Abstract
Objective
Cigarette smoking has emerged as a risk factor for development of rheumatoid arthritis (RA). Recent studies have suggested that cigarette smoking may lead to lower treatment response rates with methotrexate (MTX) and some biologic agents in RA. Knowledge of whether tobacco exposure reduces treatment efficacy is important as smoking could represent a modifiable factor in optimizing RA treatment.
Methods
Study participants included patients with early RA (<3 years duration) enrolled in the Treatment of Early Aggressive RA (TEAR) trial, a randomized, blinded, placebo-controlled clinical trial (RCT) comparing early intensive therapy (MTX + etanercept or MTX + hydroxychloroquine + sulfasalazine [triple therapy]) versus initial treatment with MTX with step-up to MTX + etanercept or to triple therapy if still active at 24 weeks. Serum cotinine was measured using a commercially available ELISA at baseline and 48 weeks with detectable concentrations at both visits serving as indicator of smoking status. Mean Disease Activity Score (DAS-28) was compared by smoking status, adjusting for baseline disease activity.
Results
Of 412 subjects included in the analysis, 293 (71%) were categorized as ‘non-smokers’ and 119 (29%) as ‘current smokers’. There were no differences in the mean DAS-28 between 48 and 102 weeks based on smoking status for the overall group (p=0.881) or by specific treatment assignment.
Conclusion
Among patients enrolled in a large RCT of early RA with poor prognostic factors, smoking status did not impact treatment responses for those receiving early combination or initial MTX with step-up therapy at 24 weeks if still active.
Cigarette smoking is now widely accepted to be a risk factor for the development of rheumatoid arthritis (RA). Both the duration and cumulative magnitude of cigarette smoking exposure have been shown to increase the risk of developing RA (1–4). In fact, smoking alone has been shown to account for almost 20% of all new cases of RA (1) with attributable risks for autoantibody-positive disease due to smoking approaching 50% in patients homozygous for HLA-DRB1 alleles containing the shared epitope alleles (5). A significant association has been shown between smoking and the presence of disease-related autoantibodies including anti-citrullinated protein antibody (ACPA) (2, 6, 7) and rheumatoid factor (RF) (8), both of which are associated with poor disease prognosis. Cigarette smoking is additionally associated with a higher prevalence of extra-articular disease manifestations in RA including subcutaneous nodules (9–13) and interstitial lung disease (14). This is particularly salient since both of these disease manifestations are associated with worse long-term outcomes in RA, including higher all-cause mortality (15, 16).
There has been recent evidence to suggest that worse outcomes in RA related to smoking may be secondary to a detrimental effect on treatment response to both biologic and non-biologic disease-modifying anti-rheumatic drugs (DMARDs). In a large observational cohort study, heavy smokers (defined as more than a 20 pack-year cumulative smoking history), had less improvement in disease activity over a three-year period of observation and more often required DMARD combinations or biologic therapies compared to those who smoked less or not at all (17). Investigators from the British Society for Rheumatology Biologics Register (BSRBR) recently reported a lower treatment response rate to the tumor necrosis factor (TNF)-α inihibitor infliximab in RA patients reporting current smoking compared to non-smokers (18). To date, however, there have been no studies examining the associations of cigarette smoking with treatment response in early RA in the context of a randomized double-blind controlled trial. Knowledge of whether cigarette smoking reduces treatment efficacy is important as smoking could represent a modifiable factor in optimizing RA treatment strategies.
Methods and Materials
Study design and participants
The Treatment of Early Aggressive RA (TEAR) trial was designed to compare the effectiveness of early intensive therapy versus step-up to one of two combinations of medications (methotrexate [MTX] + etanercept [ETN] vs. MTX + hydroxychloroquine + sulfasalazine [triple therapy]) in early, active RA (19). This was a two-year, randomized, double-blinded trial using a two-by-two factorial design in which subjects were treated initially with either MTX alone, triple therapy (MTX + sulfasalazine + hydroxychloroquine), or MTX + ETN. At 24 weeks, participants in the MTX monotherapy group with disease activity score for 28 joints (DAS-28) > 3.2, reflecting moderate to severe levels of persistent disease activity, were stepped up to either oral triple therapy or MTX + ETN. The primary outcome of TEAR was mean DAS-28 observed from week 48 to week 102.
