Abstract
Integrin-linked kinase (ILK) is a widely expressed and evolutionally conserved component of cell-extracellular matrix (ECM) adhesions. Although initially named as a kinase, ILK contains an unusual pseudoactive site that is incapable of catalyzing phosphorylation. Instead, ILK acts as a central component of a heterotrimer (the PINCH-ILK-parvin complex) at ECM adhesions mediating interactions with a large number of proteins via multiple sites including its pseudoactive site. Through higher level protein-protein interactions, this scaffold links integrins to the actin cytoskeleton and catalytic proteins and thereby regulates focal adhesion assembly, cytoskeleton organization and signaling. This review summarizes recent advances in our understanding of the structure and functions of the PINCH-ILK-parvin complex, and discuss emerging new features of the molecular mechanisms by which it regulates diverse cellular, physiological and pathological processes.
Introduction
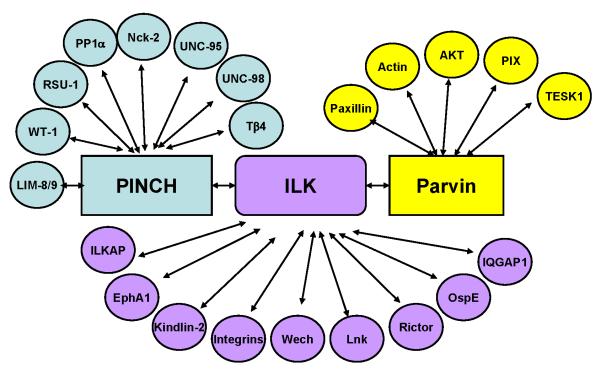
The interplay between ECM and the actin cytoskeleton is crucial for regulation of diverse cellular processes including cell-ECM adhesion, shape change, migration, proliferation and survival. This interplay depends critically on heterodimeric (α/β) transmembrane receptors, integrins, which bind to ECM proteins via their extracellular domains while connecting to actin via their cytoplasmic tails (CTs). Understanding how integrin CTs connect to actin has been a major theme of research over the past several decades [1]. Integrin-linked kinase (ILK) was identified in a search for proteins interacting with integrin β CTs [2]. ILK contains an N-terminal ankyrin repeat domain (ARD) and a C-terminal integrin β CT binding kinase-like domain (KLD). Initial studies showed that bacterially expressed ILK not only binds but also phosphorylates integrin β1 CT, suggesting an ILK-dependent phosphorylation mechanism on integrin signaling [2]. Soon after, it was discovered that ILK binds PINCH, which comprises of five LIM domains, via its ARD [3] and α- (also known as CH-ILKBP or actopaxin) or β-parvin (also known as affixin), which comprise two calponin-homology (CH) domains, via its C-terminal KLD [4,5]. PINCH, ILK and parvin form a stable ILK-centered heterotrimer (termed IPP thereafter) in cells [4], which is a prerequisite for their localization to mammalian cell-ECM adhesion sites [6,7]. Subsequently, ILK has been shown to interact with many additional proteins either directly or indirectly through PINCH or parvin (Fig 1) to mediate diverse arrays of biological events.
Figure 1.
Summary of IPP interactome. This figure depicts PINCH-, ILK- or parvin-mediated interactions of which functions have been investigated. Many of the IPP binding proteins have been listed in previous reviews [10, 27] except the recently published ones including IQGAP1 [43], PP1α [56], WT-1 [58], Ospe [59], LIM8/9 and UNC-95 [60], and Lnk [61].
In parallel to the findings that ILK functions as a key scaffold at cell-ECM adhesions, the role of ILK as a kinase has also been extensively examined. ILK was found to regulate phosphorylation of several key signaling intermediates including AKT and GSK-3 [8]. How ILK regulates protein phosphorylation, however, was elusive. It was suggested that ILK directly phosphorylates AKT, GSK-3β, and many other substrates [8]. However, ILK lacks key catalytic residues and thus its ability to function as a “true” kinase was questioned [9]. A series of genetic studies in multiple species also casted doubt on the notion that ILK functions as a kinase in vivo as putative kinase dead mutations were able to rescue ILK deficiency-induced developmental and cellular defects [10]. However, despite this, many studies have reported that ILK exerts kinase activity on numerous substrates including AKT and GSK-3 [11]. Thus, although there is a consensus that ILK is biologically important, it remained controversial as to how ILK could confer its functions. Below, we will discuss recent progress on ILK research, focusing on structure-derived pseudokinase features of ILK and how IPP functions as a scaffold in diverse cellular processes.
