Abstract
Background
We investigated the impact of breast cancer molecular subtypes and treatment on survival in a cohort of medically insured women followed for over twenty years.
Methods
We examined 934 female members of an integrated health care delivery system newly diagnosed with invasive breast cancer between 1988 and 1995 and followed them through 2008. Tumors were classified into four molecular subtypes based on their expression profile: luminal A; luminal B; basal-like; and HER2-enriched. We followed women from the surgery date to death, health plan disenrollment, or study’s end. Hazard rate ratios (HR) and 95% confidence intervals (CI) were fit using Cox proportional hazards models adjusting for cancer treatments and tumor characteristics.
Results
A total of 223 (23.9%) women died due to breast cancer during the 21-year study period. Compared to women with luminal A tumors, women with HER2-enriched (HR 2.56, 95% CI 1.53–4.29) and luminal B tumors (HR 1.96, 95% CI: 1.08–3.54) had roughly a two-fold increased adjusted risk of breast cancer mortality. In addition, the survival curves suggest that risk of late mortality persists in women with luminal A tumors.
Conclusion
Among women with healthcare coverage, molecular subtypes were important predictors of breast cancer mortality. Women with HER2-enriched tumors and luminal B subtypes had the poorest survival despite adjusting for important covariates.
Impact
In a cohort followed over 20 years, women with HER2 enriched tumors had worse survival, but interestingly, the survival curve for women with luminal A tumors continued to steadily decline after 10 years of follow-up.
Keywords: breast cancer, biologic subtypes, cancer treatment, survival, cohort
INTRODUCTION
Breast cancer will be responsible for nearly 39,510 deaths among women in 2012 in the U.S. [1]. Breast cancer is a heterogeneous disease and several biologic subtypes have been identified [2]. As conventional clinical factors such as tumor grade, size, lymph node involvement, and surgical margins are no longer sufficient as sole prognostic factors, it is important to consider breast cancer subtypes in treatment decision making. Limited knowledge exists if effectiveness of adjuvant treatment varies by subtype and how biologic subtypes affect long-term prognosis. Four main major breast cancer subtypes have been identified based on the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). These subtypes include luminal types A and B, basal-like and HER2-enriched [3]. Luminal A is the most common breast cancer subtype and is characterized by ER+ and/or PR+/HER2- status, low grade tumors, and good prognosis [4–6]. Luminal B subtype accounts for roughly 10% of all breast cancer and is distinguished by ER+ and/or PR+/HER2+ status. Breast cancer subtypes with negative ER, PR, and HER2 status are typically called “triple negative” breast cancers and approximate the basal-like category. The basal-like subtype is more common in pre-menopausal, younger, overweight, African American women and is associated with high grade tumors [4, 6, 7]. The HER2-enriched subtype (HER2+/ER−/PR−) is less common but is similarly characterized by high-grade tumors and poor outcomes [4, 5].
Most of the prior studies that examined survival by molecular subtypes lacked treatment data [2], and even fewer have examined long-term survival [8–12]. Moreover, variations in laboratory methods have made it difficult to make meaningful conclusions. Therefore, the goal of the current study was to examine the impact of breast cancer subtypes and treatment on long-term survival considering important covariates. We conducted a population-based cohort study of women diagnosed with invasive breast cancer who were members of a managed care organization in southern California and examined their survival over a 21 year period.
MATERIALS AND METHODS
Patients and Setting
The cohort included women diagnosed with invasive breast cancer (AJCC TNM stages I-IV) from January 1, 1988 to December 31, 1995 in a large integrated health care delivery system, Kaiser Permanente Southern California, San Diego (KPSC), and followed through December 31, 2008. We identified patients through the health plan’s National Cancer Institute SEER (Surveillance Epidemiology and End Results)-affiliated cancer registry. Eligible patients included those who completed their first course of treatment within the health plan. We identified a total of 1645 women aged 25 to 79 years. We excluded 66 women who did not have medical charts, 96 women with missing information on cause of death, and 549 women with missing tumor tissue or ER/PR/HER2 status. The final cohort consisted of 934 women. The design of this study has been detailed previously [13].