Eligibility criteria for TEAR enrollment included age > 18 years; satisfaction of the 1987 American College of Rheumatology (ACR) classification criteria for RA (20); disease duration < 3 years from the time of formal diagnosis; active disease defined as at least four swollen and four tender joints using the 28-joint count; positive RF or ACPA, or at least two erosions present on radiographs of hands/wrists/feet if negative for RF/ACPA; stable doses of corticosteroid therapy and < 10 mg/day of prednisone (or equivalent) if taking such therapy; and, if taken, stable doses of non-steroidal anti-inflammatory drugs (NSAIDs). Participants included in the present analysis provided additional informed consent for banking of both DNA and serum for future ancillary studies examining biomarkers as predictors of treatment response.
Smoking status
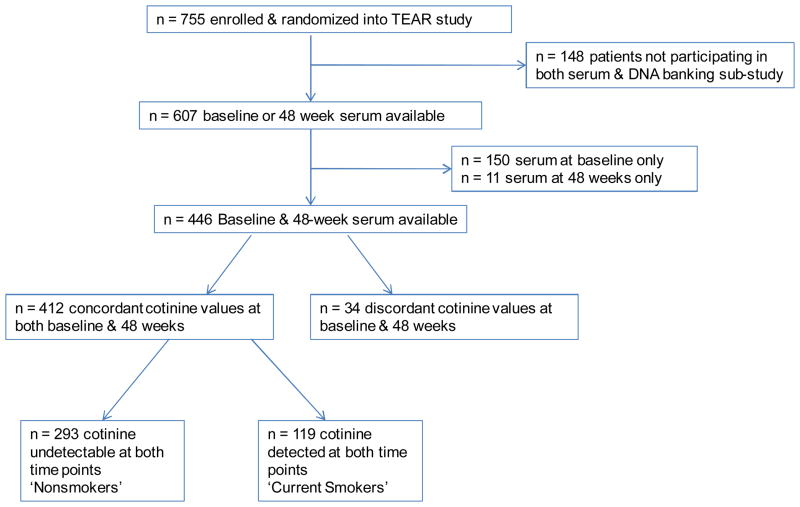
Self-reported smoking status was not collected as part of TEAR. Recognizing that the association of smoking with treatment response was not a primary objective of the parent study, participants were categorized as ‘current smokers’ or ‘non-smokers’ based on serum cotinine status. Serum cotinine is a metabolite of nicotine and is widely used in epidemiologic research as an objective measure of recent tobacco use (21, 22). Circulating cotinine was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Calbiotech, Spring Valley, CA, USA) using banked serum from baseline and from week 48 of follow-up. Of the 755 participants enrolled in the TEAR study, 148 did not participate in the serum and DNA banking sub-studies and were subsequently excluded from this analysis. Of the 607 with serum available, 161 were excluded from primary analyses due to serum available at baseline only (n = 150) or 48 weeks only (n = 11). Participants with discordant values at baseline and week 48 were excluded (n = 34), resulting in a total of 412 study participants included in the primary analyses. Study participants with detectable cotinine (> 5 ng/ml) concentrations at both visits were categorized as ‘current smokers’ and those with undetectable cotinine levels at both the baseline and 48 week visits were classified as ‘non-smokers’ (Figure 1).
Figure 1.
Disposition of Participants.