Structural basis of ILK as a pseudokinase
~10% of kinome lack one or more catalytic residues based on sequence alignment [12]. ILK belongs to this category, which was suggested as a pseudokinase [9]. However, one cannot exclude the possibility that ILK may have an unusual active site where the key missing catalytic residues are spatially compensated by some alternative residues. The compensation was indeed observed in WNK kinase, which lacks the conserved catalytic lysine residue in the subdomain II, uses K233 in its β2 strand as an alternative [13]. To address this issue, a high resolution 3D structure of ILK is necessary. This was achieved in recent crystallization of ILK KLD in complex with α-parvin C-terminal CH2 [14••,15•]. ILK KLD was found to contain conserved kinase domain fold with an ATP bound at the same location as conventional kinases. However, detailed examination revealed multiple pseudoactive site features (Figure 2): (i) an unusual ATP orientation with its γ-phosphate being far away from the putative substrate site; (ii) a nonhydrolyzed ATP in the crystal. ATP is usually hydrolyzed in either active or inactive kinases [16•]; (iii) a severely degraded catalytic loop including the absence of the key catalytic base Asp in the HRD motif and absence of other catalytic residues Lys and Asn. Unlike WNK [13], ILK contains no spatially adjacent residues to compensate these missing catalytic residues; (iv) altered magnesium coordination topology due to DVK that replaces typical metal coordinating DFG motif; (v) unusually short and rigid activation segment that lacks conserved phosphorylation site. The activation segment in a conventional kinase is typically long and flexible required for regulating the kinase activity. These structural features strongly indicated that ILK is a pseudokinase. This was confirmed by the lack of kinase activity of recombinant ILK expressed in either E. coli or mammalian cells or endogenous ILK purified from chick tissue [14••,15•]. It is also consistent with the extensive genetic data demonstrating that the kinase activity is not required for embryonic development [17,18,19,20••]. Mn2+, which was recently proposed to promote the kinase function of ILK [11,21], caused no structural difference of ILK as compared to Mg2+ [15•]. Furthermore, proteomic analysis revealed no kinase activity against any potential substrates in the presence of either Mg2+ or Mn2+, providing definitive biochemical evidence that ILK is a pseudokinase [15•]. The purity of ILK is crucial for definitively examining the kinase activity since a tiny amount of impurity kinase may lead to false-positive result [15•]. The impurity or some unknown kinase associated with partially purified ILK may explain some of the previous studies that showed kinase activity.
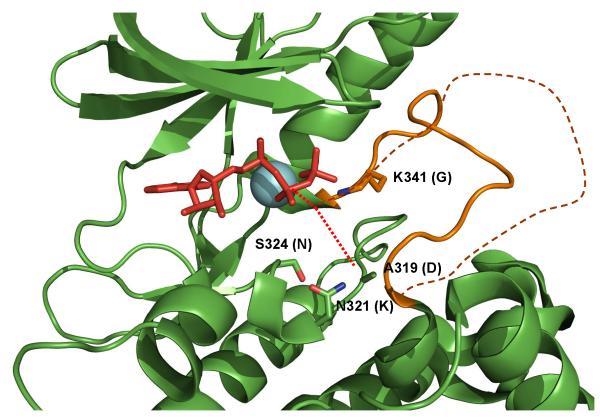
Figure 2.
Pseudoactive site features of ILK: A non-degraded ATP is bound to ILK KLD but its g-phosphate is oriented far away from A319 (see the dotted red line) – a site corresponding to the conventional catalytic base Asp. The typical DFG is replaced by DVK where K341 forms the salt-bridge with the g-phosphate of ATP, facilitating its distinct orientation. In addition to A319 that replaces the conventional catalytic Asp, multiple other catalytically important residues are missing including N321 that replaces Lys and S324 that replaces Asn. These catalytic residues are labeled and their corresponding catalytic residues are provided in the parentheses. Note that there are no surrounding residues that may alternatively substitute these catalytic residues. A single magnesium (cyan) is present in ILK KLD but coordinated differently in contrast to the two magnesium ions present in the conventional kinases. Finally, the activation segment (solid orange line) of ILK KLD is much shorter than the conventional one (dotted orange line).
