Abstract
We have combined fluorescent analogue cytochemistry with fluorescence photobleaching recovery to measure the mobility of fluorescently labeled actin and other labeled test proteins microinjected into living amoebae. Bovine serum albumin, ovalbumin, and ribonuclease A have a cytoplasmic mobility, expressed as a diffusion coefficient, that is 1/2 to 1/3 of that observed in aqueous solution; 90% of the actin has a mobility 1/2 to 1/8 of that of G-actin in aqueous solution, and approximately equal to 10% of the actin has a mobility comparable to that of F-actin in aqueous solution. Therefore, no more than 10% of the actin in the cytoplasm of amoebae can exist as static filaments. Microinjection of phalloidin decreases the diffusion coefficient of the mobile component of cytoplasmic actin, and it also increases the low-mobility fraction to 50% but has no effect on the mobility of labeled ovalbumin. By comparing the mobility of actin in different parts of amoebae and by separating cytoplasm from plasmalemma-ectoplasm, we found the low-mobility fraction of actin to be enriched in the tail, along the plasmalemma-ectoplasm, and in contracted cytoplasm.
Full text
PDF
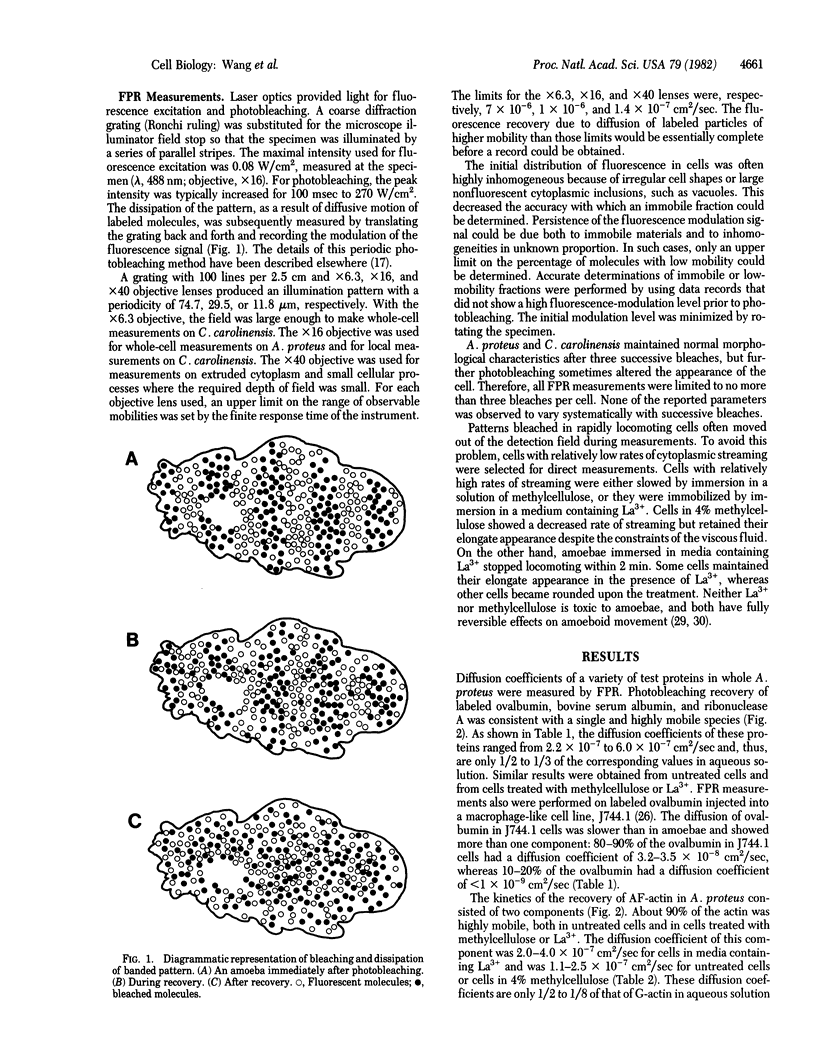
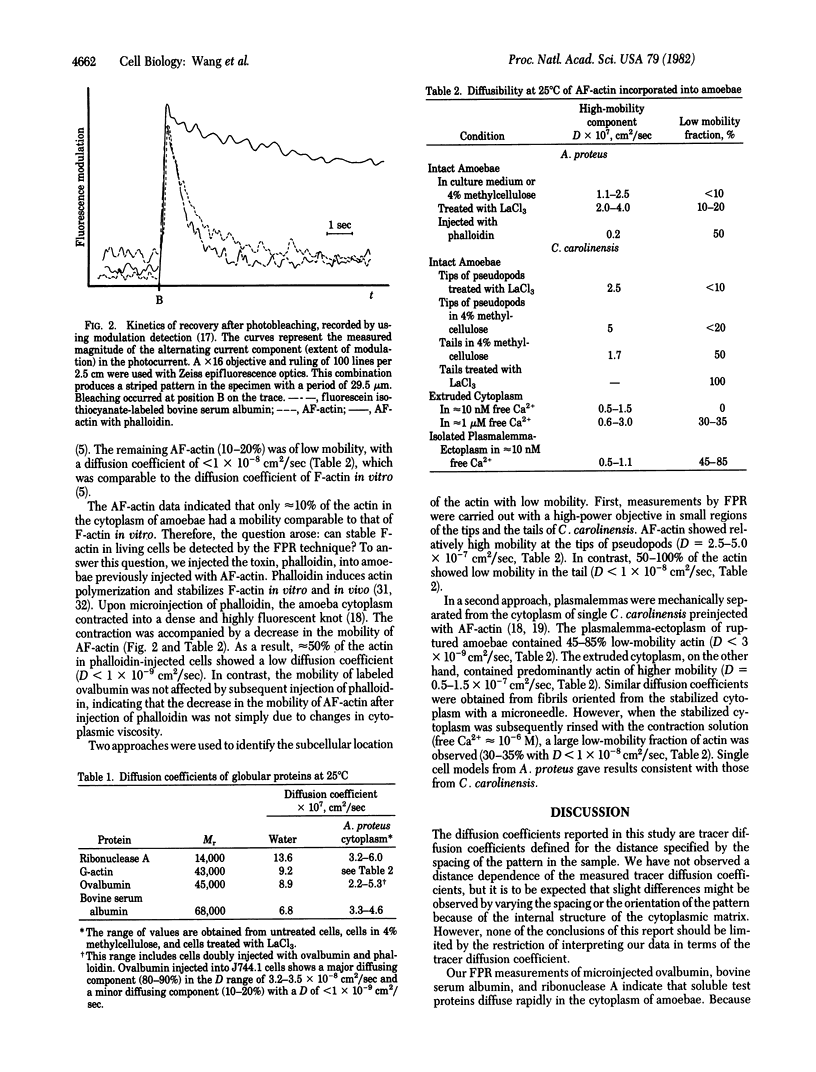


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Allen N. S. Cytoplasmic streaming in amoeboid movement. Annu Rev Biophys Bioeng. 1978;7:469–495. doi: 10.1146/annurev.bb.07.060178.002345. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Bray D., Thomas C. Unpolymerized actin in fibroblasts and brain. J Mol Biol. 1976 Aug 25;105(4):527–544. doi: 10.1016/0022-2836(76)90233-3. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Hawkes R. B., Holberton D. V. A calcium-sensitive lanthanum inhibition of amoeboid movement. J Cell Physiol. 1973 Jun;81(3):365–370. doi: 10.1002/jcp.1040810309. [DOI] [PubMed] [Google Scholar]