Data elements
Data elements were ascertained from medical charts, the electronic cancer registry and mortality databases. Date of death and cause (main outcome) were ascertained by linking our cohort with in-patient mortality files and California’s master file of death certificates. Cause of death was confirmed by medical chart review. The main exposure variables included primary tumor treatment and biologic subtype. Treatment information of the primary tumor (type of surgery, chemotherapy dates, hormonal therapy, radiotherapy) was abstracted from medical records. Biologic subtypes were determined by immunohistochemical (IHC) assays of ER, PR and HER2 markers of archived formalin fixed paraffin embedded tumor tissue. The HER2/neu proto-oncogene was also assessed for gene amplification by fluorescence in situ hybridization (FISH). The IHC and FISH assays were conducted at a single laboratory at the University of Southern California (USC) under the supervision of one of the authors (MFP) according to methods previously described [14–17]. Patients were classified into four main biologic subtypes based on previously published categories: luminal A (ER+ and/or PR+/HER2−), luminal B (ER+ and/or PR+/HER+), basal-like (ER−/PR−/HER−, “triple negative”), or HER2-enriched (HER2+/ER−/PR−) [2–3].
Covariates extracted from the patients’ charts included demographic and health factors (age at diagnosis, year of diagnosis, race/ethnicity, family history of breast cancer in a first degree relative, menopausal status at diagnosis, body mass index at diagnosis, history of tobacco use and number of live births). We also extracted tumor characteristics including grade, TNM stage, lymph node status, surgical margins and histopathology. The Institutional Review Boards (IRB) of KPSC and USC reviewed and approved the study.
Statistical analyses
Differences in demographic, health and tumor characteristics, and primary treatments by breast cancer subtype were first examined using chi-square and Fisher exact tests. P-values were two sided, and were considered significant if less than 0.05. We also examined mortality by breast cancer subtypes, stratified by stage at diagnosis. Follow-up commenced on the date of surgery (1988–1995) and ended on the date of death, termination of health plan membership, or study’s end (December 31, 2008), whichever occurred first. Although some women disenrolled from the health plan, we were able to obtain their date and cause of death by linking their social security number with the state’s electronic mortality files. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated fitting Cox proportional hazards models with time dependent treatment variables (i.e., 0 up to start date; 1 after start date), and adjusted for stage and age at diagnosis, year of diagnosis, menopausal status, lymph node status and treatment regimen. Kaplan-Meier methods were used to compare breast cancer and other cause mortality by subtype.
RESULTS
Demographic and health characteristics of the 934 study participants are listed in Table 1. Study participants were followed a maximum of 21 years (median of 13.3 years, range 0.1–21.0 years), and had a median age of 59 years at diagnosis (range of 25 to 79 years). The most common breast cancer subtype was luminal A (66%), followed by basal-like (22%), HER2-enriched (7%), and luminal B (5%). The majority of women were white non-Hispanic (86%), and roughly one-third was diagnosed with breast cancer before the age of 50 years (32%). Race varied by biologic subtype. Although non-white women comprised less than 15% of the cohort, they were diagnosed with nearly one-quarter of all luminal B cancers and more than one-fifth of all HER2-enriched cancers. In the luminal B category, a larger proportion was premenopausal at diagnosis (52.5%) than postmenopausal (47.5%). There were no significant differences in body mass index (BMI) categories, history of tobacco use, family history of breast cancer in first degree relative, or number of live births by molecular subtype.
Table 1.