In addition to WNK, a few previously proposed pseudokinases have also been shown to have residual kinase activity such as CASK and HER3/ErbB3 [16•]. However, the kinase activities of these “active” pseudokinases are extremely low and hence their physiological relevance as effective enzymes remain to be further investigated. As a comparison, ILK belongs to the pseudokinase Group 2 including STRADα and VRK3, which have severely degraded active site with no structural alternative and no residual kinase activity [16•].
IPP functions as a key interaction hub at ECM adhesions
How does ILK act as a pseudokinase to regulate cell behavior and intracellular signaling? Emerging evidences suggest that pseudokinases utilize their pseudokinase domains to recognize specific targets. In certain cases, this leads to conformational changes of the target proteins including kinases and thus triggering signaling [16•]. ILK may function in similar manners by interacting with different proteins through multiple binding sites. In particular, ILK binds tightly to PINCH via its ARD [3,6,22,23] and to parvin via its KLD [4,5, 14••], leading to formation of a stable ILK-centered IPP complex [6,10]. The formation of IPP is critical for stability of PINCH, ILK and parvin [24,25,26] and for their localization to cell-ECM adhesions in mammalian cells [6], where they engage in interactions with a large number of additional proteins [10,27]. In invertebrates such as C. elegans and Drosophila, ILK also forms a ternary complex with PINCH and parvin [18,28,29,30,31•,32•]. While the core interactions (e.g., PINCH-ILK and ILK-parvin) are well conserved, certain binding partners are gained or lost (e.g. PINCH binding partner UNC-98 [33]) during evolution. Similar to what was found in mammalian cells [6,24,25,26], PINCH, ILK and parvin are mutually dependent for their stability and localization to ECM adhesions in the wing epithelium in Drosophila [32•]. However, Drosophila ILK can localize to strong muscle attachment sites in the absence of PINCH [30,31•]. In C. elegans, ILK can localize to but fails to properly organize in muscle attachment sites in the absence of PINCH [34].
The structure of ILK KLD/α-parvin CH2 complex revealed that the CH2 binding site involves the conserved helices (αEF and αG) and part of P+1 loop, which is the substrate site for conventional kinases [14••,15•]. Thus, ILK has evolved to utilize a unique pseudoactive site to specifically engage with its target. Based on the high sequence homology of parvin family members, we expect that β- and γ-parvin CH2 bind to ILK KLD in similar manner. Consistently, the interactions of ILK with α- and β-parvin are mutually exclusive [35]. Interestingly, the ILK KLD/parvin CH2 complex can still bind integrin β1/β3 CTs, suggesting that the integrin-binding site on ILK KLD is different from that of parvin CH2 [14••]. The structure of ILK ARD/PINCH complex has also been resolved, which involves a large interface consistent with the tight affinity [22,23]. These distinct binding sites on ILK thus provide a basis for understanding the formation of IPP and its connection to integrin. Because parvin has also been shown to bind to F-actin [36], we can now envision an integrin β CT-IPP-actin pathway (Fig 3) that may mechanically link ECM with cytoskeleton. Such pathway may also communicate with other pathways through interactions of IPP with other signaling/adaptor proteins (Fig 1) thereby regulating diverse arrays of cellular events. The structures of several binding interfaces of the IPP-mediated complexes have been determined including those of PINCH LIM4 bound to Nck2 [37] and parvin CH2 bound to paxillin LD motif [38,39]. The interfaces of integrin β CT [14••], Nck2 [37], and paxillin [38,39] on the IPP complexes do not overlap, which suggest a scaffolding mechanism. More than two dozens of IPP binding proteins have been identified (Fig 1) and it is anticipated that more structures of IPP-mediated complexes will be forthcoming, which will provide a framework to design definitive mutation experiments to dissect functions of various IPP-mediated pathways.
