Abstract
Background
Oncolytic adenoviruses provide a promising alternative for cancer treatment. Recently, adjuvant α-interferon has shown significant survival benefits for pancreatic cancer, yet was impeded by systemic toxicity. To circumvent these problems adenovirus with high-level targeted IFNα expression can be generated.
Methods
Conditionally replicative adenoviruses (CRAds) with improved virulence and selectivity for pancreatic cancer were generated. The vectors were tested in vitro, in vivo, and in human pancreatic cancer and normal tissue specimens.
Results
Adenoviral death protein and fiber modifications significantly improved oncolysis. CRAds selectively replicated in vitro, in vivo and showed persistent spread in cancer xenografts. They exhibited high-level replication in human pancreatic cancer specimens, but not in normal tissues. Improved IFN-CRAd oncolytic efficiency was demonstrated.
Conclusions
Optimized Cox2CRAds show highly favorable effects in vitro and in vivo. We have the first report of a pancreatic cancer specific, highly virulent, IFN-expressing CRAd and believe this strategy to have great promise.
Keywords: Pancreatic cancer, Oncolytic virus, Adenovirus, Conditionally replicative adenovirus (CRAd), Gene therapy, Interferon alpha, Adenoviral death protein (ADP), Cox2, Krumdiek tissue slicer
INTRODUCTION
Pancreatic ductal adenocarcinoma is a highly lethal disease, with an estimated 43,140 new cases and resulting in nearly 37,000 deaths in 2010 1. Owing to the deep retroperitoneal location of the pancreas and the fact that early stage disease typically has no specific symptoms, it is uncommon for this cancer to be diagnosed at a surgically resectable stage. Of the newly diagnosed cases, approximately 85-90% will have inoperable disease at presentation due to locally advanced stage or metastases 2. Chemotherapy with gemcitabine is currently the standard of care in the adjuvant setting; however overall survival remains poor with median survival of approximately 22-24 months in selected series 3.
Oncolytic adenovirus is an extensively studied vector in cancer therapy. In particular, conditionally replicative adenoviruses (CRAds), oncolytic adenoviruses which are specifically designed for replication restricted to cancer cells, have been developed 4, 5. Clinical trials in various cancers including pancreatic, prostate, ovarian, and glioblastoma multiforme 6, 7 have established the tolerability of CRAds and such experience with its use has contributed greatly to its well-understood safety profile. However, clinical trials to date have also highlighted several significant disadvantages of CRAd therapy: limited infection of cancer cells, limited intratumoral spread, limited specificity for cancer cells with off-target effects including hepatotoxicity, and immunogenicity 5, 8-10. Thus, there is a pressing need to develop Ad vectors for cancer therapy with both increased efficacy and virulence toward pancreatic cancer while at the same time possessing high selectivity for cancer cells with the intent to avoid liver and systemic toxicity.
Pancreatic cancer cells are resistant to infection with conventional CRAds due to the profound lack of the coxsackie-adenovirus receptor (CAR) on the cell surface 13. We and others have previously reported an improved infectivity of adenoviral vectors toward pancreatic cancer cells through genetic modification of the viral capsid proteins to alter tropism by binding to alternative cell surface receptors on pancreatic cancer: integrins (RGD fiber) and Ad3 receptor (5/3 fiber) 11-13. Additionally, improved killing ability has been achieved with adenoviral death protein (ADP) overexpression, an enhancer of apoptosis and viral spread 14. A further increase in antitumor effect can be achieved by incorporating an anti-tumor transgene into the CRAd genome. This local transgene expression, coupled with strong vector spread, will significantly advance oncolytic virotherapy for pancreatic cancer and has the potential to create an entirely new class of therapy.
