and Feldman, 1993) may be attenuated and the normal interaction of serotonergic system with other neurotransmitter and neuromodulatory systems may be disrupted, with potential adverse effect on respiratory control.
Decreased 5-HT transmission renders the animals less capable of responding adequately to exogenous respiratory insults, such as hypoxia, hypercapnia, or metabolic acidosis. The insult is more severe during sleep, when respiratory activities are normally depressed (Olson and Simon, 1996) and the firing of serotonergic neurons is reduced (Veasey et al., 1996), thus compromising airway patency (Horner, 2007). Of relevance are reports of developmental abnormalities in the medullary 5-HT system, such as reduced expression and/or binding of 5-HT receptors (including 5-HT1A and 5-HT2A) in several brain stem nuclei (such as the arcuate nucleus or RTN, ROb, and NTS) in Sudden Infant Death Syndrome (SIDS) and other pathologies (Ozawa and Okado, 2002; Paterson et al., 2006). Whether human infants normally undergo a drastic reduction in their brain stem serotonergic receptor expression during the critical period is at present unknown.
5.4.2 Metabolic and ventilatory changes during the critical period
Since the bulk of neuronal energy is used to repolarize the membrane after excitatory depolarization (rather than after hyperpolarization, as it is mainly passive) (Wong-Riley, 1989), reduced excitation is also correlated with a significant decrease in the level of a key energy-generating enzyme, cytochrome oxidase, at P12 in the same brain stem respiratory-related nuclei where neurochemical changes are detected (Liu and Wong-Riley, 2002, 2003).
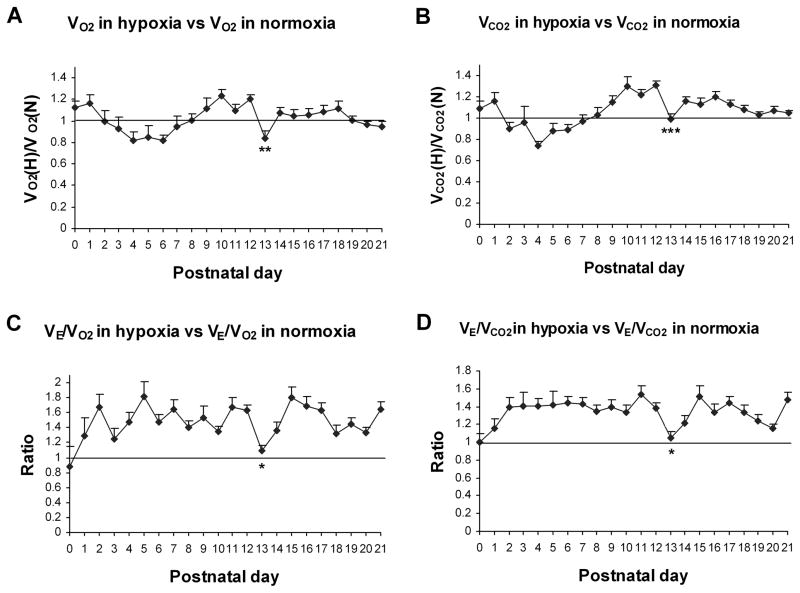
The metabolic rate and ventilation during the critical period in both normoxia and hypoxia are distinctly different from those during the rest of the 2nd and 3rd postnatal weeks. There is a sudden enhancement of the metabolic rate and V̇E/V̇O2 and V̇E/V̇CO2 ratios in normoxia, which are matched by significant increases in respiratory frequency, tidal volume, minute ventilation, as well as an abrupt rise in body temperature, all occurring at P13 (Liu et al., 2009; Liu et al., 2006). In response to acute hypoxia, the ventilatory response was weakest at P13, being lower at P12–P14 than the rest of the 3 postnatal weeks (Liu et al., 2006). Moreover, metabolic rates and V̇E/V̇O2 and V̇E/V̇CO2 ratios were significantly reduced in acute hypoxia at P13, unlike more stable trends in the rest of the 2nd and 3rd weeks (Liu et al., 2009) (Fig. 3). If hypoxia should become more severe and prolonged, the animals’ ventilatory response, metabolic rate, and energy production will not be sufficient to meet the increased demand at P13, and the survival of the animal may be at stake.
Fig. 3.
Postnatal trends of metabolic rate (V̇O2 and V̇CO2) (A and B) and V̇E/V̇O2 and V̇E/V̇CO2 ratios (C and D) in hypoxia as compared to normoxia in rats from P0 to P21. Normoxic values were adjusted to 1 for comparison. Tukey’s tests comparing one age group with its immediately adjacent younger age group indicated that P13 was the only time point in the entire 3 postnatal weeks that a day-to-day significance was found (*P < 0.05; **P < 0.01; ***P < 0.001). See text for details. (Modified from Liu et al., 2009).
In a recent paper, a critical period in the development of the respiratory control system at P12–13 in the rat was confirmed, with an emphasis that the male showed a greater significance than the female, although the same trend was also present in females (Holley et al., 2012). This raises an interesting possibility that male rats may have a weaker response to hypoxia than female ones. However, a significant reduction in ventilatory response to hypoxia at P13 reported earlier (P < 0.001), which combined male and female animals (Liu et al., 2006), was not found in Holley et al.’s study for either male or female. By grouping P12 with P13, a difference between P12 and P13 in various parameters noted in Liu et al.’s study might have been overlooked by Holley’s group, leading to some differences between the two studies. Further investigation is warranted to determine if the significantly reduced hypoxic response at P13 (Liu et al., 2006) pertains more or only to male, or is similar between genders.