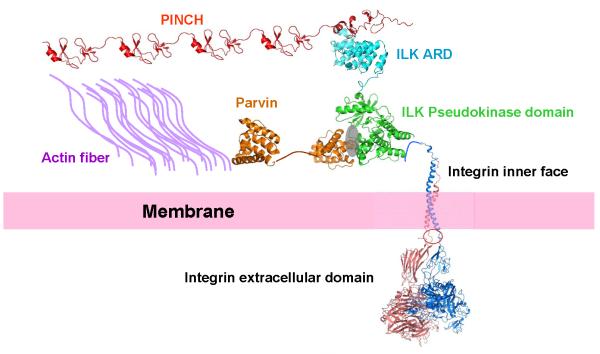
Figure 3.
A proposed structural assembly of IPP and its connection to integrin and actin filaments, allowing the linkage of ECM with actin cytoskeleton. The landscape was created using various PDB coordinates including the integrin extracellular domain (3FCU), integrin transmembrane-cytoplasmic domain (2KNC), ILK KLD/parvin CH2 complex (3KMW), ILK ARD/PINCH LIM1 complex (2KBX), PINCH LIM4 (1NYP), and Parvin CH2 (2K2R).
ILK-centered IPP mediates diverse functions in embryonic development and diseases
Functional studies in various model organisms have demonstrated that ILK, PINCH and parvin are indispensable for embryonic development [17,18,19,20••,25,32•,40,41]. Furthermore, studies in human patients, tissue- or cell-selective knockout animals and cultured cells have revealed important roles of ILK, PINCH and parvin in organ (e.g., kidney, liver, heart, muscle, etc.) functions and diseases (e.g., cancer). Although detailed discussions on this topic are beyond the scope of this review (readers are referred to several excellent reviews on this topic [10,11,42]), we highlight three general themes emerging from these studies.
First, loss of ILK, PINCH and/or parvin in both invertebrates and vertebrates always inevitably cause defects in cell-ECM adhesion, migration and/or actin cytoskeleton organization, suggesting that IPP functions as an evolutionally conserved essential scaffold in the integrin-actin network. Second, ILK plays additional important roles both in and outside of ECM contacts. For example, ILK is critical for caveolae trafficking in keratinocytes and functions in this process by binding and recruiting IQGAP1 to nascent focal adhesions at the cell cortex and thereby stabilizing microtubules and promoting caveolin trafficking to the plasma membrane [43•]. ILK can also associate with mitotic centrosomes and regulate mitotic spindle organization [44] and centrosome clustering in cancer cells [45]. A fraction of ILK can localize to the nucleus and regulate gene expression [46]. Third, ILK is often considered pro-proliferative. However, there are notable exceptions. For example, removal of ILK from hepatocytes in mice increases hepatocyte proliferation, resulting in hepatomegaly and enhanced liver regeneration [47,48]. IPP may also mediate cross-communications of integrins with other signaling pathways such as EphA signaling [49] and EGF signaling [50]. The different and sometime even opposing effects of ILK on cell proliferation and survival may reflect, at least in part, different signaling proteins with which IPP engages. At pathological level, abnormally high levels of IPP and its network components have been observed in numerous diseases such as failing hearts [51] and various cancers [52]. However, given that ILK is a pseudokinase, the therapeutic strategy that was targeted at the kinase activity should be re-considered.
Conclusions
Since the discovery of IPP more than a decade ago [4], numerous studies have affirmed a central role of this scaffold in connecting integrins to the actin cytoskeleton and signaling proteins. Furthermore, recent structural, biochemical and genetic analyses have definitively shown that ILK, a central piece of IPP, is a bona fide pseudokinase. This calls upon a significant revision of some of the previously proposed mechanisms of how ILK may operate to regulate various biological pathways. Considering that ablation or dysfunction of ILK or other components of IPP frequently affects key signaling pathways such as those of AKT and MAP kinases [53,54], the next wave of investigation may focus on the precise mechanisms by which IPP regulates these pathways. It is conceivable that multiple mechanisms might be involved. For example, α-parvin has been shown to facilitate membrane translocation of Akt [55], an obligatory step in Akt activation. Thus, IPP may regulate compartmentalization of signaling complexes and hence influence their activation. Furthermore, some of the IPP interactive proteins may possess kinase or phosphatase activities or function as adaptors linking IPP to kinases or phosphatases. For example, PINCH can associate with phosphatase 1alpha [56•], which leads to inhibition of the phosphatase activity and thus enhances Akt1 phosphorylation, suggesting another mode of Akt regulation. It is also possible that ILK directly binds to and regulates an unknown kinase. This has been shown in the pseudokinase STRADα, which activates its binding partner LKB kinase [57]. Clearly, the advances in IPP research over the past decade have attested to the power of combined use of structural, biochemical, cellular and genetic approaches for better understanding of cell-ECM adhesion and signaling in diverse biological and pathological responses. With the unraveling of the structures and functions of IPP and its partners, we are entering a new era in which the discoveries may lead to novel therapeutic approaches for pathological processes associated with abnormal ECM adhesion and signaling.