Recently, alpha interferon (IFN), a cytokine with direct and indirect antitumor effects, has shown promising improvements in survival in multimodality adjuvant therapy. This was first reported by the Virginia Mason group study15 in which they found a statistically significant improvement in survival with interferon-based adjuvant chemoradation over gemcitabine-based adjuvant therapy at 26 months of follow-up, with 84% survival at 2 years. A subsequent phase II study by Linehan and colleagues using adjuvant interferon-based chemoradiation (CRT) with post-radiation gemcitabine instead of 5-FU resulted in 56% 2 year actuarial survival 16, a survival rate identical to that reported by Picozzi and colleagues in the multicenter phase II ACOSOG Z05031 trial17. However, this regimen suffers from systemic side effects with an incidence as high as 95%, and over 25% of patients required dose reduction due to IFN systemic toxicities 15-18. Overall, this indicates a pressing need for the development of highly active agents for the treatment of pancreatic cancer. As shown by these previous studies IFN could be a powerful tool for the generation of such a modality. To use IFN as a targeted therapy, the challenge of limiting systemic IFN toxicities must be overcome. One strategy would be to design a viral vector to selectively infect pancreatic cancer cells, replicate within them, and release therapeutic amounts of IFN locally to avoid systemic effects. However, such modified viruses expressing IFN as a therapeutic transgene for pancreatic cancer have not been reported except as an early-generation nonreplicating construct 19.
In this work we have designed an adenovirus for optimal infectivity of pancreatic cancer with increased virulence due to IFN and ADP expression. This is a conditionally replicative adenovirus controlled by the tumor-specific promoter Cox2, allowing the virus to target pancreatic cancer while sparing toxicity to the liver. We hypothesize that this vector will possess superior attributes of cancer selectivity, cell infectivity, and cell killing compared to adenovirus vectors previously studied. Such a strategy will hopefully lead to a more powerful yet better tolerated means of interferon administration in pancreatic cancer patients.
MATERIALS AND METHODS
Cell lines and animals
The human pancreatic ductal adenocarcinoma (PDAc) cell lines AsPS1, S2VP10, S2013, MiaPaCa2, Panc1, and HS766T were maintained in Dulbecco’s modified Eagle medium (DMEM) (Mediatech, Herndon, VA) with 20% fetal bovine serum (FBS) for ASPC-1 and 5% FBS for all other cell lines respectively. MiaPaCa2, Panc1, AsPC1, HS766T, the Cox2-positive human nonsmall cell lung adenocarcinoma cell line A549, and the Cox2-negative human breast cancer cell line BT474, and epidermoid carcinoma cell line A431 were obtained from the American Type Culture Collection (Manassas, VA). BT474 was maintained in Roswell Park Memorial Institute medium supplemented with 15% FBS and bovine insulin (0.01 mg/ml, Life Technologies, Rockville, MD). 911 cells (a kind gift of Dr. Van Der Eb, Leiden University, Netherlands20) were maintained in DMEM supplemented with 5% FBS. Human umbilical vein endothelial cells (HUVEC) were obtained from ATCC and cultured in EGM-2 medium (Cambrex Biosciences, Walkersville, MD). All media were supplemented with penicillin (100 IU/ml) and streptomycin (100 μg/ml).
Female athymic nude mice (NCr-nu/nu, National Cancer Institute at Frederick, Frederick, MD) at 6-8 weeks of age were used for in vivo studies. All animals received humane care based on the guidelines set by the American Veterinary Association. All experimental protocols involving live animals were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Adenoviral vectors
Replication-deficient Ad vectors expressing the human interferon α gene (RGD-CMV-IFN, 5/3-CMV-IFN,) have been constructed using homologous recombination with the region E1-deleted fiber-modified adenoviral backbones designed in our lab. The human IFN encoding plasmid has been kindly provided by Dr. Aoki (Genetics Division, National Cancer Center Research Institute, Tokyo, Japan) 19. The infectivity-enhanced Cox2 promoter-controlled CRAds were generated using homologous recombination in E. coli as described previously 11, 13, 21, 22. The ADP over-expressing oncolytic viruses with ether Luciferase or IFN expression cassette were constructed using pShuttleΔE3ADPKanF2 cloning 11, 23. Replication-incompetent CMV promoter-driven luciferase expression vectors with the genetically modified RGD fiber (RGDCMV-Luc), Ad5/Ad3-chimeric fiber (5/3CMV-Luc) or native Ad5 fiber (Ad5CMV-Luc) were used to analyze infectivity enhancement and have been described previously 21, 22. Wild type Ad5 (Ad-Wt) and its RGD isogenic versions (RGD-Wt) were utilized as non-selective replicative control vectors.