Demographic and health characteristics of women at diagnosis by breast cancer subtype
| Luminal A (N=615) | Luminal B (N=42) | Basal-like (N=210) | HER2-enriched (N=67) | Total (N=934) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N | % | N | % | N | % | N | % | N | % | |
| Year of diagnosis | ||||||||||
| 1988–1989 | 90 | 14.6 | 4 | 9.5 | 34 | 16.2 | 9 | 13.4 | 137 | 14.7 |
| 1990–1991 | 124 | 20.2 | 12 | 28.6 | 39 | 18.6 | 10 | 14.9 | 185 | 19.8 |
| 1992–1993 | 191 | 31.1 | 16 | 38.1 | 68 | 32.4 | 18 | 26.9 | 293 | 31.4 |
| 1994–1995 | 210 | 34.1 | 10 | 23.8 | 69 | 32.9 | 30 | 44.8 | 319 | 34.2 |
| P Valuea=0.48 | ||||||||||
| Age at diagnosis (y) | ||||||||||
| <50 | 163 | 26.5 | 24 | 57.1 | 81 | 38.6 | 26 | 38.8 | 294 | 31.5 |
| 50–59 | 120 | 19.5 | 6 | 14.3 | 54 | 25.7 | 18 | 26.9 | 198 | 21.2 |
| 60–69 | 187 | 30.4 | 11 | 26.2 | 47 | 22.4 | 18 | 26.9 | 263 | 28.2 |
| >70 | 145 | 23.6 | 1 | 2.4 | 28 | 13.3 | 5 | 7.5 | 179 | 19.2 |
| P Valuea <0.0001 | ||||||||||
| Race | ||||||||||
| White | 540 | 87.8 | 32 | 76.2 | 177 | 84.3 | 53 | 79.1 | 802 | 85.9 |
| Other | 75 | 12.2 | 10 | 23.8 | 33 | 15.7 | 14 | 20.9 | 132 | 14.1 |
| P Valuea= 0.044 | ||||||||||
| Family history of breast cancerb | ||||||||||
| Yes | 110 | 17.9 | 9 | 21.4 | 33 | 15.7 | 9 | 13.4 | 161 | 17.2 |
| No | 416 | 67.6 | 29 | 69.0 | 148 | 70.5 | 50 | 74.6 | 643 | 68.8 |
| Unknown | 89 | 14.5 | 4 | 9.5 | 29 | 13.8 | 8 | 11.9 | 130 | 13.9 |
| P Valuea=0.62 | ||||||||||
| Menopausal status | ||||||||||
| Pre | 170 | 31.3 | 21 | 52.5 | 81 | 44.0 | 22 | 37.9 | 294 | 35.6 |
| Post | 373 | 68.7 | 19 | 47.5 | 103 | 56.0 | 36 | 62.1 | 531 | 64.4 |
| P Valuea =0.0017 | ||||||||||
| BMI (kg/m2) | ||||||||||
| <18.5 | 8 | 1.3 | 1 | 2.4 | 4 | 1.9 | 2 | 3.0 | 15 | 1.6 |
| 18.5–24.9 | 213 | 34.6 | 14 | 33.3 | 74 | 35.2 | 15 | 22.4 | 316 | 33.8 |
| 25–29.9 | 172 | 28.0 | 14 | 33.3 | 62 | 29.5 | 28 | 41.8 | 276 | 29.6 |
| ≥30 | 140 | 22.8 | 8 | 19.0 | 43 | 20.5 | 13 | 19.4 | 204 | 21.8 |
| Unknown | 82 | 13.3 | 5 | 11.9 | 27 | 12.9 | 9 | 13.4 | 123 | 13.2 |
| P Valuea=0.42 | ||||||||||
| Smoking history | ||||||||||
| Never | 284 | 46.2 | 19 | 45.2 | 107 | 51.0 | 35 | 52.2 | 445 | 47.6 |
| Smoker at dx | 94 | 15.3 | 8 | 19.0 | 21 | 10.0 | 7 | 10.4 | 130 | 13.9 |
| Former smoker | 134 | 21.8 | 8 | 19.0 | 53 | 25.2 | 14 | 20.9 | 209 | 22.4 |
| Non-smoker | 36 | 5.9 | 4 | 9.5 | 8 | 3.8 | 4 | 6.0 | 52 | 5.6 |
| Unknown | 67 | 10.9 | 3 | 7.1 | 21 | 10.0 | 7 | 10.4 | 98 | 10.5 |
| P Valuea=0.43 | ||||||||||
| Live births | ||||||||||
| 0 | 78 | 12.7 | 7 | 16.7 | 32 | 15.2 | 11 | 16.4 | 128 | 13.7 |
| ≥1 | 464 | 75.4 | 32 | 76.2 | 148 | 70.5 | 49 | 73.1 | 693 | 74.2 |
| Unknown | 73 | 11.9 | 3 | 7.1 | 30 | 14.3 | 7 | 10.4 | 113 | 12.1 |
| P Valuea=0.63 | ||||||||||
P values based on chi-square test. Unknown data were excluded in calculation of P Value. For variables with categories <5, P values are based on the Fisher exact test.
History of breast cancer in first degree blood relative (mother, sister or daughter).