Acknowledgements
This work is supported by grants from the National institute of Health to J.Q. and C.W. respectively. Useful discussions with present and past lab members of Qin and Wu labs are appreciated. We apologize that many literatures related to this topic are not cited in this manuscript due to the space restriction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Legate KR, Fässler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122(Pt 2):187–98. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- 2.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 3.Tu Y, Li F, Goicoechea S, Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol. Cell. Biol. 1999;19:2425–34. doi: 10.1128/mcb.19.3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu Y, Huang Y, Zhang Y, Hua Y, Wu C. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J. Cell Biol. 2001;153:585–598. doi: 10.1083/jcb.153.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaji S, Suzuki A, Sugiyama Y, Koide Y, Yoshida M, Kanamori H, Mohri H, Ohno S, Ishigatsubo Y. A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction. J. Cell Biol. 2001;153:1251–1264. doi: 10.1083/jcb.153.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Chen K, Tu Y, Velyvis A, Yang Y, Qin J, Wu C. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J Cell Sci. 2002;115:4777–86. doi: 10.1242/jcs.00166. [DOI] [PubMed] [Google Scholar]
- 7.Wu C. The PINCH-ILK-parvin complexes: assembly, functions and regulation. Biochim Biophys Acta. 2004;1692(2-3):55–62. doi: 10.1016/j.bbamcr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 8.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase--essential roles in physiology and cancer biology. J Cell Sci. 2008;121(Pt 19):3121–32. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 9.Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol. 2006;16:443–452. doi: 10.1016/j.tcb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Wickström SA, Lange A, Montanez E, Fässler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 2010;29(2):281–91. doi: 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannigan GE, McDonald PC, Walsh MP, Dedhar S. Integrin-linked kinase: not so ‘pseudo’ after all. Oncogene. 2011;30(43):4375–85. doi: 10.1038/onc.2011.177. [DOI] [PubMed] [Google Scholar]
- 12.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 13.Min X, Lee BH, Cobb MH, Goldsmith EJ. Crystal structure of the kinase domain of WNK1, a kinase that causes a hereditary form of hypertension. Structure. 2004;12:1303–1311. doi: 10.1016/j.str.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda K, Gupta S, Chen K, Wu C, Qin J. The pseudoactive site of ILK is essential for its .. binding to alpha-Parvin and localization to focal adhesions. Mol Cell. 2009;36(5):819–30. doi: 10.1016/j.molcel.2009.11.028. This is the first structural demonstration of ILK as a pseudokinase. While the structure of ILK KLD mimics the active conformation of a conventional kinase, it cannot exert the catalytic function due to a pseudoactive site structure including the distinctly oriented ATP γ-phosphate that is far away from the putative catalytic base, multiple missing catalytic residues with no spatial alternatives, and substantially shortened activation segment. The pseudoactive site is specifically involved in recognizing the target protein parvin.
- 15.Fukuda K, Knight JD, Piszczek G, Kothary R, Qin J. Biochemical, proteomic, structural, . and thermodynamic characterizations of integrin-linked kinase (ILK): cross-validation of the pseudokinase. J Biol Chem. 2011;286(24):21886–95. doi: 10.1074/jbc.M111.240093. This work provides three important findings: (i) Mn- and Mg-bound ILK have no structural difference and neither exhibits the kinase activity. (ii) ILK does not have any unusual substrate based on an unbiased proteomic whole cell search. (iii) Partially purified ILK sample may cause false-positive kinase activity.
- 16.Zeqiraj E, van Aalten DM. Pseudokinases-remnants of evolution or key allosteric . regulators? Curr Opin Struct Biol. 2010;20(6):772–81. doi: 10.1016/j.sbi.2010.10.001. This is an excellent review summarizing various pseudokinase features.