The approach of classifying smoking status based on serum cotinine was examined in an independent population that included 691 RA patients enrolled in the Veterans Affairs Rheumatoid Arthritis (VARA) registry (23, 24). VARA participants examined were primarily men (93%), had a mean (±SD) age of 68 (±11) years, and 26% (n = 180) self-reported current smoking status concurrent with the blood draw. Information specific to other forms of tobacco use or nicotine replacement therapy were not routinely available for VARA participants. There was excellent concordance of any detectable serum cotinine (sensitivity = 0.94; specificity 0.85) with self-reported current smoking status. The corresponding area under the receiver operator curve (AUC ROC) was 0.90. Serum cotinine has a circulating half-life of approximately 20 hours and may be detectable for several days following exposure to tobacco (25). Additionally, the manufacturer of the ELISA kit used for cotinine analysis reports performance characteristics including the ability to detect cotinine in samples taken from non-smokers exposed to passive inhalation for over 30 days.
Statistical analysis
Demographic and RA-related characteristics were compared between ‘current smokers’ and ‘non-smokers’ using the Student’s t-test for continuous values and the chi-square test for categorical values. To assess for the possibility of participation bias, we also compared the same characteristics between those with available cotinine values at any time point (n = 607) and those not participating in the DNA banking sub-study (n = 148) (Figure 1). The association of smoking status with mean DAS-28 from week 48 and week 102 was examined using ANCOVA, adjusting for baseline DAS-28. In secondary analyses using the chi-square test, we also examined the association of smoking status with treatment response based on the European League Against Rheumatism (EULAR) improvement criteria (26) including the frequency of those attaining a ‘good’ response or ‘remission.’ These analyses were done in the overall trial group as well as in different treatment arms included in the TEAR trial. Based on reports that smoking may be a risk factor for treatment-related infection (27, 28), we also explored whether smoking was associated with the occurrence of serious adverse events (SAEs), study withdrawal, and serious infections, categorized as any serious infection or a serious respiratory infection including bronchitis and/or pneumonia. Given the relatively low frequency of SAEs in TEAR and resulting modest study power, these analyses were considered exploratory. Recognizing that randomization was not stratified by smoking status for the parent study, we also examined whether the primary results changed after adjustments for factors including age, gender, race, disease duration, patient global, HAQ score, functional status, and comorbidity including self-reported cardiovascular or respiratory conditions. Additional subanalysis included the evaluation of the primary outcome limited to women. To further examine the possibility that the results may have been impacted by differential drop out / withdrawal between ‘smokers’ and ‘non-smokers,’ we performed a sensitivity analysis including all participants with available serum (n = 607) using a last observation carried forward (LOCF) imputation. We also examined whether ‘heavy smoking,’ defined as serum cotinine > 100ng/ml, was associated with treatment response using the approach defined above. All analyses were completed using SAS v9.2 (SAS Institute Inc., Cary, NC).
Results
Of the 607 participants with baseline or 48 week cotinine values available, a total of 412 participants were included in the primary analysis, with 293 (71%) categorized as ‘non-smokers,’ and 119 (29%) categorized as ‘smokers.’ Of these, 94 were classified as ‘heavy smokers’ at baseline, defined as serum cotinine concentration > 100ng/ml. There were no differences in age, gender, and other RA related factors between the 607 participants with serum available and the 148 participants randomized but not participating in the DNA / serum banking sub-study. A summary of participant baseline characteristics is shown in Table 1. Patient characteristics were similar between the two groups including age, gender, race, disease duration, body mass index, and cardiovascular disease at baseline, with the exception that ‘current smokers’ had higher baseline patient (p < 0.001) and physician (p = 0.030) global scores, and were more likely to have a history of respiratory disease (p = 0.017). Additionally, a significantly higher proportion of smokers had worse functional status compared to ‘non-smokers,’ as ‘smokers’ were more likely to be in functional class II or III. There was no significant difference in the number of participants withdrawing from the study during the two-year trial based on smoking status (data not shown).
Table 1.
