Abstract
Integrins link the extracellular matrix (ECM) to the cytoskeleton to control cell behaviors including adhesion, spreading and migration. Band 4.1 proteins contain 4.1, ezrin, radixin, moesin (FERM) domains that likely mediate signaling events and cytoskeletal reorganization via integrins. However, the mechanisms by which Band 4.1 proteins and integrins are functionally interconnected remain enigmatic. Here we have investigated roles for Band 4.1 proteins in integrin-mediated cell spreading. We demonstrate that Proteins 4.1B and 4.1G show overlapping and dynamic patterns of sub-cellular localization in astrocytes spreading on fibronectin. During early stages of cell spreading Proteins 4.1B and 4.1G are enriched in ECM adhesion sites but become more diffusely expressed in later stages of cell spreading. Combinatorial inhibition of Protein 4.1B and 4.1G expression leads to impaired astrocyte spreading. Furthermore, using exogenous expression systems we show that the isolated Protein 4.1 FERM domain significantly enhances β1 integrin-mediated cell spreading. Lastly, Protein 4.1B is dispensable for reactive astrogliosis in experimental models of cortical injury, likely due to functional compensation by related Protein 4.1 family members. Collectively, these findings reveal that Band 4.1 proteins are important intracellular components for integrin-mediated cell spreading.
Keywords: extracellular matrix, astrocyte, Band 4.1, cytoskeleton, FERM domain
Introduction
The Protein 4.1 superfamily is comprised of a diverse group of proteins that share common FERM domains [1]. Members of this superfamily include unconventional myosins [2], talins [3], kindlins [4], focal adhesion kinase [5] and the proto-typical Band 4.1 proteins [6]. The five members of the Band 4.1 family show overlapping expression in various tissues where they interact with cytoplasmic signaling effectors and link transmembrane proteins to the actin cytoskeleton [7,8,9,10].
Various studies have revealed important roles for Protein 4.1B in tumor cell growth and migration. For example, Protein 4.1B gene expression is down regulated in cancers of the brain [11,12] and lung [13]. Protein 4.1B has also been identified as a suppressor of metastasis in mouse models of sarcoma and prostate cancer [14,15]. However, mice genetically null for Protein 4.1B do not develop spontaneous malignancies, suggesting that loss of Protein 4.1B in combination with other tumor suppressors and/or activating mutations in oncogenes drive tumorigenesis. Alternatively, other related Band 4.1 family members may compensate for loss of Protein 4.1B expression in knockout mice [16]. Indeed, in various Band 4.1 knockout mouse models functional compensation by different family members has been suggested [17,18].
Protein 4.1B contains an N-terminal FERM domain that likely interacts with cell surface receptors including integrins. Here, we demonstrate that Protein 4.1B and β1 integrins associate in ECM adhesion sites in primary astrocytes spreading on fibronectin, with the Protein 4.1B FERM domain promoting β1 integrin-mediated cell spreading. Collectively, these data reveal important functional links between Protein 4.1B and β1 integrins in regulating astrocyte spreading.
Materials and Methods
Experimental mice and primary cell culture systems
4.1B+/− mice were interbred to generate wild type and 4.1B−/− littermates as previously described [16]. Genotypes were determined using PCR-based methods as described previously [19]. Primary wild type and 4.1B−/− astrocytes were isolated from P1-P3 as described previously [20,21]. Wild type and 4.1B−/− cells were transduced with retroviruses expressing E6/E7 oncogenes, and cells were selected in growth media containing one µg/ml puromycin for five days as described previously [20]. SMARTPool siRNAs targeting murine Band 4.1G gene sequences were purchased from Dharmacon, Inc.
Immunoblotting, immunofluorescence and immunoprecipitation
Generation of the anti-Protein 4.1B rabbit polyclonal antibody has been described previously [22]. The following antibodies are from commercial sources: anti-4.1G and anti-4.1N (Protein Express); anti-actin and anti-talin (Sigma), anti-α-actinin (Abcam), anti-paxillin (BD Biosciences), anti-myc (Invitrogen); anti-β1 and anti-α8 (Santa Cruz Biotech); anti-β1, anti-α2, anti-α3, anti-α4, anti-α6, anti-β1, and anti-GFAP (Millipore); anti-α2 and anti-α5 mAbs (Emfret Analytics). The anti-αv and anti-β8 integrin antibodies have been described elsewhere [23,24]. Secondary fluorescent antibodies are from Molecular Probes.
Cells on ECM-coated glass coverslips were fixed with 4% PFA/PBS and then permeabilized with PBS containing 0.5% NP40. Samples were blocked with PBS containing 10% goat serum, incubated with appropriate primary and secondary antibodies. E11.5 wild embryos were dissected, embedded in Tissue-Tek OCT (Sakura Finetek) and coronal sections were prepared. To analyze interactions between myc-tagged Protein 4.1B and β1 integrin, transfected cells were plated on fibronectin for 30 minutes. Detergent-soluble lysates were immunoprecipitated using anti-myc mAbs (Invitrogen) and immunoblotted with anti-β1 integrin pAbs (Millipore).
Biotinylation and immunoprecipitation
Primary astrocytes growing on laminin were labeled with 0.1mg/ml Sulfo-NHS-biotin (Thermo Scientific) for 30 minutes at 37°C. After briefly washing cells with PBS and TBS, cells were lysed in NP40 buffer. Lysates were pre-cleared with goat anti-rabbit-agarose (Sigma), rabbit anti-goat IgG-agarose (Research Diagnostics), or goat anti-rat IgG-agarose (Sigma) prior to immunoprecipitation with anti-integrin antibodies.