All viruses were propagated in the 911 cell line and purified by double CsCl density gradient ultracentrifugation, followed by dialysis against phosphate-buffered saline (PBS) with 10% glycerol. The vectors were titrated by plaque assay, and viral particle (vp) number was measured spectrophotomectrically with absorbance at 260 nm. Vectors were stored at −80 °C until ready for use. Viral structure was confirmed by PCR for Cox2, a mutant replication competent Ad (RCA) contamination, and fiber structure as described previously 13, 21.
In vitro analysis of infectivity with luciferase-expressing Ads
Cells (5×104 cells/well) grown in 24 well plates were infected with 100 viral particle (VP) per cell for 48 hours, followed by lysis with 100 μl of cell culture lysis buffer (Promega, Madison, WI) and Luc activity was determined with the Luciferase Assay System (Promega). All experiments were performed in triplicate.
Detection of Cox2 promoter-dependent CRAd replication
Cultured cells (5×104cells/well) were infected with 0.1 vp/cell in 100μl DMEM. The infection medium was replaced with 1ml of medium two hours later and cells were incubated for two days. At this time, the cells were lysed with cell culture lysis buffer (Promega, Madison, WI), and luciferase activity was determined with the Luciferase Assay System (Promega). Experiments were performed in triplicate and standardized with protein concentration quantitated by the DC protein assay (Bio-Rad, Hercules, CA).
In vitro quantitative analysis of cancer cell killing ability
Cells were seeded in 96-well plates at 3000 per well, then infected with Ad vectors at varying multiplicities of infection in 100 μl of appropriate medium. The cells were incubated under standard conditions and the number of living cells was measured colorimetrically at serial time points using the Cell Titer Aqueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer’s instructions. The proportion of living cells at each time point was normalized to the number of living uninfected cells. All experiments were done in triplicate.
Human tissue samples
After approval by the University of Minnesota Institutional Review Board, resection specimens of biopsy-proven pancreatic ductal adenocarcinoma, adjacent normal pancreas, and normal liver were obtained fresh, and immediately sliced to 200 μm thickness (Krumdieck tissue slicer, Alabama Specialty Products, Inc., Munford, AL).
Viral infection of human tissue slices
Tissues of thickness of 200 μm were plated in 12-well plates containing 1ml/well of medium consisting of 50% FBS Ham’s F12 (Mediatech, Herndon, VA) and 50% FBS DMEM with 1% penicillin/streptomycin, 1% amphotericin B, 15% FBS, and 10 μg/ml dexamethasone. Infection was performed at a multiplicity of infection of 100 vp/cell estimated by average weight to calculate the approximate cell number per tissue slice. The infection media was replaced 3 hours later with appropriate 50% FBS culture media. At day 4 viral DNA was extracted from the slices using a QIAamp DNA Blood Mini kit (Qiagen, Germantown, MD). Viral copy number was quantitated by SYBR Green RT-PCR (Applied Biosystems, Foster City, CA) with the adenoviral E4 primers 13 and compensated with β-actin. All experiments were triplicates.
In vivo bioluminescent imaging of CRAd replication in a mouse xenograft model
HS766T and A431 cells (1.0 ×106) were injected into both flanks of nude mice. When tumors reached 6 to 10 mm in diameter, a single virus dose of 1010vp in 50 μl PBS was injected intratumorally. Bioluminescent light imaging was performed under anesthesia with 2% isoflurane and following intraperitoneal injection of 3mg D-Luciferine (Molecular Imaging Products, Bend, OR) using an in vivo imaging system as we described previously 11,23.