- 17.Zervas CG, Gregory SL, Brown NH. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 2001;152:1007–1018. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 2002;12:787–797. doi: 10.1016/s0960-9822(02)00810-2. [DOI] [PubMed] [Google Scholar]
- 19.Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurcheno PD, Fässler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange A, Wickström SA, Jakobson M, Zent R, Sainio K, Fässler R. Integrin-linked kinase .. is an adaptor with essential functions during mouse development. Nature. 2009;461(7266):1002–6. doi: 10.1038/nature08468. This study, together with ref 17-19, provides strong genetic evidence that ILK kinase activity is not required for development.
- 21.Maydan M, McDonald PC, Sanghera J, Yan J, Rallis C, Pinchin S, Hannigan GE, Foster LJ, Ish-Horowicz D, Walsh MP, et al. Integrin-linked kinase is a functional Mn2+- dependent protein kinase that regulates glycogen synthase kinase-3β (GSK-3beta) phosphorylation. PLoS One. 2010;5(8):e12356. doi: 10.1371/journal.pone.0012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiswell BP, Zhang R, Murphy JW, Boggon TJ, Calderwood DA. The structural basis of the integrin-linked kinase-PINCH interactions. Proc. Natl. Acad. Sci. USA. 2008;105:20677–20682. doi: 10.1073/pnas.0811415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Wang X, Hawkins CA, Chen K, Vaynberg J, Mao X, Tu Y, Zuo X, Wang J, Wang YX, et al. Structural basis of focal adhesion localization of LIM-only adaptor PINCH by integrin-linked kinase. J. Biol. Chem. 2009;284:5836–5844. doi: 10.1074/jbc.M805319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda T, Chen K, Shi X, Wu C. PINCH-1-1 is an obligate partner of ILK functioning in cell shape modulation, motility and survival. J. Biol. Chem. 2003;278:51324–51333. doi: 10.1074/jbc.M309122200. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Bordoy R, Stanchi F, Moser M, Braun A, Kudlacek O, Wewer UM, Yurchenco PD, Fässler R. PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci. 2005;118(Pt 13):2913–21. doi: 10.1242/jcs.02422. [DOI] [PubMed] [Google Scholar]
- 26.Stanchi F, Grashoff C, Yonga CF Nguemeni, Grall D, Fässler R, Van Obberghen-Schilling E. Molecular dissection of the ILK-PINCH-parvin triad reveals a fundamental role for the ILK kinase domain in the late stages of focal-adhesion maturation. J Cell Sci. 2009;122(Pt 11):1800–11. doi: 10.1242/jcs.044602. [DOI] [PubMed] [Google Scholar]
- 27.Wu C. PINCH, N(i)ck and the ILK: network wiring at cell-matrix adhesions. Trends Cell Biol. 2005;15(9):460–6. doi: 10.1016/j.tcb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Lin X, Qadota H, Moerman DG, Williams BD. C. elegans PAT-6/actopaxin plays a critical role in the assembly of integrin adhesion complexes in vivo. Curr Biol. 2003;13(11):922–32. doi: 10.1016/s0960-9822(03)00372-5. [DOI] [PubMed] [Google Scholar]
- 29.Clark KA, McGrail M, Beckerle MC. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development. 2003;130(12):2611–21. doi: 10.1242/dev.00492. [DOI] [PubMed] [Google Scholar]
- 30.Kadrmas JL, Smith MA, Clark KA, Pronovost SM, Muster N, Yates JR, 3rd, Beckerle MC. The integrin effector PINCH regulates JNK activity and epithelial migration in concert with Ras suppressor 1. J Cell Biol. 2004;167(6):1019–24. doi: 10.1083/jcb.200408090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zervas CG, Psarra E, Williams V, Solomon E, Vakaloglou KM, Brown NH. A central . multifunctional role of integrin-linked kinase at muscle attachment sites. J Cell Sci. 2011;124(Pt 8):1316–27. doi: 10.1242/jcs.081422. This is an interesting study that provides a detailed analysis of the recruitment and functions of ILK and other focal adhesion proteins in muscle attachment sites in Drosophila, which helps to illustrate the hierarchy of assembly of the integrin–actin link in this model of strong ECM adhesions.