5.4.3 Electrophysiological changes during the critical period
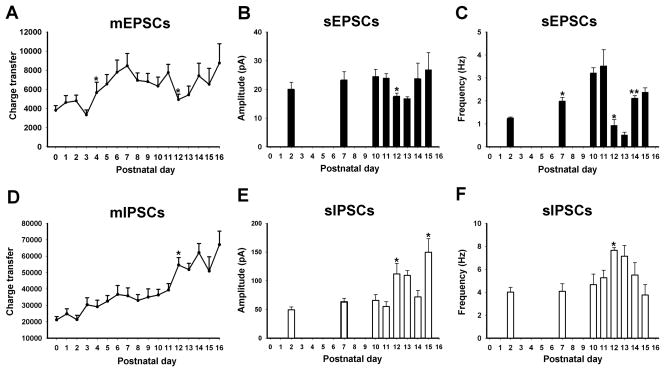
The inhibitory-excitatory neurochemical imbalance during the critical period is demonstrable at the electrophysiological level in XII. Whole-cell patch-clamp recordings of hypoglossal motoneurons in rats daily from P0 to P16 indicated that i) the amplitude and charge transfer of mEPSCs were significantly reduced at P12–13; ii) the amplitude, mean frequency and charge transfer of mIPSCs were significantly increased at P12–13; and iii) the amplitude and frequency of spontaneous EPSCs (sEPSCs) were significantly reduced at P12–13, whereas those of sIPSCs were significantly increased at P12–13 (Gao et al., 2011). Remarkably, P12–13 was the only time point from P0 to P16 that significant day-to-day changes were found (P < 0.05) (Fig. 4). These results match precisely the transient imbalance between reduced excitatory and enhanced inhibitory neurotransmitter/receptor expressions at P12 seen in many brain stem respiratory-related nuclei, including the XII (see section 5.4.1 above). The time point also corresponds exactly to the critical period of ventilatory and metabolic development (see section 5.4.2 above), when the animals’ response to hypoxia is the weakest as compared to the rest of the first three postnatal weeks.
Fig. 4.
Patch clamp recordings of hypoglossal motoneurons in normal rats from P0 to P16. A. Charge transfer of mEPSCs was significantly reduced at P12. B. Amplitude of sEPSCs was significantly reduced at P12–P13. C. Frequency of sEPSCs was significantly reduced at P12–P13 and rose again at P14. The frequency of sEPSCs at P7 was also significantly higher than that at P2. D. Charge transfer of mIPSCs was significantly increased at P12, the only time point when a day-to-day difference was found. E. The amplitude of sIPSCs was significantly increased at P12 and P15. F. The frequency of sIPSCs was significantly increased at P12. (*P < 0.05; **P < 0.01). See text for details. (Modified from Gao et al., 2011).
5.4.4 Carotid body denervation during the critical period
When carotid body denervation (removal of both carotid bodies and sectioning both carotid sinus nerves) was done at P11–13, it did not lead to a shrinkage of PBC neurons, but it caused a delay and prolongation of the metabolic maturational process, with possible delay and prolongation of the critical period (Liu et al., 2003). Thus, the carotid body definitely influences the development of the PBC and perhaps other respiratory-related nuclei. However, carotid body denervation can only modify but not eliminate the critical period, whose existence and manifestation may be based on an intrinsic and possibly a genetically determined mechanism.
5.4.5 Relationship to pathologies
Clearly, toward the end of the 2nd postnatal week (P12–13) is a highly critical period in rats, when an inhibitory dominance renders the animals less responsive to an external stressor, such as hypoxia. This is also a time when the animals are more likely to succumb to death if there is an additional vulnerable attribute. In an experiment in which rats were subjected to immune system-altering primary infection, they died only on day 12 when challenged secondarily with a sublethal dose of endotoxin (Blood-Siegfried et al., 2002). It is noteworthy that the peak incidence of SIDS is not at birth, but rather, between the 2nd and 4th months after birth (Goldberg et al., 1986). For brain development, the human postnatal months 2 – 4 have been correlated with the rat’s postnatal days P11–14 (Ballanyi, 2004). The critical period, external stressor, and a vulnerable infant constitute the triple risk factors of SIDS (Filiano and Kinney, 1994).
Besides SIDS, abnormalities in central chemosensitivity have also been implicated in diseases such as congenital central hypoventilation syndrome (CCHS) (Spengler et al., 2001), obstructive sleep apnea (Hilaire et al., 1993), and Rett syndrome (Zhang et al., 2011). Whether the incidence is higher during the critical period remains to be determined.
6. Conclusions
Much has been learned in the past few decades about peripheral and central chemosensors and their interactions at the anatomical and physiological levels. The challenge ahead includes the elucidation of: a) the true identities of all central chemoreceptors; b) the mechanisms of chemotransduction for both peripheral and central chemoreceptors; c) the mechanisms of interaction between peripheral chemoafferents and central chemosensors during development and adulthood; d) the mechanism of developmental plasticity with perturbations of peripheral and/or central chemosensors; e) a possible existence of a critical period of development in the carotid bodies; and f) the mechanism underlying abnormal chemosensitive response during the critical period of postnatal respiratory development.