Table 2 displays the distribution of tumor characteristics by molecular subtype. Overall, the majority of study participants were diagnosed with early stage breast cancer (TNM stages I–II) (90%). Although numbers were small, a larger proportion of women with HER2-enriched tumors was diagnosed with late stage disease (TNM III–IV, about 16.4%). Women with luminal B and HER2-enriched tumors were most likely to have positive lymph nodes. Compared to luminal A, women with luminal B, basal-like and HER2-enriched subtypes were more likely to have higher grade tumors (P<0.0001). Nearly all women (93% overall) had no residual tumor, and as expected, surgical margin status was not associated with subtype (P=0.66). Roughly 8% (n=78) of the cohort had invasive lobular carcinoma (ILC) histopathology and 5% (n=49) had mixed histopathology. Histopathology was related to molecular subtype; the fraction of women with IDC was highest in women with basal-like tumors and lowest in women with luminal A tumors, while the fraction of women with ILC was highest in women with luminal A tumors and lowest in HER2-enriched tumors (P=0.05).
Table 2.
Tumor characteristics by breast cancer subtype
| Luminal A (N=615) | Luminal B (N=42) | Basal-like (N=210) | HER2-enriched (N=67) | Total (N=934) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N | % | N | % | N | % | N | % | N | % | |
| Grade | ||||||||||
| 1 | 98 | 15.9 | 0 | 0 | 8 | 3.8 | 0 | 0 | 106 | 11.3 |
| 2 | 460 | 74.8 | 23 | 54.8 | 83 | 39.5 | 28 | 41.8 | 594 | 63.6 |
| 3 | 54 | 8.8 | 19 | 45.2 | 118 | 56.2 | 38 | 56.7 | 229 | 24.5 |
| Unknown | 3 | 0.5 | 0 | 0 | 1 | 0.5 | 1 | 1.5 | 5 | 0.5 |
| P Valuea<0.0001 | ||||||||||
| TNM Stage | ||||||||||
| 1 | 340 | 55.3 | 15 | 35.7 | 82 | 39.0 | 19 | 28.4 | 456 | 48.8 |
| 2 | 216 | 35.1 | 21 | 50.0 | 107 | 51.0 | 37 | 55.2 | 381 | 40.8 |
| 3 | 28 | 4.6 | 3 | 7.1 | 13 | 6.2 | 7 | 10.4 | 51 | 5.5 |
| 4 | 23 | 3.7 | 1 | 2.4 | 7 | 3.3 | 4 | 6.0 | 35 | 3.7 |
| Unknown | 8 | 1.3 | 2 | 4.8 | 1 | 0.5 | 0 | 0 | 11 | 1.2 |
| P Valuea<0.0001 | ||||||||||
| Lymph Nodes | ||||||||||
| Negative | 374 | 60.8 | 20 | 47.6 | 128 | 61.0 | 28 | 41.8 | 550 | 58.9 |
| Positive | 207 | 33.7 | 18 | 42.9 | 71 | 33.8 | 33 | 49.3 | 329 | 35.2 |
| Unknown | 34 | 5.5 | 4 | 9.5 | 11 | 5.2 | 6 | 9.0 | 55 | 5.9 |
| P Valuea=0.019 | ||||||||||
| Surgical Margins | ||||||||||
| No residual tumor | 570 | 92.7 | 39 | 92.9 | 193 | 91.9 | 63 | 94.0 | 865 | 92.6 |
| Microscopic residual tumor | 32 | 5.2 | 1 | 2.4 | 10 | 4.8 | 4 | 6.0 | 47 | 5.0 |
| Margins not evaluable | 3 | 0.5 | 1 | 2.4 | 3 | 1.4 | 0 | 0 | 7 | 0.7 |
| No primary site surgery | 4 | 0.7 | 1 | 2.4 | 2 | 1.0 | 0 | 0 | 7 | 0.7 |
| Unknown | 6 | 1.0 | 0 | 0.0 | 2 | 1.0 | 0 | 0 | 8 | 0.9 |
| P Valuea=0.66 | ||||||||||
| Histopathology | ||||||||||
| IDCb | 521 | 84.7 | 37 | 88.1 | 192 | 91.4 | 57 | 85.1 | 807 | 86.4 |
| ILCb | 61 | 9.9 | 2 | 4.8 | 12 | 5.7 | 3 | 4.5 | 78 | 8.4 |
| Unknown/Other | 33 | 5.4 | 3 | 7.1 | 6 | 2.9 | 7 | 10.4 | 49 | 5.2 |
| P Valuea=0.056 | ||||||||||
P values based on chi-square test. Unknown excluded in calculation of P Value. For variables with categories <5, P values are based on the Fisher exact test.
