Abstract
The hepatitis C virus (HCV) nonstructural protein 3 (NS3) is essential for the processing of the HCV polyprotein, the replication of HCV RNA, and to short circuit innate immunity signaling. NS3 contains an N-terminal domain with protease activity and a C-terminal domain with helicase activity. The two domains communicate with each other along with other HCV and cellular proteins. Herein we show that RNAs can bind directly to the active site cleft of the NS3 protease domain (NS3P) and inhibit proteolysis of peptide substrates. RNAs that are less apt to form intramolecular structures have a stronger inhibitory activity than RNAs with more stable base paired regions. Two mutations in the protease domain that resulted in decreased affinity to ssRNA were also defective in RNA-induced ATPase activity from the helicase domain of NS3. The coordinated effects on inhibition of protease activity and stimulation of ATPase activity raise the possibility that RNA serves as a regulatory switch for the two processes.
Keywords: Hepatitis C Virus, positive-strand RNA virus, NS3 protease, nucleic acid inhibitors, RNA binding, ATPase activation
1. Introduction
Approximately 2 to 3% of the world population has Hepatitis C, and 3 to 4 million new cases are reported every year, resulting in liver diseases including cirrhosis and hepatocellular carcinoma (Shepard et al., 2005; Tan et al., 2002). The caustic agent of hepatitis C is the hepatitis C virus (HCV) (Choo et al., 1989). While the recent development of HCV protease inhibitors has improved the treatment for patients infected with genotype 1 HCV, resistance to treatment and substantial side effects remain serious issues (Feld and Hoofnagle, 2005; Jacobo-Molina et al., 1993; Kwong et al., 2011). Better understanding of the mechanism of HCV infection will inform strategies for hepatitis C treatment.
HCV is a positive single-stranded (ss) RNA virus of the family Flaviviridae (Choo et al., 1991; Moradpour et al., 2007). HCV encodes a polyprotein that is processed into ten mature proteins by a combination of cellular and HCV-encoded proteases (Moradpour et al., 2007). The mature nonstructural proteins then assemble into a membrane-associated complex that replicates the virus RNA as well as alter the physiology of the cell (Chisari, 2005; Elazar et al., 2004; Gale and Foy, 2005; Gao et al., 2004).
Nonstructural protein 3 (NS3) is a key HCV protein with roles in both polyprotein processing and RNA replication. NS3 has a serine protease domain located in the N-terminal 180 residues and an RNA helicase domain in the remaining 453 residues. The protease domain adopts a typical chymotrypsin-like fold with two β-barrel subdomains, and its catalytic triad is composed of His57, Asp81 and Ser139 (Jurgens et al., 2006; Kim et al., 1996). Although NS3 possesses proteolytic activity, substrate cleavage is dramatically enhanced by NS4A (Kwong et al., 2008; Lam and Frick, 2006; Yan et al., 1998; Yao et al., 1999). The NS3 protease activity is also involved in counteracting cellular antiviral defense pathways by cleavage of the adaptor protein named MAVS (also known as IPS-1, CARDIF, and VISA) that is activated by cytoplasmic RNA sensors RIG-I and MDA5 (Meylan et al., 2005). NS3-4A has also been reported to proteolyze TRIF to abrogate Toll-like receptor 3 signaling (Li et al., 2005a; Lin et al., 2006).
The NS3 helicase belongs to the Superfamily 2 of the DEXH/D box RNA helicases. It has ATPase activity and unwinds double-stranded (ds) nucleic acids in a 3′ to 5′ direction in an ATP-dependent manner (Mann et al., 2008; Yi et al., 2007). Both the protease and the helicase activities are essential for HCV RNA replication and are validated targets for antiviral development (Kolykhalov et al., 2000; Lam and Frick, 2006; Mederacke et al., 2009; Pang et al., 2002; Taliani et al., 1996).
Since the protease and helicase domains reside in one protein, it is not surprising that the domains communicate with each other. Indeed, the protease domain can stimulate the helicase activity of the NS3 protein and increase RNA binding by the helicase (Frick et al., 2004; Gu et al., 2005; Zhang et al., 2005). Additionally, the helicase domain enhances the NS3 protease activity (Beran and Pyle, 2008; Beran et al., 2007). The protease domain can also mediate interactions with other subunits of the HCV replication enzyme complex (Pang et al., 2002).
Cleavage sites of NS3P, the protease domain of NS3, contain a number of conserved acidic residues, especially at the P6 position (Fig. 1A). This feature suggests that other negatively charged polymers could mimic NS3 substrates. Herein, we provide evidence that the NS3P can bind ssRNA at its active site, causing a reduction in protease activity. Furthermore, RNA binding to the protease domain enhances ATPase activity in the helicase domain.
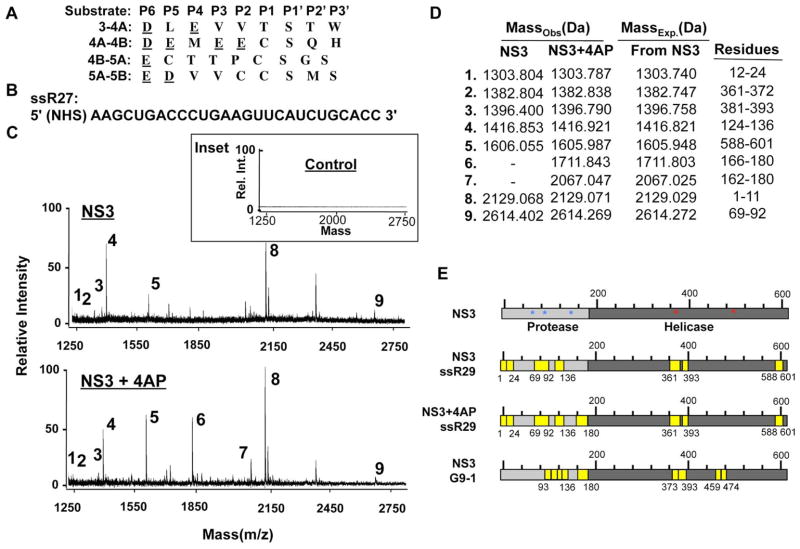
Fig. 1. RNA can be crosslinked to the protease domain of the HCV NS3 protein.
A) Alignments of the substrate sequences recognized by the NS3 protease. The amino acids are in standard one-letter codes, with the acidic residues underlined. B) Sequence of ssR27, the RNA used to generate an affinity resin for the RCAP assay. C) Mass spectra of a control reaction in which the formaldehyde was left out of a reaction (top inset), NS3 crosslinked to the RNA-resin (middle), and NS3 amended with the 4AP crosslinked to the RNA-resin (bottom). The reactions were performed as described in the Materials and Methods. The ions were resolved by a Bruker Autoflex III MALDI-TOF mass spectrometer set in reflectron mode. D) A summary of the ions observed in the mass spectra containing NS3 peptides reversibly crosslinked to the RNA-resin. The observed and expected masses of the assigned peptides are shown. E) Schematics of NS3 showing the locations of the peptides that crosslinked to the ligand indicated. G9-1, an RNA that was selected by SELEX to inhibit NS3 protease activity, was also analyzed by the RCAP assay (Urvil et al., 1997, bottom panel). Identical peptides were identified for a phosphorothioate-containing DNA (2006) as ssR27. To better discern the two domains of NS3, the helicase domain is in darker grey while the NS3P domain is in lighter color. Residues in the catalytic triad of NS3P are denoted by blue asterisk, the helicase nucleic acid binding residues identified by (Frick, 2007; Mackintosh et al., 2006) are denoted by red asterisks. The locations of NS3 that we identified to bind RNA and phosphorothioate-modified 2006 are in yellow.
2. Materials & Methods
2.1 Reagents
Oligodeoxyribonucleotides and oligoribonucleotides were chemically synthesized by IDT (Coralville, OR). The RNA aptamer G9-1 was synthesized using the AmpliScribe T7-Flash transcription kit per manufacturer’s protocol (Epicentre Biotechnologies Inc., Madison, WI). All chemicals were from Sigma-Aldrich Inc. (St. Louis, MO) unless stated otherwise. Mass spectrometry reagents were from Thermo Fisher (Waltham, MA).
2.2 Plasmid constructs
Mutations in the plasmid expressing NS3 were introduced using the QuikChange site-directed mutagenesis (Stratagene, Santa Clara, CA) following the manufacturer’s protocol. All mutant constructs used in this study were sequenced to confirm the presence of the mutation and absence of unintended mutations. Recombinant NS3P-4A was made based on the strategy used by Taremi et al., (Taremi et al., 1998). Briefly, the DNA encoding the residues 21–32 of NS4A was cloned at the 5′ end of the NS3 protease (1–181 residues) coding region separated by nucleotides that encode a Gly-Ser-Gly-Ser linker to allow proper folding of the recombinant protein.
2.3 Protein expression and purification
NS3P-4A and NS3 were expressed and purified through a Ni-NTA column according to the protocol in (Zhang et al., 2005). The proteins were subsequently purified by a polyU column. The purified proteins were divided into aliquots and stored in 20 mM Tris (pH 7.5), 400 mM NaCl, at −80°C, protein concentrations were determined by the Bradford assay using BSA as the standard.