Acknowledgments
This review is dedicated to all past and present investigators of the rat’s respiratory system. Supported by NIH Grant HD048954.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aicher SA, Kurucz OS, Reis DJ, Milner TA. Nucleus tractus solitarius efferent terminals synapse on neurons in the caudal ventrolateral medulla that project to the rostral ventrolateral medulla. Brain Res. 1995;693:51–63. doi: 10.1016/0006-8993(95)00660-i. [DOI] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529(Pt 1):215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K. Neuromodulation of the perinatal respiratory network. Curr Neuropharm. 2004;2:221–243. doi: 10.2174/1570159043476828. [DOI] [PubMed] [Google Scholar]
- Bamford OS, Sterni LM, Wasicko MJ, Montrose MH, Carroll JL. Postnatal maturation of carotid body and type I cell chemoreception in the rat. Am J Physiol. 1999;276:L875–884. doi: 10.1152/ajplung.1999.276.5.L875. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Dmitrieff EF, Young KM, Piro SE. Hypoxic ventilatory response of adult rats and mice after developmental hyperoxia. Respir Physiol Neurobiol. 2011a;177:342–346. doi: 10.1016/j.resp.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Johnson RA, Ording KM, Otis JP, Mitchell GS. Respiratory plasticity after perinatal hypercapnia in rats. Respir Physiol Neurobiol. 2005 doi: 10.1016/j.resp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Kim I, Pradhan N, Nawreen N, Dmitrieff EF, Carroll JL, Donnelly DF. Recovery of carotid body O2 sensitivity following chronic postnatal hyperoxia in rats. Respir Physiol Neurobiol. 2011b;177:47–55. doi: 10.1016/j.resp.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Vidruk EH, Bisgard GE, Mitchell GS. Level and duration of developmental hyperoxia influence impairment of hypoxic phrenic responses in rats. J Appl Physiol. 2003;95:1550–1559. doi: 10.1152/japplphysiol.01043.2002. [DOI] [PubMed] [Google Scholar]
- Belegu R, Hadziefendic S, Dreshaj IA, Haxhiu MA, Martin RJ. CO2-induced c-fos expression in medullary neurons during early development. Respir Physiol. 1999;117:13–28. doi: 10.1016/s0034-5687(99)00046-8. [DOI] [PubMed] [Google Scholar]
- Bennett AF, Hicks JW. Postprandial exercise: prioritization or additivity of the metabolic responses? J Exp Biol. 2001;204:2127–2132. doi: 10.1242/jeb.204.12.2127. [DOI] [PubMed] [Google Scholar]
- Bertin R, De Marco F, Mouroux I, Portet R. Postnatal development of nonshivering thermogenesis in rats: effects of rearing temperature. J Dev Physiol. 1993;19:9–15. [PubMed] [Google Scholar]
- Biscoe TJ, Duchen MR, Eisner DA, O’Neill SC, Valdeolmillos M. Measurements of intracellular Ca2+ in dissociated type I cells of the rabbit carotid body. J Physiol. 1989;416:421–434. doi: 10.1113/jphysiol.1989.sp017769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1391–1400. doi: 10.1152/ajpregu.2000.278.6.R1391. [DOI] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO(2) J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco LN. Mechanisms for the generation of gas-exchange surface area in rat lung. Am J Physiol. 1995;269:L698–708. doi: 10.1152/ajplung.1995.269.5.L698. [DOI] [PubMed] [Google Scholar]
- Blood-Siegfried J, Nyska A, Lieder H, Joe M, Vega L, Patterson R, Germolec D. Synergistic effect of influenza a virus on endotoxin-induced mortality in rat pups: a potential model for sudden infant death syndrome. Pediatr Res. 2002;52:481–490. doi: 10.1203/00006450-200210000-00005. [DOI] [PubMed] [Google Scholar]
- Bonham AC. Neurotransmitters in the CNS control of breathing. Respir Physiol. 1995;101:219–230. doi: 10.1016/0034-5687(95)00045-f. [DOI] [PubMed] [Google Scholar]
- Bosman LW, Rosahl TW, Brussaard AB. Neonatal development of the rat visual cortex: synaptic function of GABAA receptor alpha subunits. J Physiol. 2002;545:169–181. doi: 10.1113/jphysiol.2002.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Brorson JR, Zhang Z, Vandenberghe W. Ca(2+) permeation of AMPA receptors in cerebellar neurons expressing glu receptor 2. J Neurosci. 1999;19:9149–9159. doi: 10.1523/JNEUROSCI.19-21-09149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillette RT, Waxman DH. Evaluation of the newborn’s blood gas status. National Academy of Clinical Biochemistry. Clin Chem. 1997;43:215–221. [PubMed] [Google Scholar]
- Burri PH. The postnatal growth of the rat lung. 3. Morphology. Anat Rec. 1974;180:77–98. doi: 10.1002/ar.1091800109. [DOI] [PubMed] [Google Scholar]