Baseline participant characteristics for those with concordant cotinine values at baseline and at 48 weeks (n=412).
| Total (n=412) | ‘Non-Smokers’ (n=293) | ‘Current Smokers’ (n=119) | p-value ‘Current vs. Non-smokers’ | |
|---|---|---|---|---|
|
| ||||
| Mean (±SD) or % | p-value | |||
| Demographics | ||||
| Age, years | 49.6 (12.2) | 49.4 (12.7) | 50.1 (10.8) | 0.540 |
| Female gender | 73 | 75 | 67 | 0.089 |
| Caucasian race | 80 | 80 | 79 | 0.842 |
| BMI, kg/m2 | 30.0 (7.5) | 30.2 (7.8) | 29.4 (6.9) | 0.301 |
| CV disease | 27.4 | 27.7 | 26.9 | 0.876 |
| Respiratory disease | 18.8 | 15.9 | 26.1 | 0.017 |
| RA Characteristics | ||||
| Dis. duration, mo. | 3.7 (6.6) | 3.8 (6.5) | 3.4 (6.9) | 0.566 |
| DAS-28 | 5.8 (1.1) | 5.8 (1.0) | 5.8 (1.1) | 0.440 |
| ESR, mm/hr | 32.8 (24.0) | 32.9 (23.9) | 32.6 (24.3) | 0.894 |
| Swollen joints | 12.5 (5.7) | 12.4 (5.7) | 12.8 (5.8) | 0.515 |
| Tender joints | 14.0 (6.6) | 13.7 (6.5) | 14.7 (6.9) | 0.157 |
| Patient global (0–10) | 5.9 (2.2) | 5.6 (2.2) | 6.7 (2.0) | <0.001 |
| Physician global (0–10) | 6.4 (1.7) | 6.3 (1.7) | 6.7 (1.7) | 0.030 |
| HAQ (0-3) | 1.2 (0.4) | 1.2 (0.4) | 1.3 (0.4) | 0.067 |
| Functional Status | ||||
| I | 25 | 27 | 20 | |
| II | 54 | 55 | 52 | 0.022* |
| III | 21 | 18 | 28 | |
Mantal-Haenszel Chi-Square.
BMI = body mass index, CV = cardiovascular, RA = rheumatoid arthritis, DAS-28 = disease activity score in 28 joints, ESR = erythrocyte sedimentation rate, HAQ = health assessment questionnaire.
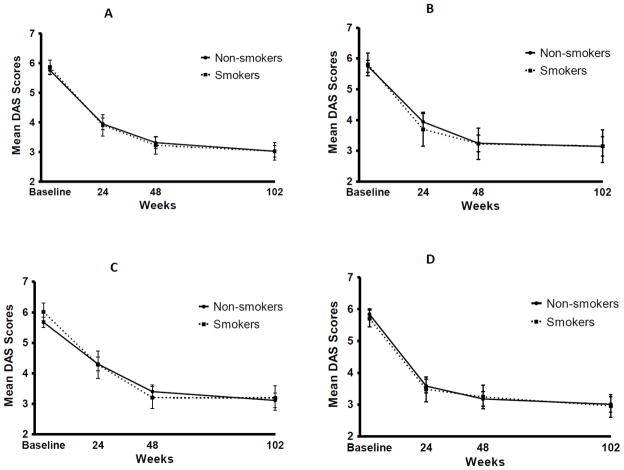
There was no significant difference in the primary outcome (mean DAS-28 score at weeks 48 and 102) based on smoking status among overall study participants (p = 0.881). Likewise, there were also no differences in outcomes stratified by TEAR treatment groups (Figure 2 and Table 2) or when multivariate analysis was performed adjusting for age, gender, race, disease duration, patient global, HAQ score, functional status, cardiovascular disease, and respiratory disease (p = 0.755). Additional subanalysis of the primary outcome after limiting to women, again showed no significant difference in treatment response based on smoking status (p = 0.962). Figure 2 shows DAS-28 values over time for: 1) participants assigned to receive etanercept + MTX as either immediate combination or as step-up; 2) participants assigned to receive triple therapy as either immediate combination or as step-up; 3) those assigned to receive initial MTX monotherapy followed by step-up as needed; and 4) those assigned initial combination therapy, either etanercept + MTX or triple therapy. Table 2 summarizes treatment results for the overall study population in addition to those specifically assigned to 1) immediate combination of ETN + MTX; 2) participants assigned to immediate triple therapy; 3) those assigned to initial MTX monotherapy with addition of ETN if needed; and 4) those assigned to