2.4 Reversible crosslinking–peptide fingerprinting (RCAP) analysis
N-hydroxysuccinimide (NHS)-agarose resin was coupled to 5′ amine-labeled oligonucleotides in 0.1 M sodium tetraborate pH 8.5, and quenched with 1 M glycine. NHS-agarose resin was also incubated in the absence of any oligonucleotide substrate to produce a control resin. After exchanging the buffer to 20 mM HEPES (pH 7.5), 4 mM MnCl2 and 10 mM dithiothreitol, NS3 was added at 2 μM final concentration and the reaction incubated at room temperature for 5 min in a final volume of 25 μL. Formaldehyde was then added to a final concentration of 0.1% and incubated for 10 min. The cross-linking reaction was stopped by the addition of glycine at a final concentration of 0.2 M. After 5 min, trypsin (Trypsin Gold; Promega, Madison, WI) was added at a protease:substrate ratio of 1:50 (w/w) in 100 mM NH4HCO3 (pH 7.8) and incubated overnight at 37°C. Unbound peptides were removed with extensive washing using 20 mM HEPES (pH 7.5), 1 M NaCl, 1 mM EDTA and 1 mM dithiothreitol. Nucleotide-peptide conjugates were then reversed by incubating the samples at 70°C for 1 h. The samples were centrifuged at 3000 × g for 5 min, and the supernatants containing the peptides were desalted using a Ziptip (Millipore, Bedford, MA) before elution in 2.5 μL of 70% acetonitrile and 0.1% trifluoroacetic acid in preparation for mass spectrometry.
2.5 Amidination of NS3
A stock solution of 200 mM S-methyl thioacetimidate was made in 20 mM HEPES buffer (pH 7.5) and mixed in equal volumes with 5 μg NS3P-4A protein. After a 1 h incubation, the reaction buffer was exchanged to 100 mM NH4HCO3, and sequencing-grade trypsin (Trypsin Gold, Promega, Madison, WI) was added at a protease/substrate ratio of 1:50 (w/w) and incubated overnight at 37°C. The samples were then stored at 4°C until analysis by reversed phase liquid chromatography coupled to electrospray ionization mass spectrometry (see below). A Dionex Ultimate 3000 chromatography system was used to deliver the gradient detailed in Table 1. A homemade fused silica trapping column (150 μm × 15 cm, Polymicro Technologies, Phoenix, AZ) with a polymerized silica frit (Wang et al., 2008) was packed with C18 beads (Michrom Bioresources Magic stationary phase, 5 μm particle size, 200 Å pore size, Auburn, CA) and used to concentrate and desalt peptides from tryptic digests. Concentrated peptides were eluted from the trap and separated using a fused silica nanospray column (75 μm × 15 cm) that had been pulled on a Sutter Instruments P-2000 Micropipette Puller (Sutter Instruments, Novato, CA) and packed with C18 beads (Michrom Magic, 5 μm particle size, 100 Å pore size).
2.6 LC-MS/MS
Liquid chromatography was performed with a flow rate of 300 nL/min using the scheme below.
| Time (min.) | %A | %B | Note |
|---|---|---|---|
| 0.0 | 95 | 5 | |
| 10.0 | 95 | 5 | |
| 10.1 | 85 | 15 | Trap switched in line with column |
| 70.0 | 45 | 55 | |
| 70.1 | 10 | 90 | |
| 80.0 | 10 | 90 | Trap switched out to waste |
| 80.1 | 95 | 5 | |
| 90.0 | 95 | 5 |
The eluting peptides were detected using a Thermo LTQ-FT Ultra hybrid ion trap/Fourier transform ion cyclotron resonance mass spectrometer (Thermo Fisher, Bremen, Germany) equipped with a nano-ESI source. The instrument was calibrated with the manufacturer’s specified mixture [caffeine, methionyl-arginyl-phenylalanyl-alanine (MRFA, acetate salt and Ultramark 1621] and tuned on the +3 charge state of bovine insulin chain B. All spectra were collected across a window of 300–2000 Th, with 1 microscan and a maximum injection time of 50 ms. Parent ion spectra were collected in the Fourier transform Ion cyclotron resonance cell with a resolution of 50,000. The five most intense masses in any parent ion spectra were subjected to collisionally-induced dissociation in the linear quadrupole ion trap using mass isolation widths of +1.5 Da/−0.5 Da, normalized collision energy of 35%, activation bandwidth of Q=0.25 and an activation time of 30 ms.
Data files containing combined MS and MS/MS spectra were converted to MASCOT Generic Format text files using the TurboRAW2MGF application of the National Center for Glycomics and Glycoproteomics ProtQuant program suite (Mann et al., 2008).
2.7 Protease assay
Protease activity was measured in real time using a fluorescence-based assay (Dahl et al., 2007) with either 0.043 μM NS3P-4A or 0.030 μM NS3 (unless stated otherwise) in 50 mM HEPES (pH 7.5), 25 mM NaCl, 10 mM dithiothreitol, 40% (w/v) glycerol, 0.1% n-octyl-β-d-glucoside). The concentrations of the proteins used were empirically determined to be optimal for cleavage. For NS3, 25 μM NS4A peptide cofactor KKGSVVIVGRIVLSGK was also added to the reaction. The mix was first incubated at 30°C for 2 min, followed by a second incubation for 10 min after the addition of nucleic acid ligand. The substrate was at a final concentration of between 0.25 and 4 μM and recorded over 10 min with a fluorescence plate reader (Fluostar Optima, BMG LACTECH). The substrate, Ac-Asp-Glu-Asp(EDANS)-Glu-Glu-Abu-w-[COO]Ala-Ser-Lys(DABCYL)-NH2 was from AnaSpec (San Jose, CA, USA; (Lam and Frick, 2006). The data were fitted by linear least-squares regression analysis to determine the reaction rates. All measurements were performed in triplicate.
2.8 ATPase assay
ATPase assay was performed in a buffer containing 20 mM HEPES (pH 7.5), 2 mM DTT and 1.5 mM MgCl2. ATPase activity was assessed using the ADP-Glo Kinase Assay per manufacturer’s protocol (Promega Inc., Madison, WI). Briefly, buffer containing 100 nM NS3 in 10 μL volume was incubated in the presence of 1 mM ATP for 30 minutes. A total of 9 μL of ADP-Glo reagent was added to remove excess ATP and terminate the ATPase activity. After a 40 min incubation, 18 μL of the ADP detection reagent was added to convert ADP to ATP and the signal was detected using a luciferase/luciferin reaction. Luminescence was quantified with the Synergy 2 Plate Reader (Biotek, Inc).
2.9 Fluorescence anisotropy assay
A fluorescence anisotropy assay was used to measure the affinity of RNA binding by NS3. All the measurements were made using a Perkin-Elmer luminescence spectrometer LS55 and cuvettes with an optical path length of 0.4 cm at 22°C as described by Bhardwaj et al. (2004). Equilibrium anisotropy was taken from fluorescein–labeled 15-mer RNA with excitation and emission wavelengths at 495 and 520 nm, respectively. F-RNA was at 0.2 μM in a buffer containing 25 mM Tris HCl (pH 7.5) and 25 mM NaCl. Proteins added during the titration did not exceed 10% of the initial sample volume. Each emission scan was repeated ten times, and all results were averaged. All data were corrected for the background intensity of the buffer and for dilution. Binding data were analyzed by nonlinear least-squares fitting using KaleidaGraph software (Synergy Software, Reading, Pa.). A simple 1:1 binding model was fitted to the binding isotherm using equation 1: ΔF/F0 = Bmax [ligand]total/(Kd + [ligand]total), where ΔF is the magnitude of the difference between the observed fluorescence intensity (arbitrary unit) at a given protein concentration and the fluorescence intensity in the absence of protein, F0 is the fluorescence intensity in the absence of protein, and Bmax is the value of the maximum relative fluorescence change.
2.10 HCV subgenomic replicon assay
HCV NS3 mutants replicon were made by site-directed mutagenesis using a NeoR HCV subgenomic replicon pFKI389/NS3-3′/WT (Lohmann et al., 1999). The RNAs were transcribed in vitro using the AmpliScribe™ T7-Flash™ Transcription Kit (Epicentre Technologies, Madison, WI), and electroporated into Huh7.5 cells as previously described (Yi et al., 2011).
Colony formation assays used 2×106 of transfected cells that were seeded into the first well of a 12 well plate, and 2-fold serial dilution was made down to the remaining wells. Twenty-four hours after electroporation, 0.5 mg/ml G418 was added to the media and the cells were maintained in the selective medium, with replacement of the media every 3 days for 21 days. Replicon harboring colonies were then stained by crystal violet, and calculated as colony formation number/μg RNA.