- Campanucci VA, Zhang M, Vollmer C, Nurse CA. Expression of multiple P2X receptors by glossopharyngeal neurons projecting to rat carotid body O2-chemoreceptors: role in nitric oxide-mediated efferent inhibition. J Neurosci. 2006;26:9482–9493. doi: 10.1523/JNEUROSCI.1672-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JL, Kim I. Postnatal development of carotid body glomus cell O2 sensitivity. Respir Physiol Neurobiol. 2005;149:201–215. doi: 10.1016/j.resp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Chavez-Valdez R, Mason A, Nunes AR, Northington FJ, Tankersley C, Ahlawat R, Johnson SM, Gauda EB. Effect of hyperoxic exposure during early development on neurotrophin expression in the carotid body and nucleus tractus solitarii. J Appl Physiol. 2012 doi: 10.1152/japplphysiol.01609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Luo T, Raymond LA. Subtype-dependence of NMDA receptor channel open probability. J Neurosci. 1999;19:6844–6854. doi: 10.1523/JNEUROSCI.19-16-06844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cream C, Li A, Nattie E. The retrotrapezoid nucleus (RTN): local cytoarchitecture and afferent connections. Respir Physiol Neurobiol. 2002;130:121–137. doi: 10.1016/s0034-5687(01)00338-3. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. A circumscribed projection from the nucleus of the solitary tract to the nucleus ambiguus in the rat: anatomical evidence for somatostatin-28-immunoreactive interneurons subserving reflex control of esophageal motility. J Neurosci. 1989;9:1668–1682. doi: 10.1523/JNEUROSCI.09-05-01668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol. 2006;101:1097–1103. doi: 10.1152/japplphysiol.00378.2006. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: an in vitro study in the rat. Neurosci Lett. 1992;143:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Dmitrieff EF, Wilson JT, Dunmire KB, Bavis RW. Chronic hyperoxia alters the expression of neurotrophic factors in the carotid body of neonatal rats. Respir Physiol Neurobiol. 2011;175:220–227. doi: 10.1016/j.resp.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF. Development of carotid body/petrosal ganglion response to hypoxia. Respir Physiol Neurobiol. 2005;149:191–199. doi: 10.1016/j.resp.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Bavis RW, Kim I, Dbouk HA, Carroll JL. Time course of alterations in pre- and post-synaptic chemoreceptor function during developmental hyperoxia. Respir Physiol Neurobiol. 2009;168:189–197. doi: 10.1016/j.resp.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci U S A. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Mayer C, Jawa A, Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS, Katz DM. Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. J Physiol. 1998;509 (Pt 2):519–526. doi: 10.1111/j.1469-7793.1998.519bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Fos-like protein is induced in neurons of the medulla oblongata after stimulation of the carotid sinus nerve in awake and anesthetized rats. Brain Res. 1991;567:11–24. doi: 10.1016/0006-8993(91)91430-9. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Fong AY. Postnatal changes in the cardiorespiratory response and ability to autoresuscitate from hypoxic and hypothermic exposure in mammals. Respir Physiol Neurobiol. 2010;174:146–155. doi: 10.1016/j.resp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Forster HV, Martino P, Hodges M, Krause K, Bonis J, Davis S, Pan L. The carotid chemoreceptors are a major determinant of ventilatory CO2 sensitivity and of PaCO2 during eupneic breathing. Adv Exp Med Biol. 2008;605:322–326. doi: 10.1007/978-0-387-73693-8_56. [DOI] [PubMed] [Google Scholar]
- Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P. Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol. 2000;119:199–208. doi: 10.1016/s0034-5687(99)00115-2. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bavis RW, Vidruk EH, Wang ZY, Olson EB, Jr, Bisgard GE, Mitchell GS. Life-long impairment of hypoxic phrenic responses in rats following 1 month of developmental hyperoxia. J Physiol. 2002;538:947–955. doi: 10.1113/jphysiol.2001.012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XP, Liu QS, Liu Q, Wong-Riley MT. Excitatory-inhibitory imbalance in hypoglossal neurons during the critical period of postnatal development in the rat. J Physiol. 2011;589:1991–2006. doi: 10.1113/jphysiol.2010.198945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ, 3rd, Zanella S, Koch H, Doi A, Ramirez JM. Chapter 3--networks within networks: the neuronal control of breathing. Prog Brain Res. 2011;188:31–50. doi: 10.1016/B978-0-444-53825-3.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central chemosensitivity. Respir Physiol Neurobiol. 2010;173:264–273. doi: 10.1016/j.resp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauda EB, Carroll JL, Donnelly DF. Developmental maturation of chemosensitivity to hypoxia of peripheral arterial chemoreceptors--invited article. Adv Exp Med Biol. 2009;648:243–255. doi: 10.1007/978-90-481-2259-2_28. [DOI] [PubMed] [Google Scholar]
- Gauda EB, Cooper R, Johnson SM, McLemore GL, Marshall C. Autonomic microganglion cells: a source of acetylcholine in the rat carotid body. J Appl Physiol. 2004;96:384–391. doi: 10.1152/japplphysiol.00897.2003. [DOI] [PubMed] [Google Scholar]
- Gaytan SP, Calero F, Nunez-Abades PA, Morillo AM, Pasaro R. Pontomedullary efferent projections of the ventral respiratory neuronal subsets of the rat. Brain Res Bull. 1997;42:323–334. doi: 10.1016/s0361-9230(96)00292-4. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Hornung R, Yamashita T, Wehrmacher W. Age at death and risk factors in sudden infant death syndrome. Aust Paediatr J. 1986;22(Suppl 1):21–28. [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Vaquero LM, Lopez-Lopez JR, Perez-Garcia MT. Oxygen-sensitive potassium channels in chemoreceptor cell physiology: making a virtue of necessity. Ann N Y Acad Sci. 2009;1177:82–88. doi: 10.1111/j.1749-6632.2009.05037.x. [DOI] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Torres JE, Gozal YM, Nuckton TJ, Hornby PJ. Nitric oxide modulates ventilatory responses to hypoxia in the developing rat. Am J Respir Crit Care Med. 1997;155:1755–1762. doi: 10.1164/ajrccm.155.5.9154888. [DOI] [PubMed] [Google Scholar]