- Heiple J. M., Taylor D. L. Intracellular pH in single motile cells. J Cell Biol. 1980 Sep;86(3):885–890. doi: 10.1083/jcb.86.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni F., Taylor D. L., Ware B. R. Fluorescence photobleaching recovery in solutions of labeled actin. Biophys J. 1981 Aug;35(2):351–364. doi: 10.1016/S0006-3495(81)84794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Microtubule treadmills--possible molecular machinery. Nature. 1981 Oct 29;293(5835):705–711. doi: 10.1038/293705a0. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Cytoskeletal functions of cytoplasmic contractile proteins. J Supramol Struct. 1976;5(3):317–334. doi: 10.1002/jss.400050306. [DOI] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature. 1975 Oct 2;257(5525):393–394. doi: 10.1038/257393a0. [DOI] [PubMed] [Google Scholar]
- Schliwa M. Proteins associated with cytoplasmic actin. Cell. 1981 Sep;25(3):587–590. doi: 10.1016/0092-8674(81)90166-5. [DOI] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Kunes S. M., Schultz D. W., Taylor A., Triman K. L. Structure of chi hotspots of generalized recombination. Cell. 1981 May;24(2):429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- Stockem W., Hoffmann H. U., Gawlitta W. Spatial organization and fine structure of the cortical filament layer in normal locomoting Amoeba proteus. Cell Tissue Res. 1982;221(3):505–519. doi: 10.1007/BF00215699. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Blinks J. R., Reynolds G. Contractile basis of ameboid movement. VII. Aequorin luminescence during ameboid movement, endocytosis, and capping. J Cell Biol. 1980 Aug;86(2):599–607. doi: 10.1083/jcb.86.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Condeelis J. S. Cytoplasmic structure and contractility in amoeboid cells. Int Rev Cytol. 1979;56:57–144. doi: 10.1016/s0074-7696(08)61821-5. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Condeelis J. S., Moore P. L., Allen R. D. The contractile basis of amoeboid movement. I. The chemical control of motility in isolated cytoplasm. J Cell Biol. 1973 Nov;59(2 Pt 1):378–394. doi: 10.1083/jcb.59.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Rhodes J. A., Hammond S. A. The contractile basis of ameboid movement. II. Structure and contractility of motile extracts and plasmalemma-ectoplasm ghosts. J Cell Biol. 1976 Jul;70(1):123–143. doi: 10.1083/jcb.70.