initial MTX monotherapy with escalation to triple therapy if needed. There were no differences in DAS-28 outcomes by smoking status using the earlier time points of 24 and 48 weeks. Using secondary outcomes of 'good' response or 'remission' as defined by EULAR response criteria, there was again no difference based on smoking status overall or by treatment group at any time point. Good EULAR response was observed in 62.5% of ‘smokers’ and 56.3% of ‘non-smokers’ at 48 weeks (p = 0.552). Remission was reached by 35% and 32.6% of ‘smokers’ and non-smokers’, respectively, at 48 weeks (p = 0.642). LOCF imputation method was used for sensitivity analysis of all subjects with serum available (n = 607), with no significant difference of change in DAS-28 observed between ‘smokers’ and ‘non-smokers’ (data not shown). The number of participants initially receiving MTX monotherapy and not reaching DAS-28 ≤ 3.2 at six months whose therapy was subsequently stepped up per study protocol was not significantly different based on smoking status (74% of ‘smokers’ received treatment escalation vs. 77% of ‘non-smokers,’ p = 0.649). We also found no difference between ‘heavy smokers’, defined as a serum cotinine > 100 ng/ml, and ‘non-smokers’ in treatment response (p = 0.446).
Figure 2.
Mean DAS-28 over time based on smoking status for each treatment group.
A. Etanercept + MTX, both immediate and step-up.
B. Triple therapy, both immediate and step-up.
C. Initial MTX monotherapy with step-up to triple therapy or MTX + ETN
D. Immediate combination therapy.
Table 2.
Mean DAS-28 in overall and treatment group among ‘smokers’ & ‘non- smokers’ (n = 412).
| Group | Baseline DAS-28 | Mean DAS-28 weeks 48-102 | p- value* | ||
|---|---|---|---|---|---|
|
| |||||
| ‘Non- smokers’ | ‘Smokers’ | ‘Non- smokers’ | ‘Smokers’ | ||
| Overall group | 5.8 (1.0) | 5.8 (1.1) | 3.17 (1.3) | 3.14 (1.3) | 0.881 |
| Active Etanercept + MTX | 5.9 (1.1) | 5.7 (1.0) | 3.01 (1.3) | 3.07 (1.2) | 0.797 |
| Active Triple Therapy | 5.7 (1.0) | 5.7 (1.3) | 3.24 (1.3) | 3.15 (1.9) | 0.809 |
| Step-Up Etanercept | 5.6 (1.1) | 6.1 (1.1) | 3.31 (1.3) | 3.20 (1.4) | 0.688 |
| Step-Up Triple therapy | 5.8 (0.9) | 5.9 (1.0) | 3.07 (1.4) | 3.23 (1.1) | 0.667 |
P-value represents difference (smokers vs. non-smokers) in mean DAS-28 at weeks 48 to 102 after adjusting for baseline value; Number of participants by treatment arm = Active Etanercept + MTX (n = 143); Active Triple Therapy (n = 69), Step-Up Etanercept (n = 136); Step-Up Triple Therapy (n = 64).
In further exploratory analyses, we found no significant differences between ‘current smokers’ and ‘non-smokers’ in the frequency of serious adverse events (SAEs) both overall and based on treatment group (Table 3). Additional subanalyses of infectious adverse events and respiratory infections (serious and non-serious) again showed no differences based on smoking status.
Table 3.