Real-time PCR assay was performed with 2.5×104 of transfected cells seeded into one well of a 24 well plate with triplicates for each time point. Total RNAs were extracted from replicon cells using the Trizol reagent (Invitrogen, Grand Island, NY) and first strand cDNA synthesis used 1 μg of total RNA along with M-MulV (New England Biolabs, Ipswich, MA) and 4 μM of a randomized 9-nt primer mix. RT-PCR used the SYBR Green kit (Bio-Rad, Hercules, CA), and primers are HCV 5′-UTRsense (5′-AGC CAT GGC GTT AGT ATG AGT GTC-3′) and 5′-UTRanti (5′-ACA AGG CCT TTC GCG ACC CAA C-3′). GAPDH was detected as internal control using the sense and antisense oligonucleotides: 5′-GAG TCA ACG GAT TTG GTC GT-3′ and 5′-TGGGAT TTC CAT TGA TGA CA-3′, respectively. All samples were heated to 95°C for 10 min, followed by 40 cycles of PCRs of 15 s at 95°C, 20 s at 55°C and 30 s at 72°C. The percent change of each mutant was compared to wild type as previously described (Livak and Schmittgen, 2001).
2.11 Cell-based assay for NS3 protease activity
Actively growing HEK293T cells were plated in CoStar White 96-well plates at 2.3×105/mL. When cells reached 80% confluence, they were transfected with a mixture of Lipofectamine 2000 reagent (Invitrogen Inc., San Diego, CA) and plasmids including pIFN-β Luc (30 ng, InvivoGen, encoding the firefly luciferase reporter regulated by the IFN-β promoter), phRL-TK (5 ng, Promega, encoding the Renilla luciferase reporter to serve as a transfection control), pNS3P-4A (0.05 ng) and pMAVS (0.5 ng). The cells were incubated for 2 h and nucleic acids were transfected into cells at 0.1 μM. After another 12 to 16 h, the cells were assayed using the Dual Glo Luciferase Assay System reagents (Promega). Luminescence signals were quantified using the Synergy 2 Plate Reader (Biotek, Inc).
Western blot analysis was performed to detect whether 2006 could affect NS3-dependent cleavage of a flag-tagged MAVS (Li et al., 2005). HEK 293T cells were transfected with pNS3P-4A (8 ng) and pMAVS (8 ng) for 2 h, then transfected with 0.1 μM 2006, or water as a control. Cells were harvested after another 16 h, washed with 1X PBS and permeabilized with PBS containing 0.019% digitonin and a protease inhibitor mix (P8340, Sigma Aldrich). After a 20 min incubation on ice, the samples were centrifuged at 15,000 × g for 15 min at 4°C. The resulting supernatant carrying the cytoplasmic proteins and pellet containing membrane proteins were diluted in 1X Laemmi sample buffer and subjected to SDS-PAGE analysis. After transfer to membrane, MAVS was detected using a monoclonal antibody specific to flag-tag antigen (F3040, Sigma Aldrich,).
3. RESULTS
3.1 NS3 protease domain can bind RNA
A reversible crosslinking-peptide fingerprinting method named RCAP was used to examine NS3 binding to RNA. This method has been used to study RNA/DNA-protein interaction, including for the HCV polymerase (Bhardwaj et al., 2008; Casula et al., 2009; Niranjanakumari et al., 2002; Vaughan et al., 2012). A 27-nt RNA named ssR27 that was empirically determined to bind NS3 in solution was chemically synthesized to contain a 5′ amine to allow covalent attachment to resin (Fig. 1B). After incubation with NS3 and crosslinking with formaldehyde, the resin-bound NS3 was digested with trypsin. Stringent washes were used to remove noncovalently-bound materials and the crosslinked peptides were eluted by heating the sample. MALDI-ToF was then used to identify the masses of the peptides. Resin that lacked RNA, or NS3 incubated with the RNA-resin but not crosslinked with formaldehyde produced much lower signals for peptides (Inset to Fig. 1C).
Several peptide peaks were reproducibly observed in the crosslinking reactions (Fig. 1C, upper panel). Only the peaks that corresponded to within 1 Da to peptides in a theoretical digest of NS3 were assigned (Fig. 1D). Interestingly, additional peaks were observed in the presence of the NS4A peptide (Fig. 1C, lower panel). Three RNA-binding peptides were from the NS3 helicase domain (Fig. 1D) and their locations are in excellent agreement to portions of NS3H previously shown to bind RNA (Mackintosh et al., 2006). Six peptides were from the NS3 protease domain, four were located adjacent to the substrate-binding pocket in the protease domain, and two were observed only in the presence of the 4A cofactor (Fig. 1D and 1E). In fact, one of the four peptides contained Asp81 of the catalytic triad (Kim et al., 1996). The same peptides were also selectively enriched with a biotinylated version of ssR27, RNAs with a U-rich sequence, or a phosphorothioated ssDNA 2006 (Fig. 1E, data not shown).
The HCV NS3P was previously used in biochemical SELEX experiments to identify high affinity RNA aptamers that inhibited protease activity (Urvil et al., 1997). One of the aptamers named G9-1 had an affinity of ~12 nM (Fukuda et al., 2000). The residues within the NS3P that contacted G9-1 were mapped by alanine scanning mutagenesis (Hwang et al., 2000a). We reasoned that G9-1 also binds to the active site of NS3P in a manner similar to the RNAs that we have used. To better map the regions in NS3P that contacts the 67-nt G9-1, the RCAP assay was used. However, lithium chloride was used to selectively co-precipitate the conjugated peptides. This alternative RNA precipitation method was previously used to map peptides that can bind longer RNAs (Hema et al., 2010; Vaughan et al., 2012; Yi et al., 2009b). An overlapping subset of peptides to those that bound to the 27-nt RNA was observed as well as additional ones (Fig. 1E). G9-1 also was crosslinked to additional peptides in both the protease and helicase domains. However, given that G9-1 is larger than our previous probe, this result is to be expected.
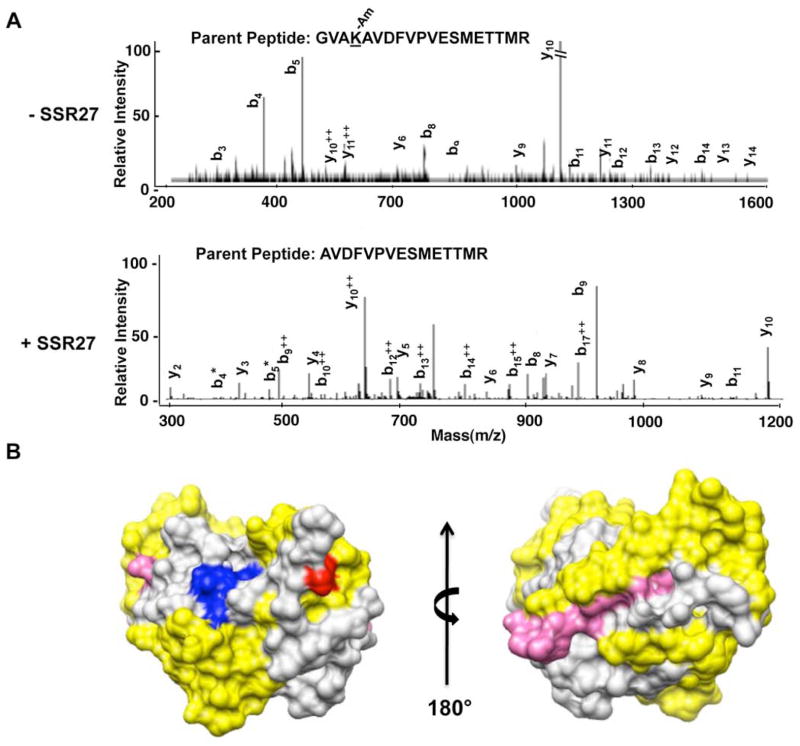
An amidination interference assay was used to confirm that the NS3 protease domain could contact RNA. This assay takes advantage of the fact that S-methyl thioacetimidate can specifically modify exposed lysines or the N-terminal amine when the residues are not bound to ligands (Janecki et al., 2005). The modified and unmodified residues were identified by proteolytic digest followed by mass spectrometry. This procedure was previously used to elucidate the contacts between the ribosomal proteins and rRNA (Lauber et al., 2009; Liu and Reilly, 2009; Running and Reilly, 2009). Figure 2A shows MS/MS spectra for tryptic peptides generated from NS3 prebound to RNA and then subjected to amidination or to a parallel processed sample that was not bound to RNA. In the absence of RNA, NS3P-4A was amidinated at K165, as determined by a 41 Da mass shift in modified peptides as well as a miss trypsin cleavage site. In the presence of RNA K165 amidination was undetectable, and an increase in the tryptic peptide cleaved at K165 was observed (Fig. 2A, data not shown). The peptide containing K165 maps adjacent to the NS3P substrate-binding pocket (Fig. 2B), in agreement with the results of the RCAP assay in Fig. 1. The amidination interference results thus confirm that substrate pocket of NS3P can bind RNA.
Fig. 2.
Amidination of lysine residues in the NS3P domain in the absence and presence of RNA.