Cell adhesion assays
Glass coverslips were coated with 3 µg/ml fibronectin (Sigma) for 16 hours at 4°C. Primary astrocytes were allowed to adhere and spread for varying times before fixation and permeabilization. Adherent cells were stained with crystal violet, and absorbance at 560nm was detected. COS7 cells were transfected with pcDNA3.1 vectors expressing 4.1B FERM-myc, full-length 4.1B (FL)-myc, and lacZ-myc fusion proteins using Effectene transfection reagents (Qiagen). Cells were fixed with 4% PFA/PBS, permeabilized with PBS containing 0.5% NP40 and immunofluorescently labeled with anti-myc antibodies. Areas encompassed by myc-expressing cells were quantified using ImageJ software (NIH).
In vivo brain injury models
To induce cortical injury in control and 4.1B−/− littermates a single incision was made from the anterior pole of the skull to the posterior ridge. A sterile scalpel blade was lowered into the brain 3 mm below the pial surface and a 3–5 mm wound was generated posterior to the initial stereotactic point of entry (1.5 mm rostral, 1.5 mm anterior). Three or seven days after the surgery control and 4.1B−/− groups (n=3 mice per genotype and time point) were sacrificed and brain sections were analyzed.
Statistical analyses
All data were analyzed using student’s t-test to determine statistically significant differences.
Results
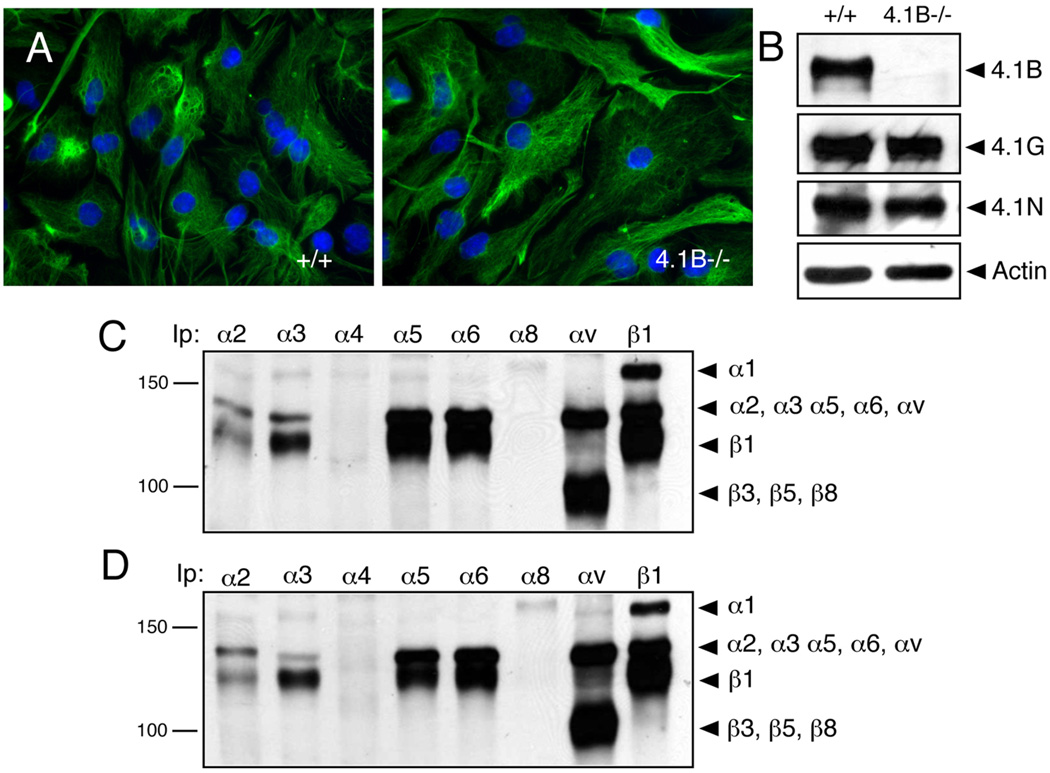
To characterize functions for Protein 4.1B and other related Band 4.1 family members in cell adhesion and spreading [25], we cultured primary brain astrocytes from wild type (control) and 4.1B−/− (mutant) newborn mice. Astrocytes from control and 4.1B−/− mice expressed robust levels of the intermediate filament proteins glial fibrillary acidic protein and nestin, and did not display obvious morphological differences (Figure 1A). Detergent-soluble lysates from control and 4.1B−/− astrocytes were immunoblotted with anti-4.1B, anti-4.1G, anti-4.1N, and anti-4.1R rabbit polyclonal antibodies. As shown in Figure 1B, in wild type cells the anti-4.1B antibody was immunoreactive with a band of approximately 145 kDa. Robust levels of protein 4.1G and 4.1N bands were also detected in wild type and 4.1B−/− astrocytes with approximate molecular weights of 150 kDa and 100 kDa, respectively (Figure 1B). No significant differences in 4.1G and 4.1N protein levels were observed between wild type and 4.1B−/− cells. Similar levels of Proteins 4.1B, 4.1G and 4.1N were detected in lysates prepared from wild type and 4.1B−/− cerebral cortices (Supplemental Figure 1). Band 4.1 proteins contain N-terminal FERM domains that are reported to interact with cell surface receptors [6], and a recent report has shown interactions between the Protein 4.1R FERM domain and β1 integrin [26]. We analyzed surface expression of various integrins in wild type and 4.1B−/− astrocytes by labeling cells with amine-reactive biotin. As shown in Figure 1C, in wild type astrocytes we detected robust levels of α1β1, α2β1, α3β1, α5β1, α6β1, and αv-containing integrins. In addition, 4.1B−/− astrocytes displayed comparable levels of integrin cell surface expression (Figure 1D).