Frequency of serious adverse events (SAEs) among ‘non-smokers’ and ‘smokers’ overall and based on treatment group.
| ‘Non-smokers’ | ‘Smokers’ | p-value | |
|---|---|---|---|
| Overall | 11.9 | 13.5 | 0.675 |
| Etanercept + MTX | 13.1 | 13.6 | 0.920 |
| Triple therapy | 9.5 | 13.2 | 0.532 |
| Step-up therapy | 11.6 | 15.1 | 0.505 |
| Immediate/Active therapy | 12.3 | 12.1 | 0.966 |
Discussion
The TEAR trial provides the only randomized, double-blind, placebo controlled study comparing oral triple therapy to combination etanercept plus methotrexate for the treatment of early RA with poor prognostic factors and thus provides an ideal context to investigate potential relationships between important environmental factors such as tobacco use on treatment response to these therapies. We observed no significant differences between participants identified as ‘current smokers’ compared to ‘non-smokers’ in the primary outcome, mean DAS-28 between weeks 48 and 102. Although few studies to date have investigated smoking as a predictor of treatment response, these results conflict with evidence that is currently available (18, 29–32). Saevarsdottir et al. observed significantly lower rates of RA treatment response with methotrexate or TNF inhibition among current smokers compared to those reporting a never-smoking status, although the primary outcome measured was response to treatment after only three months of therapy (32). Hyrich et al. specifically reported on predictors of response to selective anti-TNFα therapy in RA, and showed that current smoking was associated with a lower response rate with infliximab, although this was only significant on multivariate analysis (18). Similar results were not observed in relation to the use of other anti-TNFα agents including etanercept (18).
To date, all reported analyses of a potential relationship between smoking status and response to either DMARD or biologic therapy have come from observational or other open-label studies (17, 18, 29–32). The present analysis is unique in its use of data generated from a randomized, double blind, placebo-controlled trial design. In addition to the difference in study design of the parent studies, the reasons underpinning these discrepant results might include differences in patient populations, outcome assessment, and exposure measurements. For example, prior studies have used self-reported smoking history while the present study used an indirect measure of smoking, by measuring circulating cotinine. Regardless, the ability to identify which patients will be most likely to respond to specific therapies could be beneficial in theoretically allowing more targeted therapy to minimize unnecessary toxicities and maximize beneficial effect. However, based on our findings, smoking status may not be a targetable environmental factor in the optimization of RA treatment responses.
Limitations to this study include the number of participants lost to analysis due to unavailability of serum for cotinine analysis, related either to study withdrawal (prior to the 48-week assessment) or not participating in the study's bio-specimen banking protocol. However, using LOCF imputation method in sensitivity analysis that included all subjects for whom any serum measure was available, still no significant difference in treatment response among ‘smokers’ compared to ‘non-smokers’ was found. Although we cannot definitively exclude the possibility of a participation bias, similarities between those included in the analyses and those not included suggests that this does not serve as a major source of study bias. It is also important to recognize the possibility of exposure misclassification based on our definition of current ‘smoking’ and ‘non-smoking’, a definition that was based entirely on the presence of circulating cotinine. Although we found this measure to have excellent discrimination in an independent RA cohort, it is possible that other forms of tobacco (e.g. chewing tobacco) or nicotine replacement therapy could serve as sources of misclassification. It is possible that individuals in this study with a history of biologically irrelevant second-hand exposure to cigarette smoke could have been misclassified as current ‘smokers.’ None of the previous studies used a biologic method to determine smoking status, such as serum cotinine, but instead depended on self-reported smoking status. Additionally, because the TEAR cohort includes patients with more severe disease phenotype treated with a finite list of disease remitting therapies, results may not be generalizable to patients with less active RA or those receiving alternative therapies.
Although these results do not support an association of this exposure with treatment response, this does not diminish the paramount importance of smoking cessation in patients with RA. Among RA patients who smoke, effective cessation strategies remain essential for general health considerations including risk modification of cardiovascular disease, a major cause of morbidity and excess mortality in this patient population. However, our data would suggest smoking cessation alone per se may not be an important adjunct in lessening disease activity or modifying therapeutic response to commonly used disease-remitting treatments in RA. Additional studies will be needed in the context of randomized controlled trials to replicate these findings as well as to examine the potential impact of therapies not investigated in the TEAR study.
Significance and Innovations.
In contrast to findings from other recent studies showing that smoking may reduce treatment responses in RA, smoking status does not appear to be associated with RA treatment response with either triple DMARD therapy (MTX + HCQ + SSZ) or etanercept in combination with MTX.