In a separate experiment, A549 cells (1.0 ×106) were injected into both flanks of nude mice. When tumors reached 6 to 8 mm in diameter, a single virus dose of 1010vp in 50 μl PBS was injected systemically via the tail vein. Bioluminescent light imaging was performed under the same conditions as described above.
Statistical methods
Statistical analysis of viral effect in vitro and in vivo was carried out with Excel (Microsoft, Redmond WA). Student’s t test of means was used with a two-tailed p value of less than 0.05 taken to be statistically significant. Data are expressed as mean ± standard deviation of at least three results.
RESULTS
Adenoviral vector structure
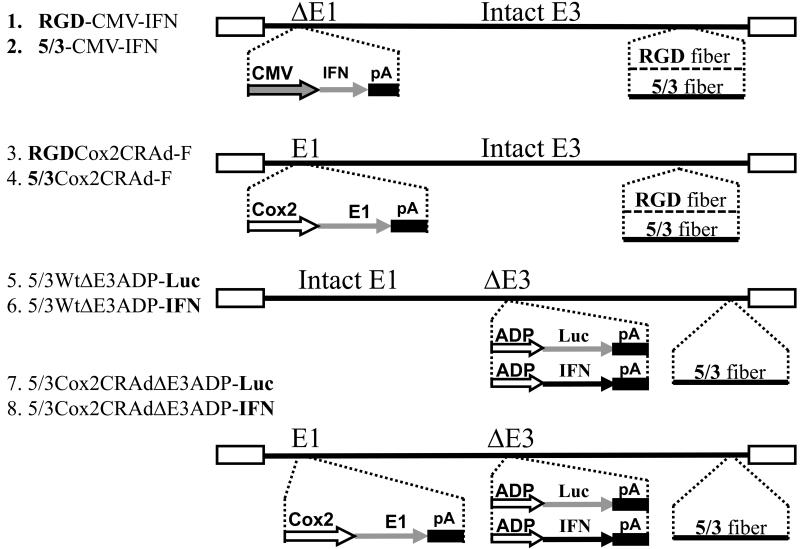
We generated multiple experimental and control vectors as shown in Figure 1. RGD-CMV-IFN and 5/3-CMV-IFN are unable to replicate owing to deletion of the critical viral E1 region (ΔE1), which is required for replication, and are designed to express the cytocidal IFN gene under control of the continuously active CMV promoter. These viruses have either the RGD or 5/3 capsid fiber modification for increased infectivity toward target cells. RGDCox2CRAd-F and 5/3Cox2CRAd-F are the replication competent oncolytic agents in which cellular Cox2 activity of the infected cell is used to trigger E1 gene expression and therefore allow the virus to replicate 11,22. Non-selective, wild type replicative 5/3WtΔE3ADP-Luc and 5/3WtΔE3ADP-IFN both have an expression cassette replacing the nonessential viral E3 region which is known to overexpress adenoviral death protein (ADP), an enhancer of viral spread21, 23, as well as either the therapeutic IFN gene or the Luc reporter. Both viruses are infectivity enhanced by changing their tropism to Ad3 receptor (5/3 fiber modification). Of note, gene expression from the adenovirus E3 region follows a late profile due to control by the major late promoter and is therefore consistent with the replication cycle. Strong transgene expression from this locale has also been previously observed to take place. The vectors 5/3Cox2CRAdΔE3ADP-Luc and 5/3Cox2CRAdΔE3ADP-IFN have the following characteristics: expression of the E1 region which is critical for replication is controlled by the Cox2 promoter, conferring cancer specificity; all E3 genes have been deleted and instead either ADP-Luc or IFN are expressed in a replication-dependent fashion; and the vectors are fiber-modified (5/3) to overcome CAR deficiency of pancreatic cancer cells.
Figure 1.