Figure 1. Multiple Band 4.1 and integrin proteins are expressed in primary brain astrocytes.
(A); Astrocytes cultured from wild type (left panel) or 4.1B−/− (right panel) neonatal brains were immunolabeled with anti-GFAP antibodies. Note that control and 4.1B mutant cells display similar cytoarchitecture. (B); Lysates from wild type and 4.1B−/− astrocytes were immunoblotted with anti-4.1B, anti-4.1G, and anti-4.1N rabbit polyclonal antibodies. (C, D); Primary astrocytes from wild type (C) and 4.1B−/− mice (D) were labeled with amine-reactive biotin and detergent-soluble lysates were immunoprecipitated with various anti-integrin antibodies.
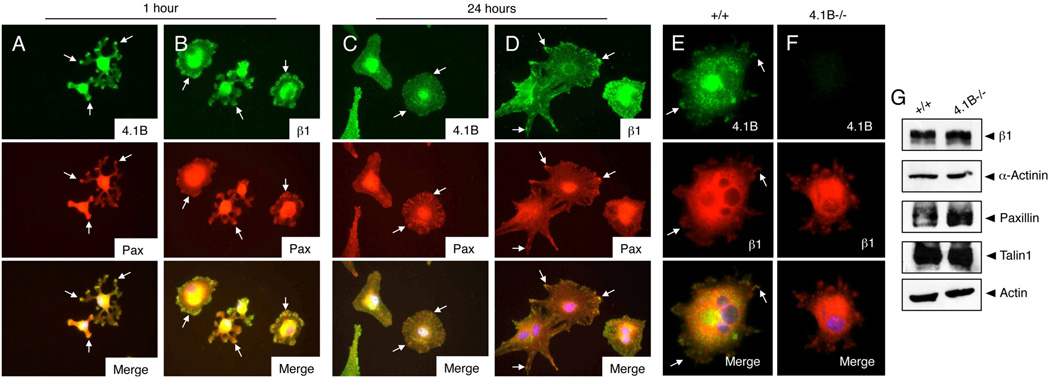
Sub-cellular localization of Protein 4.1B and β1 integrin was next analyzed in astrocytes spreading on fibronectin. Cells adhering for one hour or 24 hours were immunolabeled with anti-4.1B, anti-β1, or anti-paxillin antibodies. At one hour post-adhesion both Protein 4.1B (Figure 2A) and β1 integrin protein (Figure 2B) were detected in ECM contact sites as determined by co-localization with paxillin. However, after 24 hours when cells were well spread, Protein 4.1B was more diffusely localized (Figure 2C), whereas β1 integrin showed continued enrichment in paxillin-positive adhesion sites (Figure 2D). We next analyzed Protein 4.1B-dependent cell adhesion and spreading on fibronectin. Wild type and 4.1B−/− astrocytes plated on fibronectin were labeled with anti-4.1B polyclonal antibodies and anti-β1 integrin mAbs. Protein 4.1B and β1 integrin co-localized in spreading cells (Figure 2E), although 4.1B−/− astrocytes did not display altered β1 integrin protein enrichment in cell-ECM contact sites (Figure 2F). Furthermore, Protein 4.1B-dependent differences in expression of common focal contact proteins such as talin, paxillin, α-actinin and β1 integrin were not detected (Figure 2G). To quantify 4.1B-dependent adhesion on fibronectin, wild type and 4.1B−/− astrocytes were plated for 60 minutes and cell adhesion was measured by crystal violet staining. Morphologies of adherent wild type and 4.1B−/− astrocytes on fibronectin revealed similar actin cytoskeleton architecture as revealed by phalloidin staining (Supplemental Figure 2).
Figure 2. Protein 4.1B displays dynamic sub-cellular localization in spreading astrocytes.
(A–D); Wild type astrocytes were plated on fibronectin-coated dishes for 60 minutes (A, B) or 24 hours (C, D). Spreading cells were then labeled with anti-4.1B (A, C) or anti-β1 integrin (B, D) rabbit polyclonal antibodies in combination with anti-paxillin mAbs. (E, F); Wild type (E) and 4.1B−/− (F) astrocytes were plated on fibronectin for one hour and then immunolabeled with anti-4.1B polyclonal antibodies and anti-β1 integrin mAbs. Protein 4.1B and β1 integrin co-localize at one hour post-adhesion on fibronectin (arrows in E). Images are shown at 400×. (G); No differences in expression of common focal adhesion proteins in wild type and 4.1B−/− astrocytes.