This is the first study to our knowledge, utilizing a bioassay method to measure nicotine exposure, instead of patient-reported exposure, to investigate the possible relationship between smoking and treatment response in patients with RA.
Acknowledgments
We thank the Data Safety Monitoring Board (Michael Weinblatt, MD; David Wofsy, MD; Mark Genovese, MD; and Barbara Tilley, MD) and the external medical safety monitor, Dr. Gene Ball. We appreciate the support of all the clinical investigators, their staff, and all of the patient participants. The financial support for the trial was from Amgen and medications were provided by Amgen, (etanercept and placebo), Barr Pharmaceuticals (methotrexate), and Pharmacia (sulfasalazine and placebo. An NIH planning grant (PI: Moreland) from NIAMS supported the initial phases of the TEAR study. Cotinine assays were paid for by a grant from the Arthritis Foundation through Jeffrey Curtis, MD. Dr. Curtis’ efforts were also supported by the Arthritis Foundation, Agency for Healthcare Research and Quality (RO1HS018517), and the National Institutes of Health (AR053351). Serum samples were collected through NIH grant R01 AR052658 (SL Bridges, Jr., PI).
Footnotes
There are no financial interests or conflicts of interest to report.
Contributor Information
Leann B. Maska, University of Nebraska Medical Center at Omaha.
Harlan R. Sayles, University of Nebraska Medical Center at Omaha.
James R. O’Dell, University of Nebraska Medical Center at Omaha, and Omaha VA Medical Center.
Jeffrey R. Curtis, University of Alabama at Birmingham.
S. Louis Bridges, Jr., University of Alabama at Birmingham.
Larry W. Moreland, University of Pittsburgh.
Stacey S. Cofield, University of Alabama at Birmingham.
Ted R. Mikuls, University of Nebraska Medical Center at Omaha, and Omaha VA Medical Center.
References
- 1.Criswell LA, Merlino LA, Cerhan JR, Mikuls TR, Mudano AS, Burma M, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: Results from the iowa women's health study. Am J Med. 2002;112:465–71. doi: 10.1016/s0002-9343(02)01051-3. [DOI] [PubMed] [Google Scholar]
- 2.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 3.Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis. 2010;69:54–60. doi: 10.1136/ard.2008.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridges SL, Jr, Hughes LB, Mikuls TR, Howard G, Tiwari HK, Alarcon GS, et al. Early rheumatoid arthritis in african-americans: The CLEAR registry. Clin Exp Rheumatol. 2003;21:S138–45. [PubMed] [Google Scholar]
- 5.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503.e1–503.e9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 6.Lee HS, Irigoyen P, Kern M, Lee A, Batliwalla F, Khalili H, et al. Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: A mixed picture in three large north american rheumatoid arthritis cohorts. Arthritis Rheum. 2007;56:1745–53. doi: 10.1002/art.22703. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen M, Jacobsen S, Klarlund M, Pedersen BV, Wiik A, Wohlfahrt J, et al. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther. 2006;8:R133. doi: 10.1186/ar2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004;50:3085–92. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- 9.Nyhall-Wahlin BM, Petersson IF, Nilsson JA, Jacobsson LT, Turesson C BARFOT study group. High disease activity disability burden and smoking predict severe extra-articular manifestations in early rheumatoid arthritis. Rheumatology (Oxford) 2009;48:416–20. doi: 10.1093/rheumatology/kep004. [DOI] [PubMed] [Google Scholar]
- 10.Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: Incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62:722–7. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SK, Park SH, Shin IH, Choe JY. Anti-cyclic citrullinated peptide antibody, smoking, alcohol consumption, and disease duration as risk factors for extraarticular manifestations in korean patients with rheumatoid arthritis. J Rheumatol. 2008;35:995–1001. [PubMed] [Google Scholar]