A) MS/MS analysis of peptides from NS3P that were differentially amidinated in the presence and absence of RNA. The top panel shows the b and y ions from the MS/MS spectra for the peptide that was not cleaved by trypsin due to amidination at Lysine 165. The bottom panel shows the MS/MS spectra for the parent peptide in which amidination did not take place due to the presence of ssR27, and the peptide was cleaved at the cognate lysine. B) The model for NS3P and the location of the RCAP peptides relative to the active site of NS3P. The active site residues are in blue. The locations of peptides identified to crosslink to RNA in Fig. 1 are in yellow, and the location of the amidinated lysine (K165) is in red.
3.2 ssRNA and ssDNA can inhibit NS3P protease activity
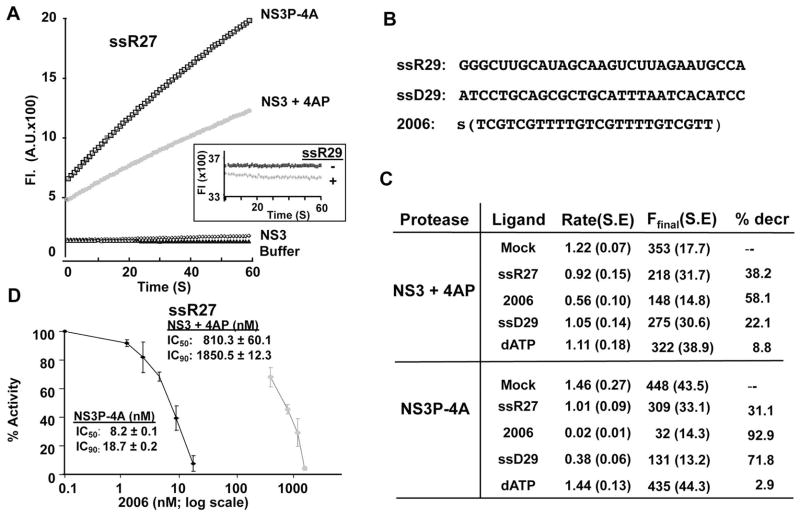
We sought to determine whether ssRNAs could affect NS3 protease activity. We also tested ssDNAs, including those with phosphorothioate-modified backbone, since they should be more resistant to degradation in cell-based assay when we used to assess for effects on NS3 protease activity later in this work. The protease assay used a peptide substrate that contained a fluorophore and a fluorescence quencher, and an increase in fluorescence is observed upon proteolysis (Lam and Frick, 2006). In the absence of ligand, both NS3 mixed with the NS4A peptide (NS3+4AP) and a fusion of the NS3 protease domain to the NS4A peptide (NS3P-4A) had robust protease activity (Fig. 3A). Fusion of full-length NS3 to the NS4A peptide was not soluble in our hands. NS3 lacking the NS4A peptide had minimal activity over the course of the reaction (Fig. 3A). The fluorescence released from a completed NS3-cleavage reaction was not quenched by a 27-nt ssRNA (ssR27), a 29-nt ssDNA, a 24-nt ssDNA with phosphorothioate backbone named 2006, or 1 mM ATP (Fig. 3A inset and Fig. 3B) demonstrating that ssRNAs and ssDNAs do not significantly interfere with the readout from the assay. ssR27, ssD29, and phosphorothioate-modified ssDNA 2006 incubated with the NS3P did inhibit the NS3 protease activity (Fig. 3C). ssD29, ssR27 and 2006 reduced protease activities by 22, 38, and 58%, respectively (Fig. 3C). Similar trends were observed with NS3P-4A, although the inhibition by 2006 was better than that of NS3 amended with the 4A peptide. As a control, dATP at 1 mM final conc. only reduced protease activity by 9%.
FIG. 3.
Effects of nucleic acid ligands on NS3 protease activity in vitro. A) A demonstration of the data generated by the fluorescent protease assay. The assay measures fluorescence releases in arbitrary units (A. U.) over a one-minute period. The presence of NS4A peptide is needed to increase catalytic activity either as a fusion to NS3P (NS3P-4A), or added in trans to the NS3 protein (NS3 + 4A). The inset shows that the RNAs added to the assay did not affect the fluorescence. This control reaction used an NS3 protease reaction that was allowed to reach saturation prior to the addition of the RNA, ssR27. The addition of ssR27 did not perturb the fluorescence, indicating that the RNA did not affect the readout for the assay. B) Sequence of the potential antagonists used in the NS3 protease assay. The letter S denotes that ssDNA 2006 contains phosphorothioates in place of phosphodiesters. C) A summary of the effects of the potential antagonists on the rates of substrate cleavage by NS3 + 4A or NS3P-4A. The rate is the slope of the curve for the fluorescence released in the first three minutes of the reaction, the F-final is the level of the fluorescence in the reaction after a ten-minute reaction period. Each reaction was done in at least triplicate and the S.E. is shown in parentheses. D) Inhibition curves for ssDNA 2006 on NS3 and NS3P-4A proteolytic activities.
The greater sensitivity of NS3P-4A protease activity to inhibition by nucleic acid ligands when compared to that of NS3+4AP suggests that the presence of the NS3 helicase domain alleviates some inhibition within the NS3P domain, perhaps by titrating away the nucleic acid antagonist. To better quantify the effect of the RNAs on the NS3 protease domain, we examined the consequence of adding increasing amounts of 2006 to NS3+4AP and NS3P-4A (Fig. 3D). The IC50 of 2006 was 810 nM for NS3+4AP and 8.2 nM for NS3P-4A, a difference of two orders of magnitude (Fig. 3D). Altogether, these results confirm that the helicase domain will titrate away some antagonists for the NS3P. Furthermore, some ligands possess yet-to-be elucidated properties that make them better inhibitors of NS3 protease activity.
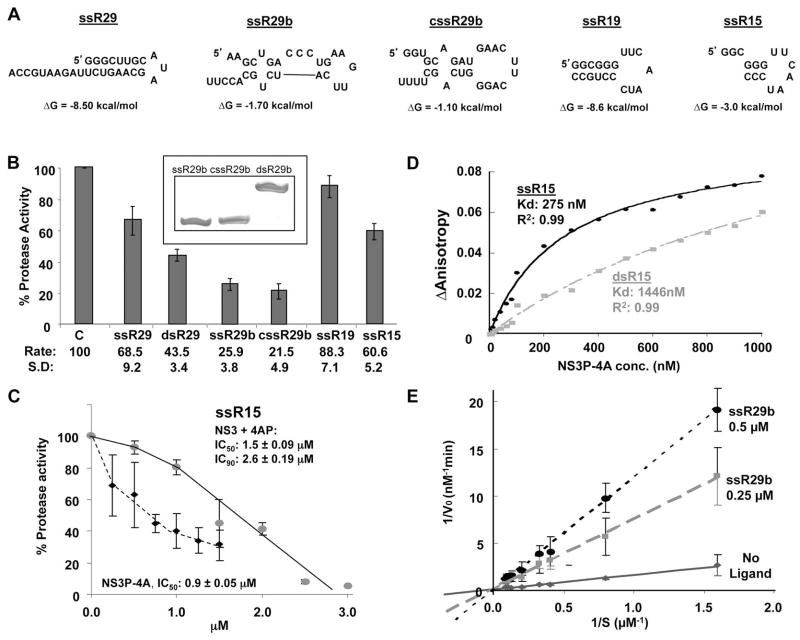
3.3 Single-stranded RNAs are better inhibitors of NS3 protease activity
We seek to identify the properties of RNAs that is inhibitory to NS3 protease activity. RNAs that differed in lengths and predicted secondary structures were tested for their ability to inhibit NS3P-4A (Fig. 4A). RNA ssR29 should form a stable hairpin (ΔG of −8.5 kcal/mol, Zuker et al., 2003) with a single-stranded 3′ tail. RNAs cssR29b and ssR29b are also 29-nt in length, but possess less stable intramolecular structures when compared to ssR29 (ΔG of −3.0 kcal/mol and −8.6 kcal/mol, respectively, Zuker, 2003). SsR29b and cssR29 were both better inhibitors of NS3P-4A activity than ssR29 (Fig. 4B), suggesting that RNAs with less stable secondary structures are better inhibitors of protease activity. To examine this trend further, we annealed cssR29b and ssR29b to form a dsRNA named dsR29b and confirmed that dsR29 was in a duplex form in a nondenaturing PAGE (Fig. 4B, inset). When tested in parallel with ssR29b and cssR29b, dsR29b had only half the inhibitory effect as either of its single-stranded progenitors (Fig. 4B). In addition, ssR15 (ΔG of −3 kcal/mol) was a better inhibitor of NS3P-4A protease activity than ssR19. The IC50 values of ssR29b for NS3+4AP and NS3P-4A were 1.5 μM and 0.9 μM, respectively (Fig. 4C). These results show that ssRNAs are better inhibitors for NS3 protease activity than those with more structured RNAs.
FIG. 4.