Schematic structure of adenoviral vectors. Several important structural features are reflected including wild type or Cox2-controlled replication, capsid fiber modification by RGD or 5/3, and insertion of ADP and transgene luciferase or human interferon α into the adenoviral E3 region: 1. RGD-CMV-IFN (replication-deficient control); 2. 5/3-CMV-IFN (replication-deficient control); 3.RGDCox2CRAd-F; 4. 5/3Cox2CRAd-F; 5. 5/3WtΔE3ADP-Luc; 6. 5/3WtΔE3ADP-IFN; 7. 5/3Cox2CRAdΔE3ADP-Luc; 8. 5/3Cox2CRAdΔE3ADP-IFN.
The RGD and 5/3 fiber modifications significantly increase adenoviral infectivity in PDAc
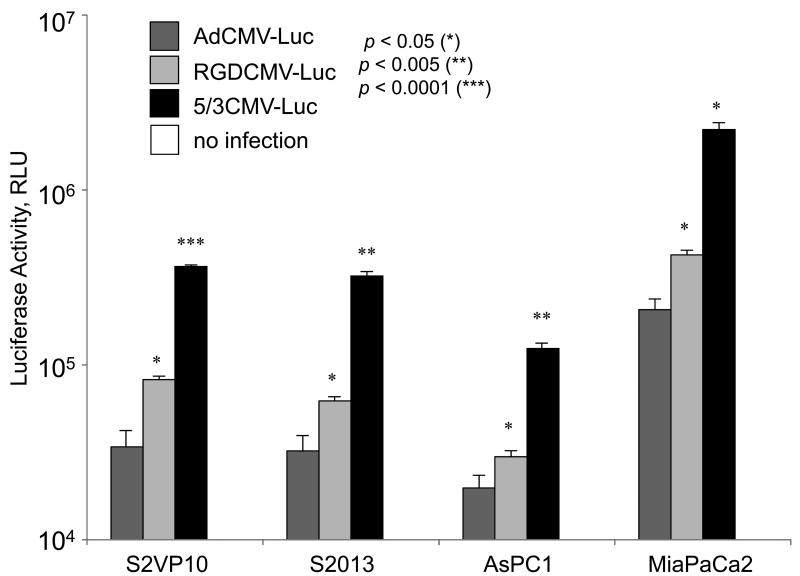
To analyze transduction efficiency of fiber-modified viral vectors in pancreatic cancer, S2VP10, S2013, AsPC1, and MiaPaca2 cells were infected with i) AdCMV-Luc vector with the native Ad5 fiber structure; ii) RGDCMV-Luc vector equipped with the RGD fiber modification; and iii) Ad5/3CMV-Luc vector with the 5/3 fiber-knob chimera. Fiber modification was the only structural change made to this identical replication-incompetent viral vectors encoding the CMV promoter-driven luciferase. The adenovirus-induced gene expression was analyzed by Luc assay (Figure 2). In all four pancreatic cancer cell lines, fiber-modified viral vectors exhibited significant increase in the Luc expression with the Ad5/3CMVLuc showing the greatest Luc activity followed by RGDCMV-Luc. Compared to fiber-unmodified AdCMV-Luc, Ad5/3CMV-Luc increased Luc activity by 91% while RGDCMV-Luc exhibited 59% increased in Luc activity in S2VP10. The Ad5/Ad3 fiber-modified vector significantly outperformed AdCMV-Luc by 90%, 84%, and 91% and RGDCMV-Luc by 48%, 34%, and 51% in S2013, AsPC1, and MiaPaca2, respectively, and showed the highest level of transduction efficiency in four out of four PDAc cell lines.
Figure 2.
Fiber-modified adenoviral vectors (5/3CMV-Luc and RGDCMV-Luc) significantly increased the infectivity of pancreatic cancer cells compared to the identical control vector with a native Ad5 fiber (AdCMV-Luc). Pancreatic cancer cell lines S2VP10, S2013, AsPC1, and MiaPaca2 were infected with replication-deficient viral vectors encoding luciferase. Both Ad5/Ad3 and RGD fiber-modified viral vectors showed significant increase in luciferase gene expression compared to the fiber-unmodified counterpart.