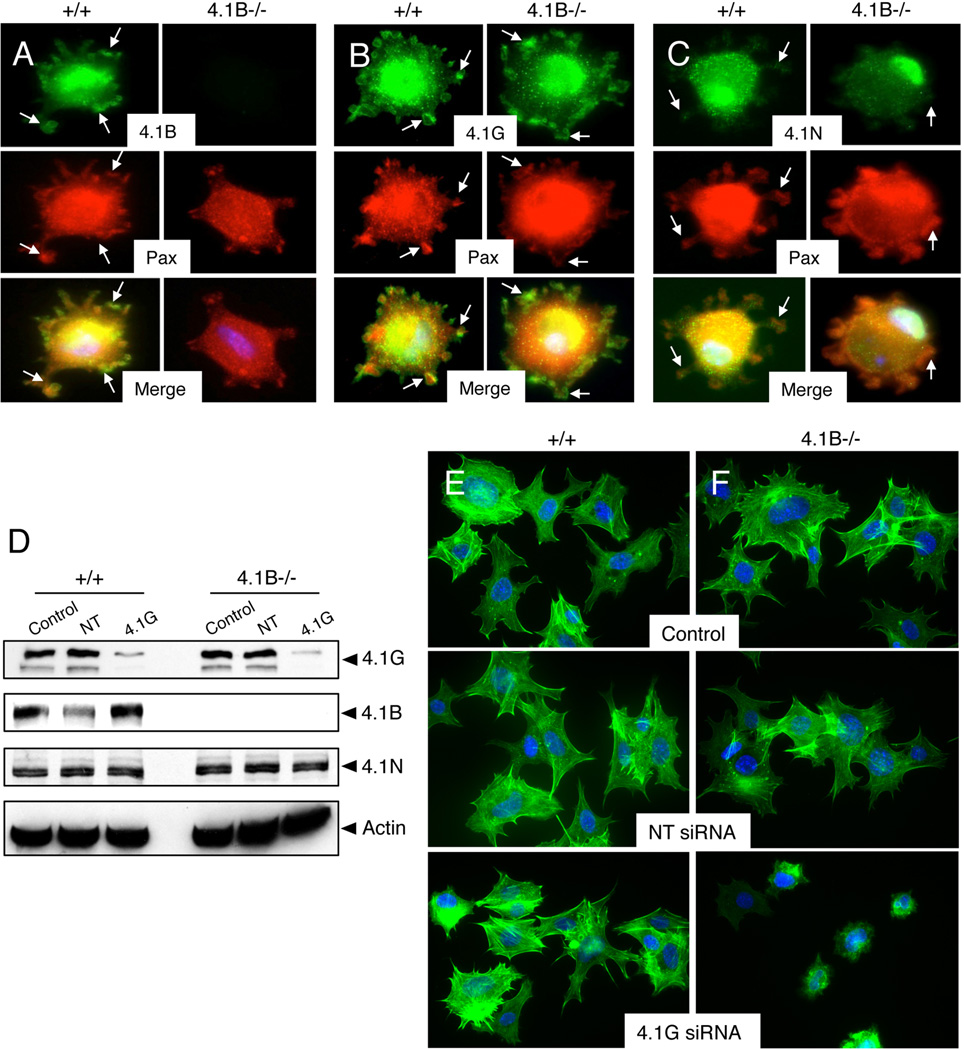
Given that Protein 4.1G and 4.1N are expressed in wild type and 4.1B−/− astrocytes (Figure 1), we next analyze spatial patterns of expression of these proteins in spreading astrocytes. Wild type and 4.1B−/− astrocytes were allowed to adhere to fibronectin for varying times, and cells were immunostained with anti-4.1B, anti-4.1G, and anti-4.1N antibodies in combination with anti-paxillin to visualize ECM adhesion sites. Protein 4.1B and 4.1G, but not Protein 4.1N, were expressed in cell-ECM contact sites (Figures 3A–C). Like wild type cells, 4.1B−/− astrocytes were adherent to fibronectin and contained paxillin-positive ECM adhesion sites, although Protein 4.1B protein did not co-localize with paxillin (Figure 3A) owing to gene deletion. Obvious differences in localization of Proteins 4.1G and 4.1N were not detected in 4.1B−/− cells (Figures 3B, C). Analysis of Protein 4.1B, 4.1G, and 4.1N sub-cellular localization in wild type and 4.1B−/− primary astrocytes at 24 hours post-adhesion to fibronectin showed diffuse, cytoplasmic expression (Supplemental Figure 3). In confluent cultures of adherent astrocytes Proteins 4.1B and 4.1G were enriched at sites of direct cell-cell contact, although Protein 4.1N remained diffusely expressed in confluent astrocyte cultures (Supplemental Figure 4). In scratch-wound migration assays we did not detect Band 4.1 proteins enriched at the leading edge of migrating astrocytes (Supplemental Figure 5A).
Figure 3. Proteins 4.1B and 4.1G, but not 4.1N, are enriched in ECM adhesion sites in spreading astrocytes.
(A–C); Wild type and 4.1B−/− astrocytes were plated on fibronectin for 60 minutes and cells were labeled with anti-4.1B (A), anti-4.1G (B), or anti-4.1N (C) rabbit polyclonal antibodies in combination with anti-paxillin mAbs. Note that Proteins 4.1B and 4.1G co-localize with paxillin in cell-ECM adhesion sites (arrows). Images are shown at 400×. (D); Wild type and 4.1B−/− astrocytes were mock transfected or transfected with non-targeting (NT) siRNAs or siRNAs targeting Protein 4.1G. Cell lysates were immunoblotted with anti-Band 4.1G, 4.1B or 4.1N antibodies. (E, F); Wild type and 4.1B−/− astrocytes that were mock transfected or transfected with non-targeting siRNAs or siRNAs targeting 4.1G were plated on fibronectin for 60 minutes and cells were stained with phalloidin to visualize the actin cytoskeleton. Images are shown at 400×.