- 32.Vakaloglou KM, Chountala M, Zervas CG. Functional analysis of parvin and different . modes of IPP-complex assembly at integrin sites during Drosophila development. J Cell Sci. 2012 doi: 10.1242/jcs.102384. [Epub ahead of print]. This paper, together with ref 17, 18, 28-31, provide strong genetic evidence showing that IPP is essential for ECM adhesion in invertebrates. Furthermore, these studies show that the mode of IPP assembly mimics that in mammalian cells in some but not other tissues in Drosophila.
- 33.Mercer KB, Flaherty DB, Miller RK, Qadota H, Tinley TL, Moerman DG, Benian GM. Caenorhabditis elegans UNC-98, a C2H2 Zn finger protein, is a novel partner of UNC-97/PINCH in muscle adhesion complexes. Mol Biol Cell. 2003;14(6):2492–507. doi: 10.1091/mbc.E02-10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norman KR, Cordes S, Qadota H, Rahmani P, Moerman DG. UNC-97/PINCH is involved in the assembly of integrin cell adhesion complexes in Caenorhabditis elegans body wall muscle. Dev Biol. 2007;309(1):45–55. doi: 10.1016/j.ydbio.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Chen K, Tu Y, Wu C. Distinct roles of two structurally closely related focal adhesion proteins, alpha-parvins and beta-parvins, in regulation of cell morphology and survival. J Biol Chem. 2004;279(40):41695–705. doi: 10.1074/jbc.M401563200. [DOI] [PubMed] [Google Scholar]
- 36.Olski TM, Noegel AA, Korenbaum E. Parvin, a 42 KLDa focal adhesion protein, related to the alpha-actinin superfamily. J Cell Sci. 2001;114(Pt 3):525–38. doi: 10.1242/jcs.114.3.525. [DOI] [PubMed] [Google Scholar]
- 37.Vaynberg J, Fukuda T, Chen K, Vinogradova O, Velyvis A, Tu Y, Ng L, Wu C, Qin J. Structure of an ultraweak protein-protein complex and its crucial role in regulation of cell morphology and motility. Mol Cell. 2005;17(4):513–23. doi: 10.1016/j.molcel.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Fukuda K, Byeon IJ, Velyvis A, Wu C, Gronenborn A, Qin J. The structure of α-parvin CH2-paxillin LD1 complex reveals a novel modular recognition for focal adhesion assembly. J. Biol. Chem. 2008;283:21113–21119. doi: 10.1074/jbc.M801270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenz S, Vakonakis I, Lowe ED, Campbell ID, Noble ME, Hoellerer MK. Structural analysis of the interactions between paxillin LD motifs and α-parvin. Structure. 2008;16:1521–1531. doi: 10.1016/j.str.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton N, Ross J, Jr, Chen J. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol. 2005;25(8):3056–62. doi: 10.1128/MCB.25.8.3056-3062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montanez E, Wickström SA, Altstätter J, Chu H, Fässler R. Alpha-parvin controls vascular mural cell recruitment to vessel wall by regulating RhoA/ROCK signalling. EMBO J. 2009;28(20):3132–44. doi: 10.1038/emboj.2009.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rooney N, Streuli CH. How integrins control mammary epithelial differentiation: a possible role for the ILK-PINCH-Parvin complex. FEBS Lett. 2011;585(11):1663–72. doi: 10.1016/j.febslet.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Wickström SA, Lange A, Hess MW, Polleux J, Spatz JP, Krüger M, Pfaller K, Lambacher A, Bloch W, Mann M, et al. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell. 2010;19(4):574–88. doi: 10.1016/j.devcel.2010.09.007. This study identifies a critical role of integrins and ILK in caveolae formation. Furthermore, it shows that ILK functions in this process through IQGAP1.