IDC=Invasive ductal carcinoma. ILC=Invasive lobular carcinoma.
Table 3 displays molecular subtype by treatment. While all women in the cohort underwent surgery (mastectomy or breast conserving surgery) for primary treatment of the initial breast cancer diagnosis, use of adjuvant treatment varied across breast cancer subtype. Overall, tamoxifen was the most common adjuvant treatment (48%, n=445), followed by chemotherapy (47%, n=439) and radiotherapy (39%, n=360). As expected, women with breast cancer subtypes that included ER+ or PR+ status (luminal A and luminal B) were more likely to use tamoxifen. Women with luminal B tumors were more likely to receive radiation (19.3%). Women with HER2-enriched tumors more often underwent chemotherapy (about 33%).
Table 3.
Receipt of treatment for initial breast cancer diagnosis in the cohort of 934 women by breast cancer molecular subtype
| Luminal A (N=615) | Luminal B (N=42) | Basal-like (N=210) | HER2- enriched (N=67) | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Treatmenta | ||||||||
| Surgery | 615 | 42.4 | 42 | 38.5 | 210 | 44.7 | 67 | 44.9 |
| Radiation | 252 | 17.4 | 21 | 19.3 | 69 | 14.7 | 18 | 12.1 |
| Chemotherapy | 231 | 15.9 | 23 | 21.1 | 136 | 28.9 | 49 | 32.9 |
| Tamoxifen | 352 | 24.3 | 23 | 21.1 | 55 | 11.7 | 15 | 10.1 |
Column percent’s exceed 100% due to multiple treatments
Table 4 presents the crude and adjusted hazard ratios (HR) for the association between breast cancer mortality and molecular subtype. Of the 934 women, 23.9% died due to breast cancer in the ensuing 21 years; 19.8% women died due to other causes; 16.6% disenrolled from the health plan; and 39.7% completed follow-up (data not shown). Treatment groups were entered as indicator variables in the multivariable models. The multivariable model included age at diagnosis, year of first breast cancer diagnosis, menopausal status, lymph node status and, for the “All stages” analysis, stage at diagnosis, as covariates. Breast cancer mortality was two-fold greater in women with luminal B (HR 1.96, 95% CI: 1.08–3.54) and HER2-enriched tumors (HR 2.56, 95% CI: 1.53–4.29) compared with women with the luminal A subtype (the referent group) when examining all stages combined. However, when examining the hazard ratios stratified by stage, the association between mortality and luminal B and HER2-enriched subtype was stronger among women with stage I disease (i.e., mortality was seven-fold higher in women with luminal B [HR 7.39, 95% CI: 1.72–31.77] and HER2-enriched [HR 6.62, 95% CI: 1.78–24.57] subtypes in comparison to women with luminal A tumors), but the confidence intervals were wide. Although we found elevated mortality for basal-like tumors, the confidence interval included the null (HR 1.20, 95% CI: 0.80–1.82 for all stages combined). The association for basal-like tumors was similar when examining the effects by stage. Data were sparse in the higher-stage categories (III-IV).
Table 4.
Hazard ratios for breast cancer mortality stratified by stage and major breast cancer subtypes (N=934)
| Alive or Died of Other Causes N |
Died of Breast Cancer N |
Univariate HR | 95% CI | Adjusted HRa | 95% CI | |
|---|---|---|---|---|---|---|
| All Stages | ||||||
| Luminal A | 490 | 128 | 1.00 | 1.00 | ||
| Luminal B | 25 | 15 | 2.01 | 1.18–3.42 | 1.96 | 1.08–3.54 |
| Basal Like | 157 | 52 | 1.30 | 0.94–1.79 | 1.20 | 0.80–1.82 |
| HER2-enriched | 41 | 26 | 2.39 | 1.57–3.64 | 2.56 | 1.53–4.29 |
| Stage 1 | ||||||
| Luminal A | 316 | 30 | 1.00 | 1.00 | ||
| Luminal B | 12 | 3 | 2.38 | 0.73–7.81 | 7.39 | 1.72–31.77 |
| Basal Like | 72 | 10 | 1.37 | 0.67–2.79 | 1.61 | 0.54–4.81 |
| HER2-enriched | 15 | 4 | 2.90 | 1.02–8.26 | 6.62 | 1.78–24.57 |
| Stage 2 | ||||||
| Luminal A | 156 | 65 | 1.00 | 1.00 | ||
| Luminal B | 12 | 9 | 1.84 | 0.92–3.69 | 2.23 | 1.01–4.96 |
| Basal Like | 78 | 29 | 1.06 | 0.68–1.64 | 1.22 | 0.71–2.11 |
| HER2-enriched | 25 | 12 | 1.26 | 0.68–2.33 | 1.85 | 0.89–3.85 |
| Stage 3–4 | ||||||
| Luminal A | 18 | 33 | 1.00 | 1.00 | ||
| Luminal B | 1 | 3 | 1.37 | 0.42–4.48 | 0.76 | 0.17–3.39 |
| Basal Like | 7 | 13 | 1.49 | 0.78–2.84 | 0.94 | 0.31–2.86 |
| HER2-enriched | 1 | 10 | 3.47 | 1.68–7.19 | 1.11 | 0.25–4.89 |
Hazard ratios are adjusted for age at diagnosis, years of first breast cancer diagnosis, menopausal status, lymph node status, stage at diagnosis, and treatment regimens.