We hypothesized that 4.1G may have overlapping functions or functionally compensate in the absence of 4.1B. Therefore, we used siRNAs to silence murine Band 4.1G gene expression in wild type and 4.1B−/− cells. Cultured primary astrocytes display limited growth properties and are not amenable for siRNA transfection (data not shown); therefore, astrocytes were immortalized with retroviruses expressing E6/E7 oncogenes as we have described previously [20]. Immortalized astrocytes displayed high transfection efficiency as determined using siRNAs targeting murine cyclophilin (>90% reduction in protein expression, data not shown). As shown in Figure 3D, we detected a significant reduction in Protein 4.1G levels in wild type and 4.1B−/− cells following siRNA transfection. Protein 4.1B and 4.1N levels were not altered following 4.1G gene silencing (Figure 3D), and we did not detect changes in the cell surface expression of β1 integrins in control and 4.1B−/− cells (data not shown). Wild type or 4.1B−/− astrocytes that were mock transfected or transfected with non-targeting siRNAs did not display differences in spreading on fibronectin. In addition, spreading differences were not detected in wild type astrocytes transfected with siRNAs targeting 4.1G (Figure 3E), likely due to expression of Protein 4.1B. However, upon silencing 4.1G gene expression in 4.1B−/− astrocytes obvious defects in cell spreading and actin cytoskeleton organization were apparent (Figure 3F). Collectively, these data reveal that Protein 4.1B and 4.1G both regulate astrocyte spreading on fibronectin, and their combined inhibition leads to spreading defects.
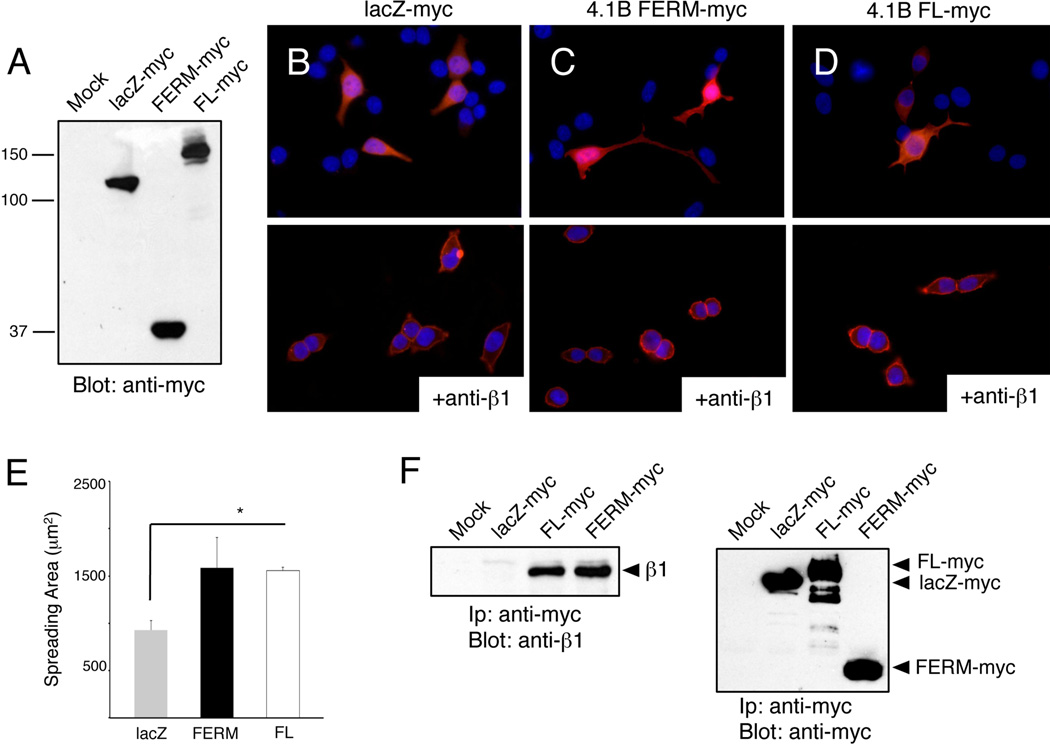
We used exogenous expression approaches to analyze 4.1B domains involved in cell spreading. COS7 cells, which express low levels of endogenous Protein 4.1B and 4.1G, were transfected with pcDNA3.1 plasmids expressing lacZ-myc, myc-tagged full length Protein 4.1B, or the myc-tagged Protein 4.1B FERM domain (Figure 4A). After 48 hours cells were plated on fibronectin for one hour and then stained with anti-myc mAbs. Unlike COS7 cells transfected with lacZ-myc (Figure 4B, upper panel), cells expressing 4.1B FERM-myc and full-length 4.1-Bmyc displayed increased spreading on fibronectin (Figures 4C, D upper panels). ImageJ software was used to quantify myc-positive cell surface areas, revealing increased spreading in cells expressing Protein 4.1B or the isolated FERM domain (Figure 4E). Cells transfected with the 4.1B FERM-myc, full-length 4.1B-myc, and lacZ-myc constructs were also treated with anti-β1 integrin neutralizing antibodies prior to plating on fibronectin. Protein 4.1B-mediated enhancement of cell spreading was inhibited by anti-β1 integrin blocking antibodies (Figures 4B–D, lower panels). We next analyzed associations between Protein 4.1B and β1 integrin in COS7 cells spreading on fibronectin by co-immunoprecipitation strategies. As shown in Figure 4F, in COS7 cells we detect interactions between endogenous β1 integrin and transfected myc-tagged full-length Protein 4.1B and the isolated FERM domain. We performed similar experiments with HEK-293 cells, which express low levels of endogenous Protein 4.1B, and found similar 4.1B FERM domain-dependent increases in spreading on fibronectin (data not shown).