Effects of RNAs on NS3 protease activity. A) Schematics of the RNAs used in the assays. The secondary structures and predicted ΔG values of the RNAs were generated using Mfold (Zuker, 2003). B) Effects of the RNAs on NS3P-4A protease activity. Each bar represents at least three independent protease assays. The names of the RNAs used are indicated below the bars of the graph. dsR29 represents the annealed products from ssR29b and cssR29b. A demonstration that the annealing of ssR29b and cssR29b resulted in dsRNAs is shown as an inset above the bar graphs. The RNAs were electrophoresed in a nondenaturing 20% polyacrylamide gel and stained with Toluidine Blue. 100 pmoles each of the RNAs were used in the electrophoresis. C) The effects and IC50/90 of the best RNA inhibitor in panel B, ssR29b, on inhibition of NS3-4A or NS3P-4A protease activities. D) Affinities of NS3P-4A for RNAs ssR15 and dsR15. ssR15 was chemically synthesized to have a fluoresceine at the 5′ terminus and then added to a fluorescence anisotropy assay performed in an HP LS55 fluorimeter. The same assay was also performed with the labeled ssR15 annealed to the complementary RNA to generate dsR15. E) Analysis of the mechanism of inhibition of ssR29b on NS3P-4A protease activity. A standard NS3P-4A protease activity assay was performed in the presence of 4 μM substrate. The effects of two concentrations of ssR29b on the rates of cleavage by NS3P were graphed in a double reciprocal plot. The intercept for the inhibitors was approximately at zero, suggesting that the RNA antagonists for NS3P-4A were competing with the peptide substrate.
3.4 Inhibitory RNAs act by binding in the NS3 protease substrate pocket
We hypothesized that ssRNA could fit better than dsRNA into the active site cleft of NS3P. To examine this, ssR15 was synthesized with a 3′ fluorescein for use as a probe in anisotropy experiments. In addition, the labeled ssR15 was annealed with its complementary RNA to generate dsR15. Both probes were titrated against NS3P-4A and the binding isotherms revealed that ssR15 had a Kd of 275 nM while the Kd for dsR15 was five-fold higher, 1446 nM (Fig. 4D). These results support the idea that that ssRNAs bind NS3P-4A better than dsRNAs.
To test whether the negatively charged RNA could compete for binding with the protein substrate, which has at least one invariant acidic residue (Fig. 1A), we performed a titration of ssR29b at two concentrations and plotted the results on a double reciprocal plot. The intercept of the isotherms was close to zero (Fig. 4E), consistent with ssR29b acting as a competitive inhibitor to the peptide substrate for binding NS3P-4A.
3.5 The RNA-binding pocket in NS3P
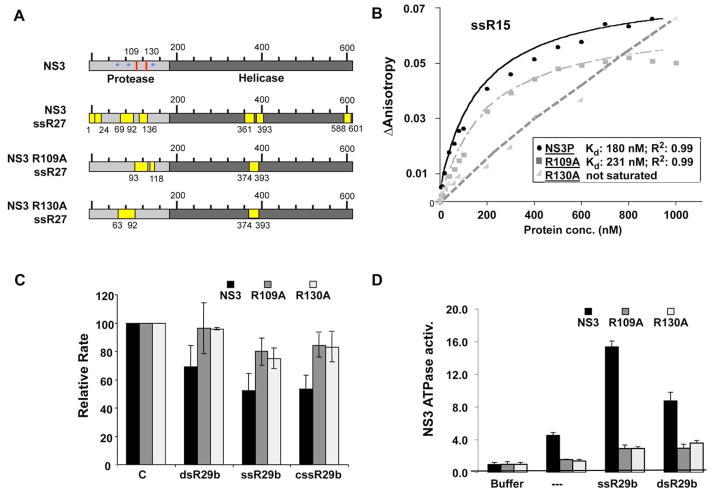
The results so far suggest that mutations in the NS3P active site pocket should affect RNA binding. Several alanine substitutions in positively-charged residues of NS3 were made, and mutants R109A and R130A were stable to storage at low millimolar concentrations while the remainder was not. Residue R130 lies in one of the peptides that was crosslinked to RNA and residue R109 was crosslinked to G9-1 (Fig. 1E, 5A). The RCAP assay was used to confirm that the mutants were affected for RNA binding. WT NS3 and both mutants crosslinked to a peptide spanning residues 374–393, but the mutants did not crosslink a peptide containing residues 1–24 in the protease domain. In addition, each mutant was crosslinked to one unique peptide from the protease domain, suggesting that RNA contact in the protease domain was altered with the two mutants (Fig. 5A). Interestingly, two peptides from the helicase domain of NS3 (residues 361–372, and 588–601) were not observed with the mutants, consistent with communication through RNA binding by the helicase and protease domains (Fig. 5A). These results demonstrate that the mutant proteins exhibit changes in their interaction with RNA.
FIG. 5.
Residues near the NS3P active site pocket can affect RNA binding. A) Schematic of peptides purified by the RCAP assay of two protease mutants, R109A and R130A, compared to those identified by the same procedure with the wild-type NS3 protein. Locations of these two residues are identified by red lines on the schematic, and active site residues with blue asterisks. B) Fluorescence anisotropy analysis of binding to fluoresceine-labeled ssR15 by NS3P, R109A, and R130A. The data derived from the binding isotherms are summarized in the inset. C) A comparison of the effects of the mutations in the active site of NS3P on the inhibition of NS3P protease activity. Three RNA ligands were tested on the three proteins and the results are shown in bar graph form. Each sample was assayed a minimum of three independent times, and the range for one standard error is shown. D) RNA-induced ATPase activity from NS3. ATPase activity used a luminescence assay from Promega Inc. The ligands denoted below the bars were tested at 2 μM. The standard errors denote the range of ATPase activities from three independent assays. The background of the assay with only the buffer is shown on the left, and normalized to 1.0 fold induction.
Fluorescence anisotropy was used to determine the affinity constant for RNA binding by the two mutant proteins. When compared to the WT, NS3P had a Kd of 180 nM, mutant R109A had a slight defect in RNA binding (Kd of 231 nM) while R130A could not reach saturation in these assays, even at the highest ligand concentration tested (2.7 μM; Fig. 5B). The latter result is consistent with those of Fukuda et al. (2000) who also showed that R130A was defective in binding to the RNA aptamer G9-1. Finally, protease activities of mutants R109A and R130A were evaluated in the presence of ssRNAs. Both mutants were less sensitive to inhibition when compared to wild-type NS3 (Fig. 5C). Results from mutational analysis of the NS3 protease domain support our proposition that RNA can bind the protein substrate-binding pocket.
3.6 NS3 protease-helicase domain interactions
Since the protease domain can modulate the NS3 helicase activity (Frick et al., 2004; Gu et al., 2005; Zhang et al., 2005), we tested whether ligands that inhibit protease activity will affect the ATPase activity of the NS3 helicase. A luminescent ATPase activity assay was used, and the background with buffer alone was normalized to one. In the presence of ssR29b, ATPase activity increased by over 3-fold. RNA-induced ATPase activity was reduced for both mutants compared to wild-type NS3, especially for R130A, the more defective of the two mutants in RNA binding (Fig. 5D). Reduced enhancement of ATPase activity was seen using dsR29b, which is in good agreement with the affinity measurements (Fig. 4D). These results suggest that the portion of the protease domain that contacts RNA can also modulate ATPase activation.
3.7 NS3 protease interaction with inhibitory nucleic acids affected MAVS activity
We seek to determine whether nucleic acids could inhibit NS3 protease activity in cells. NS3 had been reported to cleave the adaptor protein MAVS (Li et al., 2005b; Love et al., 1996). MAVS can signal constitutively when overexpressed in HEK293T cells, and the signaling can be repressed by NS3 cleavage (Meylan et al., 2005). We reasoned that in the presence of nucleic acids, inhibition of NS3 protease activity would occur and MAVS-dependent signaling would increase. We measured MAVS activity through the activation of luciferase reporter expression driven by an IFN-β promoter. In addition, a TK-Renilla luciferase reporter was also assessed as a transfection and viability control.
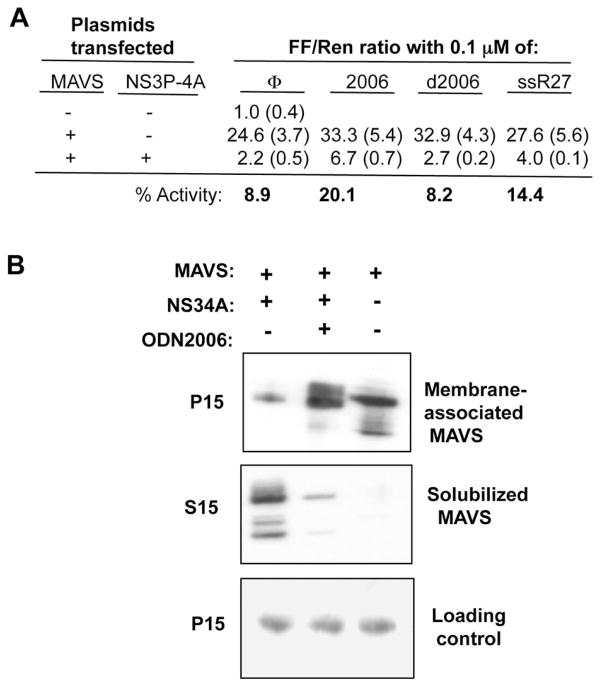
A 25-fold increase in IFN-β reporter activity was observed by the expression of MAVS relative to the control and the co-expression of NS3P-4A reduced the MAVS-dependent IFN-β reporter activity to 2.2-fold of the control (Fig. 6A). These results were consistent with previous reports (Li et al., 2005b; Meylan et al., 2005) and allowed us to examine the effects of the NS3 protease antagonists. Transfection of the NS3 inhibitor ssR27 into the cells increased MAVS-dependent INF-β signaling only modestly (Fig. 6A). We reasoned that unmodified short RNAs would rapidly turnover in cells. Therefore, we tested 2006, a phosphorothioated ssDNA that is a potent inhibitor of NS3 protease activity (Fig. 3C). 2006 present at 0.1 μM final concentration increased MAVS-dependent IFN-β reporter activity in the presence of NS3P-4A in six independent experiments, by a range of two- to three-fold above the controls (p <0.05). The modified backbone of 2006 was required for the inhibition since an unmodified 2006 named d2006 was not able to partially restore IFN-β signaling (Fig. 6A).