Note: 7 dichotomous variables were created to represent eight breast cancer treatment categories:
1) surgery+radiation+chemotherapy+tamoxifen; 2) surgery+radiation+chemotherapy; 3) surgery+radiation+tamoxifen; 4) surgery+ chemotherapy+tamoxifen; 5) surgery+radiation; 6) surgery+chemotherapy; 7) surgery+ tamoxifen; 8) surgery only.
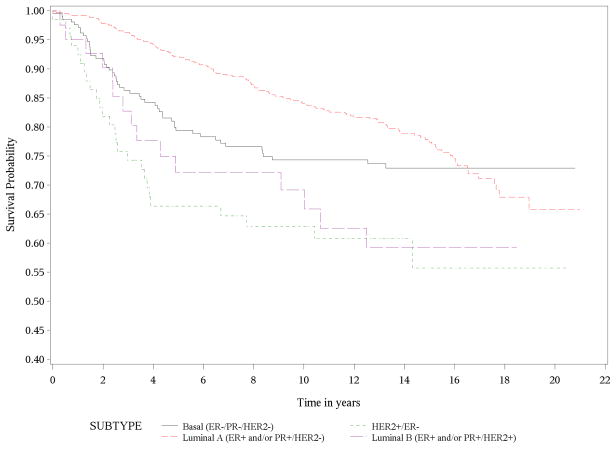
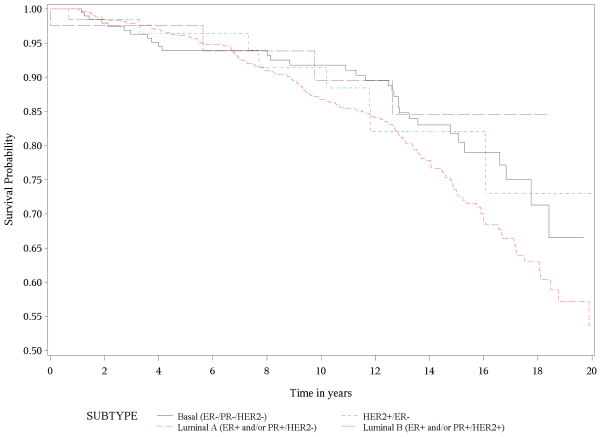
Figure 1 shows the Kaplan-Meier survival curves for breast cancer specific mortality. Women with luminal A tumors had the longest survival, while women with HER2-enriched and luminal B tumors had much shorter survival times (P < 0.0001). Women with basal-like tumors had intermediate survival times, with deaths occurring earlier than women with luminal A tumors. Survival declined precipitously during the first 3 to 4 years of follow-up for both HER2+ subtypes (HER2-enriched and luminal B), followed by a slowing in the decline over subsequent years of follow-up. The basal-like subtype showed a similar early decline over the first 2 to 2.5 years with a more gradual decline to about 13 years of follow-up. Interestingly, the curve for luminal A continues to decline steadily after 10 years of follow-up suggesting that the risk of late mortality persists in this group. As expected, Figure 2 demonstrates that breast cancer subtype had no impact on death due to all other causes of mortality (P=0.16).
Figure 1.
Kaplan-Meier Survival Curve for Breast Cancer Mortality
Figure 2.