Figure 4. The Protein 4.1B FERM domain enhances cell spreading and associates with β1 integrin.
(A); Detergent-soluble lysates from mock-transfected COS7 cells or cells overexpressing lacZ-myc, 4.1B FERM-myc, or full-length 4.1B-myc fusion proteins were immunoblotted with anti-myc antibodies. Note the similar levels of myc fusion protein expression. (B–D); COS7 cells were transiently transfected with plasmids expressing lacZ-myc (B), 4.1B FERM-myc (C), or full-length 4.1B-myc (D) fusion proteins. After 48 hours cells were plated on fibronectin for 30 minutes and then immunolabeled with an anti-myc antibodies. Alternatively, cells were pre-incubated with anti-β1 integrin blocking mAbs (B-D, lower panels) prior to adhesion to fibronectin. Note that β1 integrin inhibition blocks 4.1B-mediated cell spreading. Images are shown at 400×. (E); Surface areas of transfected COS7 cells, as visualized by anti-myc immunofluorescence, were quantified using ImageJ software, revealing that cells expressing 4.1B FERM-myc or full length 4.1B-myc proteins showed statistically significant increases in spreading in comparison to lacZ-myc controls, *p<0.01. (F); COS7 cells overexpressing lacZ-myc, 4.1B FERM-myc, or full-length 4.1B-myc proteins were plated on fibronectin for 30 minutes. Lysates were immunoprecipitated with anti-myc antibodies and immunoblotted with anti-β1 integrin (left panel) or anti-myc polyclonal antibodies (right panel).
Next, co-localization of Protein 4.1B and β1 integrin was analyzed by immunofluorescently labeling brain sections with anti-Protein 4.1B rabbit polyclonal antibodies and anti-β1 integrin mAbs. Within the developing cerebral cortex Protein 4.1B and β1 integrin co-localized in neuroepithelial cells (Supplemental Figure 6), which give rise to most astrocytes and neurons in the post-natal brain [27]. In addition, robust expression of β1 integrin protein, but not Protein 4.1B, was detected in cerebral blood vessels (arrows in Supplemental Figure 6).
To analyze functions for Protein 4.1B in astrocytes in vivo we induced cortical stab-wounds [28]. Upon brain injury astrocytes develop ‘reactive’ morphologies and upregulate expression of the intermediate filament proteins nestin and GFAP. Over several days reactive astrocytes polarize and invade through the brain microenvironment to form a ‘glial scar’ surrounding the wound [29,30]. We induced stab wounds in the cerebral cortices of P60 wild type and 4.1B−/− mice (n=6 mice per genotype). Three and 7 days after injury mice (n=3 per genotype per time point) were sacrificed and brain sections were labeled with anti-GFAP antibodies. As shown in Supplemental Figures 7A and 7B, in the non-injured cerebral cortex we detected GFAP expression in perivascular astrocytes of wild type and 4.1B−/− mice. Following cortical injury astrocytes displayed reactive morphologies and expressed elevated levels of GFAP; however, in wild type and 4.1B−/− mice no apparent differences in numbers of reactive GFAP-expressing astrocytes were detected at three days post-injury (Supplemental Figures 7C, D). In both control and 4.1B−/− mice astrocytes formed a well-defined glial scar around the wound region. Similar patterns of GFAP-expressing astrocytes were detected at 14 days post-injury in wild type and 4.1B−/− brains (Supplemental Figure 8). Consistent with the lack of Protein 4.1B-dependent astrocyte migration defects in vivo, 4.1B−/− astrocytes also showed apparently normal migration behaviors in scratch-wound assays (Supplemental Figures 5B, C). These data reveal that Protein 4.1B is dispensable for reactive astrogliosis following experimental-induced brain injury.
Discussion
Protein 4.1B localization is highly dynamic in spreading cells, showing enrichment in cell-ECM adhesions during early stages of spreading and displaying a more diffuse localization patterns during later stages of spreading. Similar to Protein 4.1B, β1 integrin localizes to paxillin-containing ECM adhesions during initial stages of spreading; however, unlike Protein 4.1B, β1 integrin protein remains in focal adhesions beyond early stages of spreading. These data suggest that Proteins 4.1B and 4.1G may regulate focal adhesion assembly. Alternatively, Protein 4.1B may link β1 integrins to the actin cytoskeleton or serve as a binding intermediate between β1 integrin and other protein components of focal adhesions. Indeed, Protein 4.1R has been shown to interact directly with the intracellular signaling effectors β-catenin [31] and IQGAP1 [32] to promote cell adhesion and spreading.