FIG. 6.
NS3 activities within human cell lines A) Effects of the NS3 protease antagonists on NS3 modulation of MAVS signaling in transiently transfected HEK293T cells. MAVS activity was measured through the activation of luciferase reporter expression driven by an IFN-b promoter. TK-Renilla luciferase reporter was also assessed as a transfection and viability control. Averages were taken from 6 different wells, and standard errors are shown in parenthesis. B) Effects of 2006 on cleavage of MAVS by NS3. The top and middle images are from Western blots to detect a flag-tagged MAVS in a membrane-enriched P15 fraction and the soluble S15 fraction. The bottom image contains a sample protein that served as a loading control in the Western blot membrane from the P15 fractions.
Next, we examined whether 2006 could affect the NS3-mediated proteolysis of MAVS. MAVS is usually localized to the mitochondrial membrane, but could be released into cytoplasm by NS3-4A cleavage (Lin et al., 2006). Cells expressing MAVS and NS3-4A were solubilized with nonionic detergent and the lysates centrifuged to generate a soluble fraction (S15) and membrane-enriched pellet (P15). The location of MAVS in the fractions was detected using Western blots. As is to be expected, the coexpression of NS3-4A resulted in the release of MAVS into the solubilized fraction (Fig. 6B). However, in the presence of 2006, the majority of MAVS remained in the membrane fraction. These results are consistent with our observation that 2006 can inhibit the protease activity of NS3-4A.
3.8 Effect of NS3 mutants on the HCV replicon
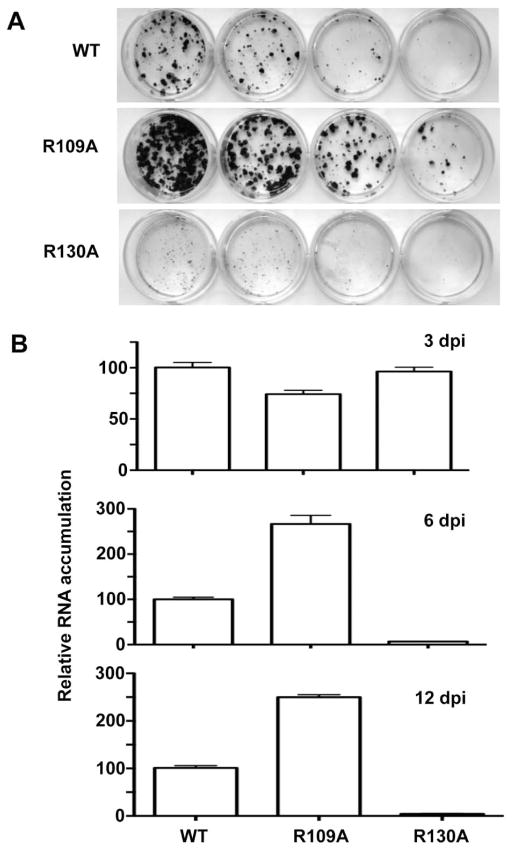
We engineered the R109A and R130A mutations into the HCV subgenomic replicon RNA and examined the consequence on HCV replication in Huh7.5 cells. The first assay used was colony formation by the neomycin-resistant HCV subgenomic replicon after three weeks of G418 selection (Fig. 7A). Mutant R130A, which was severely defective in RNA binding (Fig. 5B), was also severely reduced replicon-dependent colony formation (Hwang et al., 2000b, Fig. 6B). These results are in agreement with the previous report of Fukuda et al. (2000), who had independently tested the R130A mutant in replicons. However, to our surprise, mutant R109A, which was only modestly affected in RNA binding as a recombinant protein, enhanced the number of colonies formed (Fig. 7A).
FIG. 7.
A) Colony formation by the subgenomic replicon in Huh7.5 cells. One μg of replicon RNA was electroporated into Huh7.5 cells and plated to assess for the presence of G418-resistant colonies. Resistant colonies were stained with crystal violet after 3 weeks. The same four dilutions from the cells independently transfected with the WT and NS3 mutant replicons are shown. The results are consistent in two additional independent colony formation assays. B) Amount of replicon RNAs in Huh7.5 cells quantified by real time qRT-PCR. Total RNA extracted from replicon-containing cells at 3, 6, and 12 days post-transfection were used as indicated in the upper right corner of the graphs. The percent change of each mutant was done in triplicate and normalized to wild-type RNA levels for each set of samples.
To confirm the results from the colony formation assay, we performed semi-quantitative RT-PCR of the RNAs formed by the replicons for up to 12 days after transfection of the replicons into Huh7.5 cells. At three days, relative levels of the three replicons were fairly similar. However, by six days, the amount of RNAs for the R130A mutant decreased while the R109A mutant accumulated up to 2.5-fold higher than the WT. Similar results were observed at 9 and 12 days after transfection (Fig. 7B and data not shown). This suggests that these mutations that altered RNA binding by the NS3 protease domain to varying degrees can have both stimulatory as well as inhibitory effects on HCV RNA replication.
4. DISCUSSION
The protease and helicase activities of NS3 are both required for successful HCV infection, but are required at different times during the replication process. The NS3 protease plays a role early in the infection process in polyprotein processing and the inactivation of innate immune signaling. The NS3 helicase activity is required for RNA synthesis, which takes place after the assembly of the HCV replicase in membranous vesicles (Egger et al., 2002). The distinct roles in the chronology of a typical HCV infection should require the domains to communicate extensively during the viral infection process (Yao et al., 1999). Consistent with this idea, elegant work from Moradpour’s lab demonstrated a serial dynamic conformational coordination between NS3 protease and helicase domains allowing proper positioning of the protease active site on the membrane and accomplishing processing of the polyprotein in a sequential and spatial fashion (Brass et al., 2008).
In this study, we demonstrate that RNA, DNA, and phosphorothioate-modified ssDNAs can bind to the protease domain by using a reversible crosslinking-peptide fingerprinting assay and by a complementary amidination interference assay. These results are consistent and extend previous observations of binding by in vitro selected RNA aptamers that bind in the same region of the NS3P (Fukuda et al., 2004; Fukuda et al., 2000; Umehara et al., 2005). In fact, we showed that aptamer G9-1 can crosslink residues in/near the active site of the NS3P. G9-1 contacts a larger region of the NS3 protease active site than did the shorter ssRNAs we used. Furthermore, we observed that RNAs that are less stably structured are better inhibitors for NS3P activity while RNAs with more stable structures bind less efficiently. Since ssRNA is the product of the NS3 helicase, identifying that ssRNA are better protease inhibitors suggests that product of the helicase domain could bind to the protease active site to down regulate the protease activity.
A model where ssRNA could down regulate NS3P activity is supported by the observation that NS3 protease substrates contain several acidic residues, including an invariant aspartate at the P6 position (Fig. 1A). Another argument in support of an evolved RNA regulatory switch is that RNA binding by the protease domain increased the activation of the ATPase activity of the helicase domain. Lastly, two mutations in the active site pocket of the NS3P that affected nucleic acid binding also reduced the ability of the nucleic acids to activate ATPase activity.
The ability of ssRNA to inhibit NS3 protease activity could function in cells. Using MAVS-activated reporter expression, we observed that 2006, a modified ssDNA, could partially reverse the activity of the NS3 protease activity in HEK293T cells. Furthermore, both Hwang et al. (2000a) and we have confirmed that an alanine substitution at R130A will affect NS3P’s interaction with RNA, and that this mutation will severely affect HCV replication in cells. Interestingly, we found that the R109A mutation increases replication. The reason for this is unclear, but given that the R109A substitution resulted in only a mild decrease in RNA binding, it may be that the degree of protease binding to RNA could modulate the level of RNA accumulation.
Translation and RNA replication are mutually exclusive processes for positive-strand RNA viruses since the ribosome reads the RNA from 5′ to 3′ and the replicase reads the RNA from 3′ to 5′. A number of mechanisms have been proposed to regulate the timing of these processes in other positive-strand RNA viruses (Dahl et al., 2007; Isken et al., 2003; Jurgens et al., 2006; Yi et al., 2009a; Yi et al., 2009b). Indeed, for picornaviruses, the protease 3C is responsible for binding of the origin of PV replication (Fukuda et al., 2000; Shen et al., 2007; Yin et al., 2003). The HCV NS3 is poised to participate in both processes and our results suggest that RNA binding to the protease domain of NS3 could differentially regulate the two activities. Along with the recent observation that NS3 also participates in the assembly of HCV virions (Moradpour et al., 2007), it is likely that NS3 will prove a key regulator ensuring the proper timing of the steps in HCV RNA infection.