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L. The contractile basis of amoeboid movement. IV. The viscoelasticity and contractility of amoeba cytoplasm in vivo. Exp Cell Res. 1977 Mar 15;105(2):413–426. doi: 10.1016/0014-4827(77)90138-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Wang Y. L. Fluorescently labelled molecules as probes of the structure and function of living cells. Nature. 1980 Apr 3;284(5755):405–410. doi: 10.1038/284405a0. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Wang Y. L., Heiple J. M. Contractile basis of ameboid movement. VII. The distribution of fluorescently labeled actin in living amebas. J Cell Biol. 1980 Aug;86(2):590–598. doi: 10.1083/jcb.86.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Wang Y. L. Molecular cytochemistry: incorporation of fluorescently labeled actin into living cells. Proc Natl Acad Sci U S A. 1978 Feb;75(2):857–861. doi: 10.1073/pnas.75.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Taylor D. L. Distribution of fluorescently labeled actin in living sea urchin eggs during early development. J Cell Biol. 1979 Jun;81(3):672–679. doi: 10.1083/jcb.81.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Taylor D. L. Preparation and characterization of a new molecular cytochemical probe: 5-iodoacetamidofluorescein-labeled actin. J Histochem Cytochem. 1980 Nov;28(11):1198–1206. doi: 10.1177/28.11.6107318. [DOI] [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976 Nov;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- Wehland J., Stockem W., Weber K. Cytoplasmic streaming in Amoeba proteus is inhibited by the actin-specific drug phalloidin. Exp Cell Res. 1978 Sep;115(2):451–454. doi: 10.1016/0014-4827(78)90307-5. [DOI] [PubMed] [Google Scholar]
- Wieland T. Modification of actins by phallotoxins. Naturwissenschaften. 1977 Jun;64(6):303–309. doi: 10.1007/BF00446784. [DOI] [PubMed] [Google Scholar]
- Wojcieszyn J. W., Schlegel R. A., Wu E. S., Jacobson K. A. Diffusion of injected macromolecules within the cytoplasm of living cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4407–4410. doi: 10.1073/pnas.78.7.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]