We propose functions for Protein 4.1 family members that are partially distinct from other FERM domain-containing proteins such as talin, which activates integrins to enable ECM adhesion [33], but is dispensable for focal adhesion assembly [34]. It is possible that Protein 4.1B and related members are important for promoting focal adhesion formation prior to talin providing essential roles in stabilizing these sub-cellular structures. For example, we do not detect 4.1B-dependent differences in expression of common focal adhesion proteins (Figure 2G). Indeed, while this manuscript was in preparation An and colleagues reported associations between the Protein 4.1R FERM domain and β1 integrins [26]. Protein 4.1R was shown to modulate β1 integrin protein levels in primary mouse keratinocytes, with 4.1R−/− cells showing decreased integrin cell surface expression. In vitro scratch-wound assays and in vivo wound models also revealed essential roles for Protein 4.1R in keratinocyte adhesion and migration. The data we report herein for Protein 4.1B in astrocytes differs in some ways from the Protein 4.1R study. First, integrin surface expression levels are not altered in 4.1B−/− astrocytes (Figure 1). Second, unlike 4.1R−/− keratinocytes, 4.1B−/− astrocytes do not display migration defects in scratch-wound assays (Supplemental Figure 5) and Protein 4.1B in astrocytes appears to be dispensable for reactive astrogliosis in vivo (Supplemental Figures 7–8). Therefore, characterization of in vivo functional overlap between Protein 4.1B and related family members, particularly Protein 4.1G, may require combinatorial gene deletion or protein inhibition in astrocytes.
Supplementary Material
Highlights.
Band 4.1 proteins contain N-terminal FERM domains but their roles in cell adhesion and spreading remain enigmatic.
In primary astrocytes Band 4.1B and 4.1G proteins co-localize with β1 integrins in ECM adhesion sites.
Combined inactivation of Band 4.1B and 4.1G lead to impaired astrocyte spreading in vitro.
Band 4.1B is dispensable for reactive astrogliosis in vivo likely due to compensation by related family members.
Acknowledgements
We thank Dr. Joseph Kissil (Wistar Institute) for providing 4.1B+/− mice. This research was supported by grants awarded to J.H.M. from The Ellison Medical Foundation (AG-NS-0324-06) and the National Institutes of Neurological Disease and Stroke (R01NS059876).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tepass U. FERM proteins in animal morphogenesis. Current opinion in genetics & development. 2009;19:357–367. doi: 10.1016/j.gde.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Sousa AD, Cheney RE. Myosin-X: a molecular motor at the cell's fingertips. Trends in cell biology. 2005;15:533–539. doi: 10.1016/j.tcb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Ye F, Kim C, Ginsberg MH. Molecular mechanism of inside-out integrin regulation. Journal of thrombosis and haemostasis : JTH. 2011;9(Suppl 1):20–25. doi: 10.1111/j.1538-7836.2011.04355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malinin NL, Plow EF, Byzova TV. Kindlins in FERM adhesion. Blood. 2010;115:4011–4017. doi: 10.1182/blood-2009-10-239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nature reviews. Molecular cell biology. 2010;11:802–814. doi: 10.1038/nrm2996. [DOI] [PubMed] [Google Scholar]
- 6.Sun CX, Robb VA, Gutmann DH. Protein 4.1 tumor suppressors: getting a FERM grip on growth regulation. J Cell Sci. 2002;115:3991–4000. doi: 10.1242/jcs.00094. [DOI] [PubMed] [Google Scholar]
- 7.Diakowski W, Grzybek M, Sikorski AF. Protein 4.1, a component of the erythrocyte membrane skeleton and its related homologue proteins forming the protein 4.1/FERM superfamily, Folia histochemica et cytobiologica / Polish Academy of Sciences. Polish Histochemical and Cytochemical Society. 2006;44:231–248. [PubMed] [Google Scholar]
- 8.Okumura K, Mochizuki E, Yokohama M, Yamakawa H, Shitara H, Mburu P, Yonekawa H, Brown SD, Kikkawa Y. Protein 4.1 expression in the developing hair cells of the mouse inner ear. Brain research. 2010;1307:53–62. doi: 10.1016/j.brainres.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Ramez M, Blot-Chabaud M, Cluzeaud F, Chanan S, Patterson M, Walensky LD, Marfatia S, Baines AJ, Chasis JA, Conboy JG, Mohandas N, Gascard P. Distinct distribution of specific members of protein 4.1 gene family in the mouse nephron. Kidney international. 2003;63:1321–1337. doi: 10.1046/j.1523-1755.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- 10.Rose M, Dutting E, Enz R. Band 4.1 proteins are expressed in the retina and interact with both isoforms of the metabotropic glutamate receptor type 8. Journal of neurochemistry. 2008;105:2375–2387. doi: 10.1111/j.1471-4159.2008.05331.x. [DOI] [PubMed] [Google Scholar]
- 11.Gutmann DH, Donahoe J, Perry A, Lemke N, Gorse K, Kittiniyom K, Rempel SA, Gutierrez JA, Newsham IF. Loss of DAL-1, a protein 4.1-related tumor suppressor, is an important early event in the pathogenesis of meningiomas. Human molecular genetics. 2000;9:1495–1500. doi: 10.1093/hmg/9.10.1495. [DOI] [PubMed] [Google Scholar]