- Gozal D, Reeves SR, Row BW, Neville JJ, Guo SZ, Lipton AJ. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med. 2003;167:1540–1547. doi: 10.1164/rccm.200208-963OC. [DOI] [PubMed] [Google Scholar]
- Gozal D, Simakajornboon N, Czapla MA, Xue YD, Gozal E, Vlasic V, Lasky JA, Liu JY. Brainstem activation of platelet-derived growth factor-beta receptor modulates the late phase of the hypoxic ventilatory response. J Neurochem. 2000;74:310–319. doi: 10.1046/j.1471-4159.2000.0740310.x. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol. 2010;173:244–255. doi: 10.1016/j.resp.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RM, Gozal D, Bandler R, Spriggs D, Lee J, Alger J. Regional brain activation in humans during respiratory and blood pressure challenges. Clin Exp Pharmacol Physiol. 1998;25:483–486. doi: 10.1111/j.1440-1681.1998.tb02240.x. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Erokwu B, Bhardwaj V, Dreshaj IA. The role of the medullary raphe nuclei in regulation of cholinergic outflow to the airways. J Auton Nerv Syst. 1998;69:64–71. doi: 10.1016/s0165-1838(98)00009-5. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hertzberg T, Hellstrom S, Lagercrantz H, Pequignot JM. Development of the arterial chemoreflex and turnover of carotid body catecholamines in the newborn rat. J Physiol. 1990;425:211–225. doi: 10.1113/jphysiol.1990.sp018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire G, Bou C, Monteau R. Serotonergic modulation of central respiratory activity in the neonatal mouse: an in vitro study. Eur J Pharmacol. 1997;329:115–120. [PubMed] [Google Scholar]
- Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol Rev. 1999;79:325–360. doi: 10.1152/physrev.1999.79.2.325. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Morin D, Lajard AM, Monteau R. Changes in serotonin metabolism may elicit obstructive apnoea in the newborn rat. J Physiol. 1993;466:367–381. [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Opansky C, Qian B, Davis S, Bonis JM, Krause K, Pan LG, Forster HV. Carotid body denervation alters ventilatory responses to ibotenic acid injections or focal acidosis in the medullary raphe. J Appl Physiol. 2005;98:1234–1242. doi: 10.1152/japplphysiol.01011.2004. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol. 2010;173:256–263. doi: 10.1016/j.resp.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley HS, Behan M, Wenninger JM. Age and sex differences in the ventilatory response to hypoxia and hypercapnia in awake neonatal, pre-pubertal and young adult rats. Respir Physiol Neurobiol. 2012;180:79–87. doi: 10.1016/j.resp.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman JR, Jr, Marion LJ, Speck DF. Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neuroscience. 1990;37:541–552. doi: 10.1016/0306-4522(90)90422-z. [DOI] [PubMed] [Google Scholar]
- Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–853. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- Horner RL. Respiratory motor activity: influence of neuromodulators and implications for sleep disordered breathing. Can J Physiol Pharmacol. 2007;85:155–165. doi: 10.1139/y06-089. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol. 2005;19:327–341. doi: 10.1177/0269881105053281. [DOI] [PubMed] [Google Scholar]
- Jordan D. Central nervous pathways and control of the airways. Respir Physiol. 2001;125:67–81. doi: 10.1016/s0034-5687(00)00205-x. [DOI] [PubMed] [Google Scholar]
- Julien C, Bairam A, Joseph V. Chronic intermittent hypoxia reduces ventilatory long-term facilitation and enhances apnea frequency in newborn rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1356–1366. doi: 10.1152/ajpregu.00884.2007. [DOI] [PubMed] [Google Scholar]
- Katz DM, Finley JC, Polak J. Dopaminergic and peptidergic sensory innervation of the rat carotid body: organization and development. Adv Exp Med Biol. 1993;337:43–49. doi: 10.1007/978-1-4615-2966-8_7. [DOI] [PubMed] [Google Scholar]
- Kazemi H, Hoop B. Glutamic acid and gamma-aminobutyric acid neurotransmitters in central control of breathing. J Appl Physiol. 1991;70:1–7. doi: 10.1152/jappl.1991.70.1.1. [DOI] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Randall M, Nattie EE. CO(2) microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol. 1999;87:910–919. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol. 2006;577:307–318. doi: 10.1113/jphysiol.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response. Respir Physiol. 1997;110:261–268. doi: 10.1016/s0034-5687(97)00091-1. [DOI] [PubMed] [Google Scholar]