- 12.Mikuls TR, Hughes LB, Westfall AO, Holers VM, Parrish L, van der Heijde D, et al. Cigarette smoking, disease severity and autoantibody expression in african americans with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2008;67:1529–34. doi: 10.1136/ard.2007.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyhall-Wahlin BM, Jacobsson LT, Petersson IF, Turesson C BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601–6. doi: 10.1136/ard.2005.039172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168:159–66. doi: 10.1001/archinternmed.2007.59. [DOI] [PubMed] [Google Scholar]
- 15.Olson AL, Swigris JJ, Sprunger DB, Fischer A, Fernandez-Perez ER, Solomon J, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183:372–8. doi: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62–7. [PubMed] [Google Scholar]
- 17.Westhoff G, Rau R, Zink A. Rheumatoid arthritis patients who smoke have a higher need for DMARDs and feel worse, but they do not have more joint damage than non-smokers of the same serological group. Rheumatology (Oxford) 2008;47:849–54. doi: 10.1093/rheumatology/ken057. [DOI] [PubMed] [Google Scholar]
- 18.Hyrich KL, Watson KD, Silman AJ, Symmons DP British Society for Rheumatology Biologics Register. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: Results from the british society for rheumatology biologics register. Rheumatology (Oxford) 2006;45:1558–65. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 19.Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, William St Clair E, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early, aggressive rheumatoid arthritis. Arthritis Rheum. 2012 doi: 10.1002/art.34498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Koru-Sengul T, Clark JD, Fleming LE, Lee DJ. Toward improved statistical methods for analyzing cotinine-biomarker health association data. Tob Induc Dis. 2011;9:11. doi: 10.1186/1617-9625-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: Focus on developmental toxicology. Ther Drug Monit. 2009;31:14–30. doi: 10.1097/FTD.0b013e3181957a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikuls TR, Kazi S, Cipher D, Hooker R, Kerr GS, Richards JS, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34:1480–4. [PubMed] [Google Scholar]
- 24.Mikuls TR, Fay BT, Michaud K, Sayles H, Thiele GM, Caplan L, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: Results from the VARA registry. Rheumatology (Oxford) 2011;50:101–9. doi: 10.1093/rheumatology/keq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: Implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988;78:696–8. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–50. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46:2294–300. doi: 10.1002/art.10529. [DOI] [PubMed] [Google Scholar]
- 28.Franklin J, Lunt M, Bunn D, Symmons D, Silman A. Risk and predictors of infection leading to hospitalisation in a large primary-care-derived cohort of patients with inflammatory polyarthritis. Ann Rheum Dis. 2007;66:308–12. doi: 10.1136/ard.2006.057265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abhishek A, Butt S, Gadsby K, Zhang W, Deighton CM. Anti-TNF-alpha agents are less effective for the treatment of rheumatoid arthritis in current smokers. J Clin Rheumatol. 2010;16:15–8. doi: 10.1097/RHU.0b013e3181ca4a2a. [DOI] [PubMed] [Google Scholar]
- 30.Mattey DL, Brownfield A, Dawes PT. Relationship between pack-year history of smoking and response to tumor necrosis factor antagonists in patients with rheumatoid arthritis. J Rheumatol. 2009;36:1180–7. doi: 10.3899/jrheum.081096. [DOI] [PubMed] [Google Scholar]
- 31.Saevarsdottir S, Wallin H, Seddighzadeh M, Ernestam S, Geborek P, Petersson IF, et al. Predictors of response to methotrexate in early DMARD naive rheumatoid arthritis: Results from the initial open-label phase of the SWEFOT trial. Ann Rheum Dis. 2011;70:469–75. doi: 10.1136/ard.2010.139212. [DOI] [PubMed] [Google Scholar]
- 32.Saevarsdottir S, Wedren S, Seddighzadeh M, Bengtsson C, Wesley A, Lindblad S, et al. Patients with early rheumatoid arthritis who smoke are less likely to respond to treatment with methotrexate and tumor necrosis factor inhibitors: Observations from the epidemiological investigation of rheumatoid arthritis and the swedish rheumatology register cohorts. Arthritis Rheum. 2011;63:26–36. doi: 10.1002/art.27758. [DOI] [PubMed] [Google Scholar]