- 12.Kittiniyom K, Mastronardi M, Roemer M, Wells WA, Greenberg ER, Titus-Ernstoff L, Newsham IF. Allele-specific loss of heterozygosity at the DAL-1/4.1B (EPB41L3) tumor-suppressor gene locus in the absence of mutation. Genes, chromosomes & cancer. 2004;40:190–203. doi: 10.1002/gcc.20034. [DOI] [PubMed] [Google Scholar]
- 13.Tran YK, Bogler O, Gorse KM, Wieland I, Green MR, Newsham IF. A novel member of the NF2/ERM/4.1 superfamily with growth suppressing properties in lung cancer. Cancer research. 1999;59:35–43. [PubMed] [Google Scholar]
- 14.Cavanna T, Pokorna E, Vesely P, Gray C, Zicha D. Evidence for protein 4.1B acting as a metastasis suppressor. Journal of cell science. 2007;120:606–616. doi: 10.1242/jcs.000273. [DOI] [PubMed] [Google Scholar]
- 15.Wong SY, Haack H, Kissil JL, Barry M, Bronson RT, Shen SS, Whittaker CA, Crowley D, Hynes RO. Protein 4.1B suppresses prostate cancer progression and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12784–12789. doi: 10.1073/pnas.0705499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi C, McCarty JH, Troutman SA, Eckman MS, Bronson RT, Kissil JL. Loss of the putative tumor suppressor band 4.1B/Dal1 gene is dispensable for normal development and does not predispose to cancer. Mol Cell Biol. 2005;25:10052–10059. doi: 10.1128/MCB.25.22.10052-10059.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horresh I, Bar V, Kissil JL, Peles E. Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. J Neurosci. 2010;30:2480–2489. doi: 10.1523/JNEUROSCI.5225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wozny C, Breustedt J, Wolk F, Varoqueaux F, Boretius S, Zivkovic AR, Neeb A, Frahm J, Schmitz D, Brose N, Ivanovic A. The function of glutamatergic synapses is not perturbed by severe knockdown of 4.1N and 4.1G expression. J Cell Sci. 2009;122:735–744. doi: 10.1242/jcs.037382. [DOI] [PubMed] [Google Scholar]
- 19.Jung Y, Kissil JL, McCarty JH. beta8 integrin and band 4.1B cooperatively regulate morphogenesis of the embryonic heart. Dev Dyn. 2011;240:271–277. doi: 10.1002/dvdy.22513. [DOI] [PubMed] [Google Scholar]
- 20.Tchaicha JH, Mobley AK, Hossain MG, Aldape KD, McCarty JH. A mosaic mouse model of astrocytoma identifies alphavbeta8 integrin as a negative regulator of tumor angiogenesis. Oncogene. 2010;29:4460–4472. doi: 10.1038/onc.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchaicha JH, Reyes SB, Shin J, Hossain MG, Lang FF, McCarty JH. Glioblastoma angiogenesis and tumor cell invasiveness are differentially regulated by {beta}8 integrin. Cancer research in press. 2011 doi: 10.1158/0008-5472.CAN-11-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarty JH, Cook AA, Hynes RO. An interaction between {alpha}v{beta}8 integrin and Band 4.1B via a highly conserved region of the Band 4.1 C-terminal domain. Proc Natl Acad Sci U S A. 2005;102:13479–13483. doi: 10.1073/pnas.0506068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 24.Mobley AK, Tchaicha JH, Shin J, Hossain MG, McCarty JH. {beta}8 integrin regulates neurogenesis and neurovascular homeostasis in the adult brain. J Cell Sci. 2009;122:1842–1851. doi: 10.1242/jcs.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parra M, Gascard P, Walensky LD, Gimm JA, Blackshaw S, Chan N, Takakuwa Y, Berger T, Lee G, Chasis JA, Snyder SH, Mohandas N, Conboy JG. Molecular and functional characterization of protein 4.1B, a novel member of the protein 4.1 family with high level, focal expression in brain. The Journal of biological chemistry. 2000;275:3247–3255. doi: 10.1074/jbc.275.5.3247. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Hughes RA, Baines AJ, Conboy J, Mohandas N, An X. Protein 4.1R regulates cell adhesion, spreading, migration and motility of mouse keratinocytes by modulating surface expression of beta1 integrin. Journal of cell science. 2011;124:2478–2487. doi: 10.1242/jcs.078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norton WT. Cell reactions following acute brain injury: a review. Neurochemical research. 1999;24:213–218. doi: 10.1023/a:1022505903312. [DOI] [PubMed] [Google Scholar]
- 29.Burns KA, Murphy B, Danzer SC, Kuan CY. Developmental and post-injury cortical gliogenesis: a genetic fate-mapping study with Nestin-CreER mice. Glia. 2009;57:1115–1129. doi: 10.1002/glia.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 31.Yang S, Guo X, Debnath G, Mohandas N, An X. Protein 4.1R links E-cadherin/beta-catenin complex to the cytoskeleton through its direct interaction with beta-catenin and modulates adherens junction integrity. Biochimica et biophysica acta. 2009;1788:1458–1465. doi: 10.1016/j.bbamem.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruiz-Saenz A, Kremer L, Alonso MA, Millan J, Correas I. Protein 4.1R regulates cell migration and IQGAP1 recruitment to the leading edge. Journal of cell science. 2011;124:2529–2538. doi: 10.1242/jcs.083634. [DOI] [PubMed] [Google Scholar]
- 33.Kim C, Ye F, Ginsberg MH. Regulation of Integrin Activation. Annual review of cell and developmental biology. 2011 doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nature cell biology. 2008;10:1062–1068. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.