Kaplan-Meier Survival Curve for Other Causes of Mortality
DISCUSSION
In this cohort of nearly one thousand women followed a maximum of 21 years, we determined that overall, women with HER2-enriched and luminal B tumors had a two-fold increased adjusted risk of breast cancer mortality compared to women with luminal A tumors; these risks were seen after accounting for adjuvant treatments and other important covariates. These results are consistent with previous findings showing that women with HER2-enriched breast cancers have worse prognosis than those with luminal A tumors, although they were based on much shorter follow-up times [2]. It is possible that aromatase inhibitors might have improved survival in this group; however, the drug was not available until the mid-2000s. It is also possible that the women with luminal B or HER2-enriched tumors died earlier than other patients because of unavailability of trastuzumab at that time, which was approved by the FDA for adjuvant treatment in 2005. The survival curve analysis (Figure 1) also suggests that risk of late breast-cancer specific mortality persists women with luminal A tumors even after 10 years of follow-up. In addition, although previous studies focused on women with the more common basal-like subtype and reported poorer outcomes among those women compared to women with luminal A tumors, our study indicated reduced survival among women with luminal B and HER2-enriched tumors.
Our study has a number of strengths. As women for this study were identified through a large community-based health care delivery system in southern California, results may be more applicable to the wider community than studies which have drawn subjects from academic settings. In addition, the care the patients received should reflect the general cancer treatment patients received in other integrated delivery systems in the U.S at that time. Unlike other studies that followed patients five to ten years [2, 5], the managed care setting afforded a rare opportunity for very long-term follow up of breast cancer patients. Health plan membership sustainment was high, with more than 4 of every 5 members continuing membership until either death or the end of the 21 year follow-up period. Furthermore, we minimized bias due to loss of follow-up by ascertaining mortality status of all patients, regardless of disenrollment status. While others found reduced breast cancer survival due to poor healthcare access and lack of insurance coverage [18–23], we were able to examine differences in survival without the confounding effects of variable medical insurance coverage [24].
Certain limitations of the study must also be considered. Although we mainly examined IHC markers, which may misclassify subtypes, the use of IHC is more common in general community hospitals. Moreover, other studies have demonstrated the concordance of the IHC and gene expression profiles to assess subtype [5, 8, 9]. Another limitation was the lack of treatment data for recurrences. However, because the cohort consisted of a fully insured population with long-term membership sustainment, it is unlikely that survival rates by biologic subtype were highly dependent on treatment for recurrences. Because the cohort was assembled before the availability of trastuzumab and AIs, , these results more closely reflect the natural history of the disease in the absence of these targeted therapies, but they may not be generalizable to current practices. However, given the high costs of trastuzumab (roughly $100,000 per course) and AIs (up to $5,000 per month), these therapies may not be accessible to all breast cancer survivors. Another challenge was the small cell sizes in some of the analyses due to low numbers of deaths. Also, as staging definitions were slightly different in the mid-1990s, it is possible that some of the cases would have actually been categorized as having a higher TMN stage. In addition, the greater incidence of early stage disease in this insured cohort may be due to greater access to screening. Although we captured the types of cancer treatments, we did not have the data to quantify dose or duration.
In summary, our results extend the findings of prior studies given our long observation period. While most survival studies are limited to 5 to 10 years of follow-up, we followed cohort members over 20 years, revealing distinct changes in survival patterns by subtypes. Despite its markedly higher survival probabilities in earlier years of follow-up, luminal A subtype was the only subtype that continued a steady drop in survival over the 20 year period with little leveling off in later years. Future studies should examine how the association between molecular subtypes and survival varies by race/ethnicity, particularly in minority women who are more likely to have aggressive tumor subtypes, as well as identify factors to enhance survival in women with luminal A tumors.
Acknowledgments
The authors would like to thank Angela Santiago, Roberta Guzman and Ivonne Villalobos at USC for technical assistance with biomarker assays and data management.
GRANT SUPPORT
The original cohort study was funded through the California Breast Cancer Research Program (SME, MFP). Additional work was supported by NIH/NCI R01CA136743 (RH) and the Breast Cancer Research Foundation (MFP).