CONCLUSIONS
This work tested the hypothesis that the NS3 protease could bind RNA. Mapping studies with multiple RNAs demonstrated that the substrate binding pocket of the NS3 protease could bind RNA. RNAs that lacked stable secondary structures are bound better than RNAs with stable secondary structures. The addition of the RNAs to biochemical assays for the NS3 protease inhibited cleavage of peptide substrates. Furthermore, mutations in the substrate binding pocket that reduced binding to the RNA decreased both the inhibition of protease activity as well as the RNA-induced ATPase activity of the NS3 helicase. Nucleic acid inhibitors of NS3 could decrease NS3 cleavage of the innate immune adaptor, MAVS as well as affect HCV replication. The results support the notion that the single-stranded RNAs that are generated by the NS3 helicase domain could bind the NS3 protease domain and regulate the helicase and protease activities of the NS3 protein.
HIGHLIGHTS.
Mapping of RNA binding by the HCV NS3 protein.
RNA inhibitors of NS3 protease activity
Model for regulation of NS3 protease and helicase activities by single-stranded RNA.
Acknowledgments
We thank Dr. Stanley Lemon for the plasmid encoding the flag-tagged MAVS, S. Chinnaswamy for cDNAs of NS3 mutants, William Running for a tutorial on the ORBITRAP mass spectrometer, and Laura Kao for editing the manuscript.
This work was funded by the National Institute of Allergy and Infectious Diseases for grant 1RO1AI073335 to CK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beran RK, Pyle AM. Hepatitis C viral NS3-4A protease activity is enhanced by the NS3 helicase. J Biol Chem. 2008;283(44):29929–29937. doi: 10.1074/jbc.M804065200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran RK, Serebrov V, Pyle AM. The serine protease domain of hepatitis C viral NS3 activates RNA helicase activity by promoting the binding of RNA substrate. J Biol Chem. 2007;282(48):34913–34920. doi: 10.1074/jbc.M707165200. [DOI] [PubMed] [Google Scholar]
- Bhardwaj K, Guarino L, Kao CC. The severe acute respiratory syndrome coronavirus Nsp15 protein is an endoribonuclease that prefers manganese as a cofactor. J Virol. 2004;78(22):12218–12224. doi: 10.1128/JVI.78.22.12218-12224.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj K, Palaninathan S, Alcantara JM, Yi LL, Guarino L, Sacchettini JC, Kao CC. Structural and functional analyses of the severe acute respiratory syndrome coronavirus endoribonuclease Nsp15. J Biol Chem. 2008;283(6):3655–3664. doi: 10.1074/jbc.M708375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass V, Berke JM, Montserret R, Blum HE, Penin F, Moradpour D. Structural determinants for membrane association and dynamic organization of the hepatitis C virus NS3-4A complex. Proc Natl Acad Sci U S A. 2008;105(38):14545–14550. doi: 10.1073/pnas.0807298105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula S, Zolotarev AS, Stuart-Tilley AK, Wilhelm S, Shmukler BE, Brugnara C, Alper SL. Chemical crosslinking studies with the mouse Kcc1 K-Cl cotransporter. Blood cells, molecules & diseases. 2009;42(3):233–240. doi: 10.1016/j.bcmd.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari FV. Unscrambling hepatitis C virus-host interactions. Nature. 2005;436(7053):930–932. doi: 10.1038/nature04076. [DOI] [PubMed] [Google Scholar]
- Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo QL, Richman KH, Han JH, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby R, Barr PJ, et al. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G, Sandstrom A, Akerblom E, Danielson UH. Effects on protease inhibition by modifying of helicase residues in hepatitis C virus nonstructural protein 3. FEBS J. 2007;274(22):5979–5986. doi: 10.1111/j.1742-4658.2007.06120.x. [DOI] [PubMed] [Google Scholar]
- Egger D, Wolk B, Gosert R, Bianchi L, Blum HE, Moradpour D, Bienz K. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76(12):5974–5984. doi: 10.1128/JVI.76.12.5974-5984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazar M, Liu P, Rice CM, Glenn JS. An N-terminal amphipathic helix in hepatitis C virus (HCV) NS4B mediates membrane association, correct localization of replication complex proteins, and HCV RNA replication. J Virol. 2004;78(20):11393–11400. doi: 10.1128/JVI.78.20.11393-11400.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436(7053):967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- Frick DN, Rypma RS, Lam AM, Gu B. The nonstructural protein 3 protease/helicase requires an intact protease domain to unwind duplex RNA efficiently. J Biol Chem. 2004;279(2):1269–1280. doi: 10.1074/jbc.M310630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Umehara T, Sekiya S, Kunio K, Hasegawa T, Nishikawa S. An RNA ligand inhibits hepatitis C virus NS3 protease and helicase activities. Biochem Biophys Res Commun. 2004;325(3):670–675. doi: 10.1016/j.bbrc.2004.10.089. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Vishnuvardhan D, Sekiya S, Hwang J, Kakiuchi N, Taira K, Shimotohno K, Kumar PK, Nishikawa S. Isolation and characterization of RNA aptamers specific for the hepatitis C virus nonstructural protein 3 protease. Eur J Biochem. 2000;267(12):3685–3694. doi: 10.1046/j.1432-1327.2000.01400.x. [DOI] [PubMed] [Google Scholar]
- Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436(7053):939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- Gao L, Aizaki H, He JW, Lai MM. Interactions between viral nonstructural proteins and host protein hVAP-33 mediate the formation of hepatitis C virus RNA replication complex on lipid raft. J Virol. 2004;78(7):3480–3488. doi: 10.1128/JVI.78.7.3480-3488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Pruss CM, Gates AT, Khandekar SS. The RNA-unwinding activity of hepatitis C virus non-structural protein 3 (NS3) is positively modulated by its protease domain. Protein Pept Lett. 2005;12(4):315–321. doi: 10.2174/0929866053765716. [DOI] [PubMed] [Google Scholar]
- Hema M, Murali A, Ni P, Vaughan RC, Fujisaki K, Tsvetkova I, Dragnea B, Kao CC. Effects of amino-acid substitutions in the Brome mosaic virus capsid protein on RNA encapsidation. Molecular plant-microbe interactions : MPMI. 2010;23(11):1433–1447. doi: 10.1094/MPMI-05-10-0118. [DOI] [PubMed] [Google Scholar]
- Hwang J, Fauzi H, Fukuda K, Sekiya S, Kakiuchi N, Shimotohno K, Taira K, Kusakabe I, Nishikawa S. The RNA aptamer-binding site of hepatitis C virus NS3 protease. Biochem Biophys Res Commun. 2000a;279(2):557–562. doi: 10.1006/bbrc.2000.4007. [DOI] [PubMed] [Google Scholar]
- Hwang J, Fauzi H, Fukuda K, Sekiya S, Kakiuchi N, Taira K, Kusakabe I, Nishikawa S. Analysis of aptamer binding site for HCV-NS3 protease by alanine scanning mutagenesis. Nucleic acids symposium series. 2000b;(44):253–254. doi: 10.1093/nass/44.1.253. [DOI] [PubMed] [Google Scholar]
- Isken O, Grassmann CW, Sarisky RT, Kann M, Zhang S, Grosse F, Kao PN, Behrens SE. Members of the NF90/NFAR protein group are involved in the life cycle of a positive-strand RNA virus. EMBO J. 2003;22(21):5655–5665. doi: 10.1093/emboj/cdg562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo-Molina A, Ding J, Nanni RG, Clark AD, Jr, Lu X, Tantillo C, Williams RL, Kamer G, Ferris AL, Clark P, et al. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc Natl Acad Sci U S A. 1993;90(13):6320–6324. doi: 10.1073/pnas.90.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecki DJ, Beardsley RL, Reilly JP. Probing protein tertiary structure with amidination. Anal Chem. 2005;77(22):7274–7281. doi: 10.1021/ac050891z. [DOI] [PubMed] [Google Scholar]
- Jurgens CK, Barton DJ, Sharma N, Morasco BJ, Ogram SA, Flanegan JB. 2Apro is a multifunctional protein that regulates the stability, translation and replication of poliovirus RNA. Virology. 2006;345(2):346–357. doi: 10.1016/j.virol.2005.09.067. [DOI] [PubMed] [Google Scholar]
- Kim JL, Morgenstern KA, Lin C, Fox T, Dwyer MD, Landro JA, Chambers SP, Markland W, Lepre CA, O’Malley ET, Harbeson SL, Rice CM, Murcko MA, Caron PR, Thomson JA. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell. 1996;87(2):343–355. doi: 10.1016/s0092-8674(00)81351-3. [DOI] [PubMed] [Google Scholar]