- Liu J, Wei X, Zhao C, Hu S, Duan J, Ju G, Wong-Riley MT, Liu Y. 5-HT induces enhanced phrenic nerve activity via 5-HT(2A) receptor/PKC mechanism in anesthetized rats. Eur J Pharmacol. 2011;657:67–75. doi: 10.1016/j.ejphar.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Liu Q, Fehring C, Lowry TF, Wong-Riley MT. Postnatal development of metabolic rate during normoxia and acute hypoxia in rats: implication for a sensitive period. J Appl Physiol. 2009;106:1212–222. doi: 10.1152/japplphysiol.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kim J, Cinotte J, Homolka P, Wong-Riley MT. Carotid body denervation effect on cytochrome oxidase activity in pre-Botzinger complex of developing rats. J Appl Physiol. 2003;94:1115–1121. doi: 10.1152/japplphysiol.00765.2002. [DOI] [PubMed] [Google Scholar]
- Liu Q, Lowry TF, Wong-Riley MT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol. 2006;577:957–970. doi: 10.1113/jphysiol.2006.121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Botzinger complex. J Appl Physiol. 2002;92:923–934. doi: 10.1152/japplphysiol.00977.2001. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in cytochrome oxidase expressions in brain stem nuclei of rats: implications for sensitive periods. J Appl Physiol. 2003;95:2285–2291. doi: 10.1152/japplphysiol.00638.2003. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in the rat pre-Botzinger complex. J Appl Physiol. 2004;96:1825–1831. doi: 10.1152/japplphysiol.01264.2003. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol. 2005;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Developmental changes in the expression of GABA(A) receptor subunits alpha1, alpha2, and alpha3 in brain stem nuclei of rats. Brain Res. 2006;1098:129–138. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in the expression of serotonin 2A receptors in various brain stem nuclei of the rat. J Appl Physiol. 2008;104:1801–1808. doi: 10.1152/japplphysiol.00057.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: implication for a sensitive period. Neuroscience. 2010a;165:61–78. doi: 10.1016/j.neuroscience.2009.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in tryptophan hydroxylase and serotonin transporter immunoreactivity in multiple brainstem nuclei of the rat: implications for a sensitive period. J Comp Neurol. 2010b;518:1082–1097. doi: 10.1002/cne.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal development of N-methyl-D-aspartate receptor subunits 2A, 2B, 2C, 2D, and 3B immunoreactivity in brain stem respiratory nuclei of the rat. Neuroscience. 2010c;171:637–654. doi: 10.1016/j.neuroscience.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Wong-Riley MT. Developmental study of cytochrome oxidase activity in the brain stem respiratory nuclei of postnatal rats. J Appl Physiol. 2001;90:685–694. doi: 10.1152/jappl.2001.90.2.685. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeschcke HH, Mitchell RA, Katsaros B, Perkins JF, Konig A. Interaction of intracranial chemosensitivity with peripheral afferents to the respiratory centers. Ann N Y Acad Sci. 1963;109:651–660. doi: 10.1111/j.1749-6632.1963.tb13495.x. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Cardiovascular control from neurons in the ventrolateral medulla. In: Taylor EW, editor. The neurobiology of the cardiorespiratory system. Manchester University Press; Manchester: 1987. pp. 199–208. [Google Scholar]
- Lowe AA. The neural regulation of tongue movements. Prog Neurobiol. 1980;15:295–344. doi: 10.1016/0301-0082(80)90008-8. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Kamiya Y, Matsuda S, Yuzaki M. Cloning and characterization of a novel NMDA receptor subunit NR3B: a dominant subunit that reduces calcium permeability. Brain Res Mol Brain Res. 2002;100:43–52. doi: 10.1016/s0169-328x(02)00173-0. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. J Appl Physiol. 1963;18:523–533. doi: 10.1152/jappl.1963.18.3.523. [DOI] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol. 1994;478(Pt 1):55–66. doi: 10.1113/jphysiol.1994.sp020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP. Breathing pattern in newborns. J Appl Physiol. 1984;56:1533–1540. doi: 10.1152/jappl.1984.56.6.1533. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Dynamics of breathing in newborn mammals. Physiol Rev. 1987;67:187–243. doi: 10.1152/physrev.1987.67.1.187. [DOI] [PubMed] [Google Scholar]
- Mortola JP. How newborn mammals cope with hypoxia. Respir Physiol. 1999;116:95–103. doi: 10.1016/s0034-5687(99)00038-9. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Respiratory Physiology of Newborn Mammals. The Johns Hopkins University Press; Baltimore, MD: 2001. [Google Scholar]
- Mortola JP. Implications of hypoxic hypometabolism during mammalian ontogenesis. Respir Physiol Neurobiol. 2004;141:345–356. doi: 10.1016/j.resp.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Lanthier C. The ventilatory and metabolic response to hypercapnia in newborn mammalian species. Respir Physiol. 1996;103:263–270. doi: 10.1016/0034-5687(95)00093-3. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Morgan CA, Virgona V. Respiratory adaptation to chronic hypoxia in newborn rats. J Appl Physiol. 1986;61:1329–1336. doi: 10.1152/jappl.1986.61.4.1329. [DOI] [PubMed] [Google Scholar]
- Mtui EP, Anwar M, Gomez R, Reis DJ, Ruggiero DA. Projections from the nucleus tractus solitarii to the spinal cord. J Comp Neurol. 1993;337:231–252. doi: 10.1002/cne.903370205. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E. Multiple sites for central chemoreception: their roles in response sensitivity and in sleep and wakefulness. Respir Physiol. 2000;122:223–235. doi: 10.1016/s0034-5687(00)00161-4. [DOI] [PubMed] [Google Scholar]