Footnotes
Financial disclosures: None
References
- 1.American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Perou CM, Borresen-Dale AL. Systems Biology and Genomics of Breast Cancer. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 7.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang HR, Glaspy J, Allison MA, Kass FC, Elashoff R, Chung DU, et al. Differential response of triple-negative breast cancer to a docetaxel and carboplatin-based neoadjuvant treatment. Cancer. 2010;116:4227–37. doi: 10.1002/cncr.25309. [DOI] [PubMed] [Google Scholar]
- 9.Yerushalmi R, Hayes MM, Gelmon KA, Chia S, Bajdik C, Norris B, et al. A phase II trial of a neoadjuvant platinum regimen for locally advanced breast cancer: pathologic response, long-term follow-up, and correlation with biomarkers. Clin Breast Cancer. 2009;9:166–72. doi: 10.3816/CBC.2009.n.027. [DOI] [PubMed] [Google Scholar]
- 10.Gabos Z, Thoms J, Ghosh S, Hanson J, Deschênes J, Sabri S, et al. The association between biological subtype and locoregional recurrence in newly diagnosed breast cancer. Breast Cancer Res Treat. 2010;124:187–94. doi: 10.1007/s10549-010-1135-1. [DOI] [PubMed] [Google Scholar]
- 11.Santana-Davila R, Perez EA. Treatment Options for Patients with Triple-Negative Breast Cancer. J Hematol Oncol. 2010;3:42. doi: 10.1186/1756-8722-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawood S. Triple-negative breast cancer: epidemiology and management options. Drugs. 2010;70:2247–58. doi: 10.2165/11538150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Enger SM, Greif JM, Polikoff J, Press M. Body weight correlates with mortality in early-stage breast cancer. Arch Surg. 2004;139:954–58. doi: 10.1001/archsurg.139.9.954. [DOI] [PubMed] [Google Scholar]
- 14.Press MF, Slamon DJ, Flom KJ, Park J, Zhou J-Y, Bernstein L. Evaluation of HER-2/neu Gene Amplification and Overexpression: Comparison of Frequently Used Assay Methods in a Molecularly Characterized Cohort of Breast Cancer Specimens. J Clin Oncol. 2002;20:3095–105. doi: 10.1200/JCO.2002.09.094. [DOI] [PubMed] [Google Scholar]
- 15.Press MF, Spaulding B, Groshen S, Kaminsky D, Hagerty M, Sherman L, et al. Monoclonal antibodies designed for immunohistochemical detection of progesterone receptor in archival breast cancer specimens. Steroids. 2002;67:799–813. doi: 10.1016/s0039-128x(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 16.Press MF, Sauter G, Bernstein L, Villalobos IE, Mirlacher M, Zhou JY, et al. Diagnostic Evaluation of HER-2 as a Molecular Target: An Assessment of Accuracy and Reproducibility of Laboratory Testing in Large, Prospective, Randomized Clinical Trials. Clin Cancer Res. 2005;11:6598–607. doi: 10.1158/1078-0432.CCR-05-0636. [DOI] [PubMed] [Google Scholar]
- 17.Press MF, Finn RS, Cameron D, Di Leo A, Geyer CE, Villalobos IE, et al. HER2 Gene Amplification, HER2 and EGFR Messenger RNA and Protein Expression and Lapatinib Efficacy in Women with Metastatic Breast Cancer. Clin Cancer Res. 2008;14:7861–70. doi: 10.1158/1078-0432.CCR-08-1056. [DOI] [PubMed] [Google Scholar]
- 18.Ihemelandu CU, Leffall LD, Jr, Dewitty RL, Naab TJ, Mezghebe HM, Makambi KH, et al. Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: age-specific prevalence and survival. J Surg Res. 2007;143:109–18. doi: 10.1016/j.jss.2007.03.085. [DOI] [PubMed] [Google Scholar]
- 19.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 22.Schneider EC, Zaslavshy AM, Epstein AM. Racial disparities in the quality of care for enrollees in medicare managed care. JAMA. 2002;287:1288–94. doi: 10.1001/jama.287.10.1288. [DOI] [PubMed] [Google Scholar]
- 23.Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, et al. Race and hormone receptor positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104:406–14. doi: 10.1093/jnci/djr543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haque R, Achacoso NS, Fletcher SW, Nekhlyudov L, Collins LC, Schnitt SJ, et al. Treatment of Ductal Carcinoma In Situ Among Patients Cared for in Large Integrated Health Plans. Am J of Manag Care. 2010;16:351–60. [PMC free article] [PubMed] [Google Scholar]