- 44.Fielding AB, Dobreva I, McDonald PC, Foster LJ, Dedhar S. Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization. J Cell Biol. 2008;180(4):681–9. doi: 10.1083/jcb.200710074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fielding AB, Lim S, Montgomery K, Dobreva I, Dedhar S. A critical role of integrin-linked kinase, ch-TOG and TACC3 in centrosome clustering in cancer cells. Oncogene. 2011;30(5):521–34. doi: 10.1038/onc.2010.431. [DOI] [PubMed] [Google Scholar]
- 46.Acconcia F, Barnes CJ, Singh RR, TaluKLDer AH, Kumar R. Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proc Natl Acad Sci U S A. 2007;104(16):6782–7. doi: 10.1073/pnas.0701999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y, Yu YP, Orr A, St-Arnaud R, Dedhar S, et al. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology. 2008;48(6):1932–41. doi: 10.1002/hep.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apte U, Gkretsi V, Bowen WC, Mars WM, Luo JH, Donthamsetty S, Orr A, Monga SP, Wu C, Michalopoulos GK. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology. 2009;50(3):844–51. doi: 10.1002/hep.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamazaki T, Masuda J, Omori T, Usui R, Akiyama H, Maru Y. EphA1 interacts with integrin-linked kinase and regulates cell morphology and motility. J Cell Sci. 2009;122(Pt 2):243–55. doi: 10.1242/jcs.036467. [DOI] [PubMed] [Google Scholar]
- 50.Azimifar SB, Böttcher RT, Zanivan S, Grashoff C, Krüger M, Legate KR, Mann M, Fässler R. Induction of membrane circular dorsal ruffles requires co-signalling of integrin-ILK-complex and EGF receptor. J Cell Sci. 2012;25(Pt 2):435–48. doi: 10.1242/jcs.091652. [DOI] [PubMed] [Google Scholar]
- 51.Sopko N, Qin Y, Finan A, Dadabayev A, Chigurupati S, Qin J, Penn MS, Gupta S. Significance of thymosin β4 and implication of PINCH-1-ILK-α-parvin (IPP) complex in human dilated cardiomyopathy. PLoS One. 2011;6(5):e20184. doi: 10.1371/journal.pone.0020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer. 2010;10(12):858–70. doi: 10.1038/nrc2967. [DOI] [PubMed] [Google Scholar]
- 53.White DE, Coutu P, Shi YF, Tardif JC, Nattel S, St Arnaud R, Dedhar S, Muller WJ. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006;20(17):2355–60. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang X, Sun Y, Ye M, Scimia MC, Cheng H, Martin J, Wang G, Rearden A, Wu C, Peterson KL, et al. Targeted ablation of PINCH1 and PINCH2 from murine myocardium results in dilated cardiomyopathy and early postnatal lethality. Circulation. 2009;120(7):568–76. doi: 10.1161/CIRCULATIONAHA.109.864686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuda T, Guo L, Shi X, Wu C. CH-ILKBP regulates cell survival by facilitating the membrane translocation of protein kinase B/Akt. J Cell Biol. 2003;160(7):1001–8. doi: 10.1083/jcb.200212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eke I, Koch U, Hehlgans S, Sandfort V, Stanchi F, Zips D, Baumann M, Shevchenko A, Pilarsky C, Haase M, et al. PINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1alpha. J Clin Invest. 2010;120(7):2516–27. doi: 10.1172/JCI41078. This is an interesting study that provides an example of how ILK may indirectly regulate AKT signaling via PINCH-PP1alpha interaction.
- 57.Zeqiraj E, Filippi BM, Deak M, Alessi DR, van Aalten DM. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science. 2009;326(5960):1707–11. doi: 10.1126/science.1178377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang D, Li Y, Wu C, Liu Y. PINCH1 is transcriptional regulator in podocytes that interacts with WT1 and represses podocalyxin expression. PLoS One. 2011;6(2):e17048. doi: 10.1371/journal.pone.0017048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim M, Ogawa M, Fujita Y, Yoshikawa Y, Nagai T, Koyama T, Nagai S, Lange A, Fässler R, Sasakawa C. Bacteria hijack integrin-linked kinase to stabilize focal adhesions and block cell detachment. Nature. 2009;459(7246):578–82. doi: 10.1038/nature07952. [DOI] [PubMed] [Google Scholar]
- 60.Devallière J, Chatelais M, Fitau J, Gérard N, Hulin P, Velazquez L, Turner CE, Charreau B. LNK (SH2B3) is a key regulator of integrin signaling in endothelial cells and targets α-parvin to control cell adhesion and migration. FASEB J. 2012 doi: 10.1096/fj.11-193383. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qadota H, Mercer KB, Miller RK, Kaibuchi K, Benian GM. Two LIM domain proteins and UNC-96 link UNC-97/pinch to myosin thick filaments in Caenorhabditis elegans muscle. Mol Biol Cell. 2007;18(11):4317–26. doi: 10.1091/mbc.E07-03-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]