- Nattie E, Forster HV. Special Issue on Central Chemoreception. Resp Physiol Neurobiol. 2010;173:193–336. doi: 10.1016/j.resp.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Neylon M, Marshall JM. The role of adenosine in the respiratory and cardiovascular response to systemic hypoxia in the rat. J Physiol. 1991;440:529–545. doi: 10.1113/jphysiol.1991.sp018723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS. Severe Acute Intermittent Hypoxia Elicits Phrenic Long-Term Facilitation by a Novel Adenosine-Dependent Mechanism. J Appl Physiol. 2012 doi: 10.1152/japplphysiol.00060.2012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y. Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci. 2001;21:RC185. doi: 10.1523/JNEUROSCI.21-23-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Abades PA, Morillo AM, Pasaro R. Brainstem connections of the rat ventral respiratory subgroups: afferent projections. J Auton Nerv Syst. 1993;42:99–118. doi: 10.1016/0165-1838(93)90042-s. [DOI] [PubMed] [Google Scholar]
- Nurse CA. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol. 2010;95:657–667. doi: 10.1113/expphysiol.2009.049312. [DOI] [PubMed] [Google Scholar]
- Ohtake PJ, Simakajornboon N, Fehniger MD, Xue YD, Gozal D. N-Methyl-D-aspartate receptor expression in the nucleus tractus solitarii and maturation of hypoxic ventilatory response in the rat. Am J Respir Crit Care Med. 2000;162:1140–1147. doi: 10.1164/ajrccm.162.3.9903094. [DOI] [PubMed] [Google Scholar]
- Ohtake PJ, Torres JE, Gozal YM, Graff GR, Gozal D. NMDA receptors mediate peripheral chemoreceptor afferent input in the conscious rat. J Appl Physiol. 1998;84:853–861. doi: 10.1152/jappl.1998.84.3.853. [DOI] [PubMed] [Google Scholar]
- Olson EJ, Simon PM. Sleep-wake cycles and the management of respiratory failure. Curr Opin Pulm Med. 1996;2:500–506. [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomori Y, Murabayashi H, Ishikawa K, Miyakawa K, Nakaya K, Tanaka H. Neuropeptide Y- and catecholamine-synthesizing enzymes: immunoreactivities in the rat carotid body during postnatal development. Anat Embryol (Berl) 2002;206:37–47. doi: 10.1007/s00429-002-0275-4. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. Jama. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Patwari PP, Carroll MS, Rand CM, Kumar R, Harper R, Weese-Mayer DE. Congenital central hypoventilation syndrome and the PHOX2B gene: a model of respiratory and autonomic dysregulation. Respir Physiol Neurobiol. 2010;173:322–335. doi: 10.1016/j.resp.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol. 2008;104:1287–1294. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C, Wyatt CN, Evans AM. Mechanisms for acute oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2010;174:292–298. doi: 10.1016/j.resp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyronnet J, Roux JC, Geloen A, Tang LQ, Pequignot JM, Lagercrantz H, Dalmaz Y. Prenatal hypoxia impairs the postnatal development of neural and functional chemoafferent pathway in rat. J Physiol. 2000;524(Pt 2):525–537. doi: 10.1111/j.1469-7793.2000.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. NO and CO as second messengers in oxygen sensing in the carotid body. Respir Physiol. 1999;115:161–168. doi: 10.1016/s0034-5687(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Sensory plasticity of the carotid body: role of reactive oxygen species and physiological significance. Respir Physiol Neurobiol. 2011;178:375–380. doi: 10.1016/j.resp.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ, Kumar GK, Pawar A. Altered carotid body function by intermittent hypoxia in neonates and adults: relevance to recurrent apneas. Respir Physiol Neurobiol. 2007;157:148–153. doi: 10.1016/j.resp.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Semenza GL. Gaseous messengers in oxygen sensing. J Mol Med (Berl) 2012;90:265–272. doi: 10.1007/s00109-012-0876-1. [DOI] [PubMed] [Google Scholar]
- Prasad M, Fearon IM, Zhang M, Laing M, Vollmer C, Nurse CA. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signalling. J Physiol. 2001;537:667–677. doi: 10.1111/j.1469-7793.2001.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Conrad SC, Gdovin MJ, Erlichman JS, Leiter JC. Neonatal maturation of the hypercapnic ventilatory response and central neural CO(2) chemosensitivity. Respir Physiol Neurobiol. 2005 doi: 10.1016/j.resp.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Salgueiro JL, Gaytan SP, Ribas J, Pasaro R. Characterization of efferent projections of chemosensitive neurons in the caudal parapyramidal area of the rat brain. Brain Res Bull. 2005;66:235–248. doi: 10.1016/j.brainresbull.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. discussion 266–259. [DOI] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514(Pt 2):567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeser JC, Brackett DG, van Heerden ES, Young KM, Bavis RW. Potentiation of the hypoxic ventilatory response by 1 day of hyperoxia in neonatal rats. Respir Physiol Neurobiol. 2011;176:50–56. doi: 10.1016/j.resp.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Reis DJ. Projections from the nucleus tractus solitarii to the rostral ventrolateral medulla. J Comp Neurol. 1985;242:511–534. doi: 10.1002/cne.902420405. [DOI] [PubMed] [Google Scholar]