- Kolykhalov AA, Mihalik K, Feinstone SM, Rice CM. Hepatitis C virus-encoded enzymatic activities and conserved RNA elements in the 3′ nontranslated region are essential for virus replication in vivo. J Virol. 2000;74(4):2046–2051. doi: 10.1128/jvi.74.4.2046-2051.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong AD, Kauffman RS, Hurter P, Mueller P. Discovery and development of telaprevir: an NS3-4A protease inhibitor for treating genotype 1 chronic hepatitis C virus. Nature biotechnology. 2011;29(11):993–1003. doi: 10.1038/nbt.2020. [DOI] [PubMed] [Google Scholar]
- Kwong AD, McNair L, Jacobson I, George S. Recent progress in the development of selected hepatitis C virus NS3.4A protease and NS5B polymerase inhibitors. Curr Opin Pharmacol. 2008;8(5):522–531. doi: 10.1016/j.coph.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Lam AM, Frick DN. Hepatitis C virus subgenomic replicon requires an active NS3 RNA helicase. J Virol. 2006;80(1):404–411. doi: 10.1128/JVI.80.1.404-411.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber MA, Running WE, Reilly JP. B. subtilis ribosomal proteins: structural homology and post-translational modifications. Journal of proteome research. 2009;8(9):4193–4206. doi: 10.1021/pr801114k. [DOI] [PubMed] [Google Scholar]
- Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M, Jr, Lemon SM. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A. 2005a;102(8):2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci U S A. 2005b;102(49):17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Lacoste J, Nakhaei P, Sun Q, Yang L, Paz S, Wilkinson P, Julkunen I, Vitour D, Meurs E, Hiscott J. Dissociation of a MAVS/IPS-1/VISA/Cardif-IKKepsilon molecular complex from the mitochondrial outer membrane by hepatitis C virus NS3-4A proteolytic cleavage. J Virol. 2006;80(12):6072–6083. doi: 10.1128/JVI.02495-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Reilly JP. Correlating the chemical modification of Escherichia coli ribosomal proteins with crystal structure data. Journal of proteome research. 2009;8(10):4466–4478. doi: 10.1021/pr9002382. [DOI] [PubMed] [Google Scholar]
- Love RA, Parge HE, Wickersham JA, Hostomsky Z, Habuka N, Moomaw EW, Adachi T, Hostomska Z. The crystal structure of hepatitis C virus NS3 proteinase reveals a trypsin-like fold and a structural zinc binding site. Cell. 1996;87(2):331–342. doi: 10.1016/s0092-8674(00)81350-1. [DOI] [PubMed] [Google Scholar]
- Mackintosh SG, Lu JZ, Jordan JB, Harrison MK, Sikora B, Sharma SD, Cameron CE, Raney KD, Sakon J. Structural and biological identification of residues on the surface of NS3 helicase required for optimal replication of the hepatitis C virus. J Biol Chem. 2006;281(6):3528–3535. doi: 10.1074/jbc.M512100200. [DOI] [PubMed] [Google Scholar]
- Mann B, Madera M, Sheng Q, Tang H, Mechref Y, Novotny MV. ProteinQuant Suite: a bundle of automated software tools for label-free quantitative proteomics. Rapid Commun Mass Spectrom. 2008;22(23):3823–3834. doi: 10.1002/rcm.3781. [DOI] [PubMed] [Google Scholar]
- Mederacke I, Wedemeyer H, Manns MP. Boceprevir, an NS3 serine protease inhibitor of hepatitis C virus, for the treatment of HCV infection. Curr Opin Investig Drugs. 2009;10(2):181–189. [PubMed] [Google Scholar]
- Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437(7062):1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5(6):453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- Niranjanakumari S, Lasda E, Brazas R, Garcia-Blanco MA. Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions in vivo. Methods. 2002;26(2):182–190. doi: 10.1016/S1046-2023(02)00021-X. [DOI] [PubMed] [Google Scholar]
- Pang PS, Jankowsky E, Planet PJ, Pyle AM. The hepatitis C viral NS3 protein is a processive DNA helicase with cofactor enhanced RNA unwinding. EMBO J. 2002;21(5):1168–1176. doi: 10.1093/emboj/21.5.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Running WE, Reilly JP. Ribosomal proteins of Deinococcus radiodurans: their solvent accessibility and reactivity. Journal of proteome research. 2009;8(3):1228–1246. doi: 10.1021/pr800544y. [DOI] [PubMed] [Google Scholar]
- Shen M, Wang Q, Yang Y, Pathak HB, Arnold JJ, Castro C, Lemon SM, Cameron CE. Human rhinovirus type 14 gain-of-function mutants for oriI utilization define residues of 3C(D) and 3Dpol that contribute to assembly and stability of the picornavirus VPg uridylylation complex. J Virol. 2007;81(22):12485–12495. doi: 10.1128/JVI.00972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Taliani M, Bianchi E, Narjes F, Fossatelli M, Urbani A, Steinkuhler C, De Francesco R, Pessi A. A continuous assay of hepatitis C virus protease based on resonance energy transfer depsipeptide substrates. Anal Biochem. 1996;240(1):60–67. doi: 10.1006/abio.1996.0331. [DOI] [PubMed] [Google Scholar]
- Tan SL, Pause A, Shi Y, Sonenberg N. Hepatitis C therapeutics: current status and emerging strategies. Nat Rev Drug Discov. 2002;1(11):867–881. doi: 10.1038/nrd937. [DOI] [PubMed] [Google Scholar]
- Taremi SS, Beyer B, Maher M, Yao N, Prosise W, Weber PC, Malcolm BA. Construction, expression, and characterization of a novel fully activated recombinant single-chain hepatitis C virus protease. Protein Sci. 1998;7(10):2143–2149. doi: 10.1002/pro.5560071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara T, Fukuda K, Nishikawa F, Kohara M, Hasegawa T, Nishikawa S. Rational design of dual-functional aptamers that inhibit the protease and helicase activities of HCV NS3. Journal of biochemistry. 2005;137(3):339–347. doi: 10.1093/jb/mvi042. [DOI] [PubMed] [Google Scholar]
- Urvil PT, Kakiuchi N, Zhou DM, Shimotohno K, Kumar PK, Nishikawa S. Selection of RNA aptamers that bind specifically to the NS3 protease of hepatitis C virus. Eur J Biochem. 1997;248(1):130–138. doi: 10.1111/j.1432-1033.1997.t01-1-00130.x. [DOI] [PubMed] [Google Scholar]
- Vaughan R, Running WE, Qi R, Kao C. Mapping protein-RNA interactions. Virus Adaptation and Treatment. 2012;4(1):29–41. [Google Scholar]
- Wang LC, Okitsu CY, Kochounian H, Rodriguez A, Hsieh CL, Zandi E. A simple and inexpensive on-column frit fabrication method for fused-silica capillaries for increased capacity and versatility in LC-MS/MS applications. Proteomics. 2008;8(9):1758–1761. doi: 10.1002/pmic.200700931. [DOI] [PubMed] [Google Scholar]
- Yan Y, Li Y, Munshi S, Sardana V, Cole JL, Sardana M, Steinkuehler C, Tomei L, De Francesco R, Kuo LC, Chen Z. Complex of NS3 protease and NS4A peptide of BK strain hepatitis C virus: a 2.2 A resolution structure in a hexagonal crystal form. Protein Sci. 1998;7(4):837–847. doi: 10.1002/pro.5560070402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N, Reichert P, Taremi SS, Prosise WW, Weber PC. Molecular views of viral polyprotein processing revealed by the crystal structure of the hepatitis C virus bifunctional protease-helicase. Structure. 1999;7(11):1353–1363. doi: 10.1016/s0969-2126(00)80025-8. [DOI] [PubMed] [Google Scholar]
- Yi G, Letteney E, Kim CH, Kao CC. Brome mosaic virus capsid protein regulates accumulation of viral replication proteins by binding to the replicase assembly RNA element. RNA. 2009a;15(4):615–626. doi: 10.1261/rna.1375509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G, Vaughan RC, Yarbrough I, Dharmaiah S, Kao CC. RNA binding by the brome mosaic virus capsid protein and the regulation of viral RNA accumulation. J Mol Biol. 2009b;391(2):314–326. doi: 10.1016/j.jmb.2009.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Ma Y, Yates J, Lemon SM. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J Virol. 2007;81(2):629–638. doi: 10.1128/JVI.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Paul AV, Wimmer E, Rieder E. Functional dissection of a poliovirus cis-acting replication element [PV-cre(2C)]: analysis of single- and dual-cre viral genomes and proteins that bind specifically to PV-cre RNA. J Virol. 2003;77(9):5152–5166. doi: 10.1128/JVI.77.9.5152-5166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Cai Z, Kim YC, Kumar R, Yuan F, Shi PY, Kao C, Luo G. Stimulation of hepatitis C virus (HCV) nonstructural protein 3 (NS3) helicase activity by the NS3 protease domain and by HCV RNA-dependent RNA polymerase. J Virol. 2005;79(14):8687–8697. doi: 10.1128/JVI.79.14.8687-8697.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31(13):3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]