- Saetta M, Mortola JP. Interaction of hypoxic and hypercapnic stimuli on breathing pattern in the newborn rat. J Appl Physiol. 1987;62:506–512. doi: 10.1152/jappl.1987.62.2.506. [DOI] [PubMed] [Google Scholar]
- Sant’Anna GM, Mortola JP. Thermal and respiratory control in young rats with altered caloric intake during postnatal development. Respir Physiol Neurobiol. 2002;133:215–227. doi: 10.1016/s1569-9048(02)00148-9. [DOI] [PubMed] [Google Scholar]
- Serra A, Brozoski D, Hedin N, Franciosi R, Forster HV. Mortality after carotid body denervation in rats. J Appl Physiol. 2001;91:1298–1306. doi: 10.1152/jappl.2001.91.3.1298. [DOI] [PubMed] [Google Scholar]
- Serra A, Brozoski D, Hodges M, Roethle S, Franciosi R, Forster HV. Effects of carotid and aortic chemoreceptor denervation in newborn piglets. J Appl Physiol. 2002;92:893–900. doi: 10.1152/japplphysiol.00819.2001. [DOI] [PubMed] [Google Scholar]
- Simakajornboon N, Kuptanon T. Maturational changes in neuromodulation of central pathways underlying hypoxic ventilatory response. Respir Physiol Neurobiol. 2005;149:273–286. doi: 10.1016/j.resp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J Neurophysiol. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol. 2010;173:288–297. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, O’Neal MH., 3rd CO(2)/H(+) chemoreception in the cat pre-Botzinger complex in vivo. J Appl Physiol. 2000;88:1996–2007. doi: 10.1152/jappl.2000.88.6.1996. [DOI] [PubMed] [Google Scholar]
- Spengler CM, Gozal D, Shea SA. Chemoreceptive mechanisms elucidated by studies of congenital central hypoventilation syndrome. Respir Physiol. 2001;129:247–255. doi: 10.1016/s0034-5687(01)00294-8. [DOI] [PubMed] [Google Scholar]
- St-John WM. Neurogenesis of patterns of automatic ventilatory activity. Prog Neurobiol. 1998;56:97–117. doi: 10.1016/s0301-0082(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Sterni LM, Bamford OS, Wasicko MJ, Carroll JL. Chronic hypoxia abolished the postnatal increase in carotid body type I cell sensitivity to hypoxia. Am J Physiol. 1999;277:L645–652. doi: 10.1152/ajplung.1999.277.3.L645. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pieribone VA, Aston-Jones G. Diverse afferents converge on the nucleus paragigantocellularis in the rat ventrolateral medulla: retrograde and anterograde tracing studies. J Comp Neurol. 1989;290:561–584. doi: 10.1002/cne.902900410. [DOI] [PubMed] [Google Scholar]
- Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol. 1993;264:R41–50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am J Respir Crit Care Med. 1996;153:776–786. doi: 10.1164/ajrccm.153.2.8564132. [DOI] [PubMed] [Google Scholar]
- Verna A, Schamel A, Pequignot JM. Noradrenergic glomus cells in the carotid body: an autoradiographic and immunocytochemical study in the rabbit and rat. Adv Exp Med Biol. 1993;337:93–100. doi: 10.1007/978-1-4615-2966-8_14. [DOI] [PubMed] [Google Scholar]
- Vizek M, Pickett CK, Weil JV. Biphasic ventilatory response of adult cats to sustained hypoxia has central origin. J Appl Physiol. 1987;63:1658–1664. doi: 10.1152/jappl.1987.63.4.1658. [DOI] [PubMed] [Google Scholar]
- Von Euler C, Soderberg U. Medullary chemosensitive receptors. J Physiol. 1952;118:545–554. doi: 10.1113/jphysiol.1952.sp004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol. 2001a;434:128–146. doi: 10.1002/cne.1169. [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511(Pt 2):433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience. 1999;90:1001–1011. doi: 10.1016/s0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001b;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Wickstrom R, Hokfelt T, Lagercrantz H. Development of CO(2)-response in the early newborn period in rat. Respir Physiol Neurobiol. 2002;132:145–158. doi: 10.1016/s1569-9048(02)00076-9. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol. 1998;510(Pt 1):1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon A. Innervation of serotonergic medullary raphe neurons from cells of the rostral ventrolateral medulla in rats. Neuroscience. 1993;55:849–867. doi: 10.1016/0306-4522(93)90446-m. [DOI] [PubMed] [Google Scholar]
- Zec N, Kinney HC. Anatomic relationships of the human nucleus paragigantocellularis lateralis: a DiI labeling study. Auton Neurosci. 2001;89:110–124. doi: 10.1016/S1566-0702(01)00258-2. [DOI] [PubMed] [Google Scholar]
- Zhan G, Shaheen F, Mackiewicz M, Fenik P, Veasey SC. Single cell laser dissection with molecular beacon polymerase chain reaction identifies 2A as the predominant serotonin receptor subtype in hypoglossal motoneurons. Neuroscience. 2002;113:145–154. doi: 10.1016/s0306-4522(02)00137-9. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol. 2000;525(Pt 1):143–158. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Su J, Cui N, Gai H, Wu Z, Jiang C. The disruption of central CO2 chemosensitivity in a mouse model of Rett syndrome. Am J Physiol Cell Physiol. 2011;301:C729–738. doi: 10.1152/ajpcell.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RWt, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]