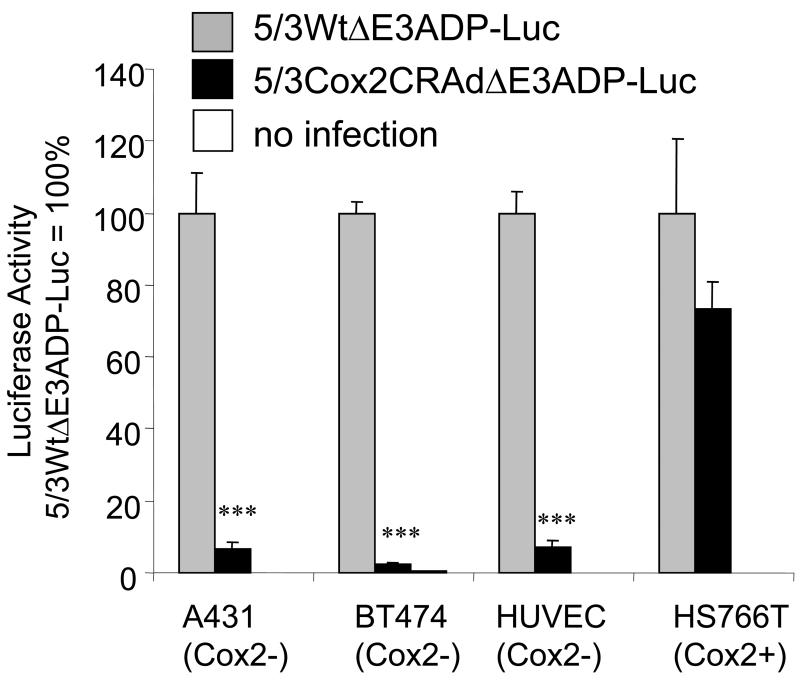
The targeted Cox2-controlled virus avoids replication in Cox2-nonexpressing cell populations
To understand the ability of the Cox2-controlled virus to selectively avoid replication (and therefore replication injury) in Cox2-negative cell populations including hepatocytes, the organ of greatest concern, representative cell lines with both low Cox2 activity A431, BT474, HUVEC) and high Cox2 (HS766T) were infected with 5/3WtΔE3ADP-Luc and 5/3Cox2CRAdΔE3ADP-Luc (Figure 3). These viruses have the most complex structure and are designed for the highest efficacy, incorporating ADP overexpression, massive transgene expression, and fiber modifications to enhance infectious ability. Reporter gene activity in Cox2-positive HS766T pancreatic cancer cells using 5/3Cox2CRAdΔE3ADP-Luc approaches that of 5/3WtΔE3ADP-Luc. Minimal reporter gene activity of 8% or less was detected using 5/3Cox2CRAdΔE3ADP-Luc in cells of low Cox2 activity (p<0.0001 compared to 5/3WtΔE3ADP-Luc). Importantly, reporter gene activity using 5/3Cox2CRAdΔE3ADP-Luc in HUVEC cells, a normal and untransformed human cell line with low Cox2 activity, was found to be significantly less than activity using 5/3WtΔE3ADP-Luc. These data emphasize the close dependence on Cox2 status of the target cell for replication, and therefore the ability of the Cox2 CRAd to differentially replicate in normal versus cancerous tissue in a highly selective fashion.
Figure 3.
The Cox2-controlled virus selectively avoids replication in Cox2-negative cell populations. The Cox2-negative cell lines A431, BT474, HUVEC cells, and the strongly Cox2- positive pancreatic cancer cell line HS766T were infected with fiber-modified viruses with wild-type replication or Cox2-promoter dependent replication. Cox2-negative cells fail to support viral replication of Cox2-controlled virus. ***: p<0.0001.
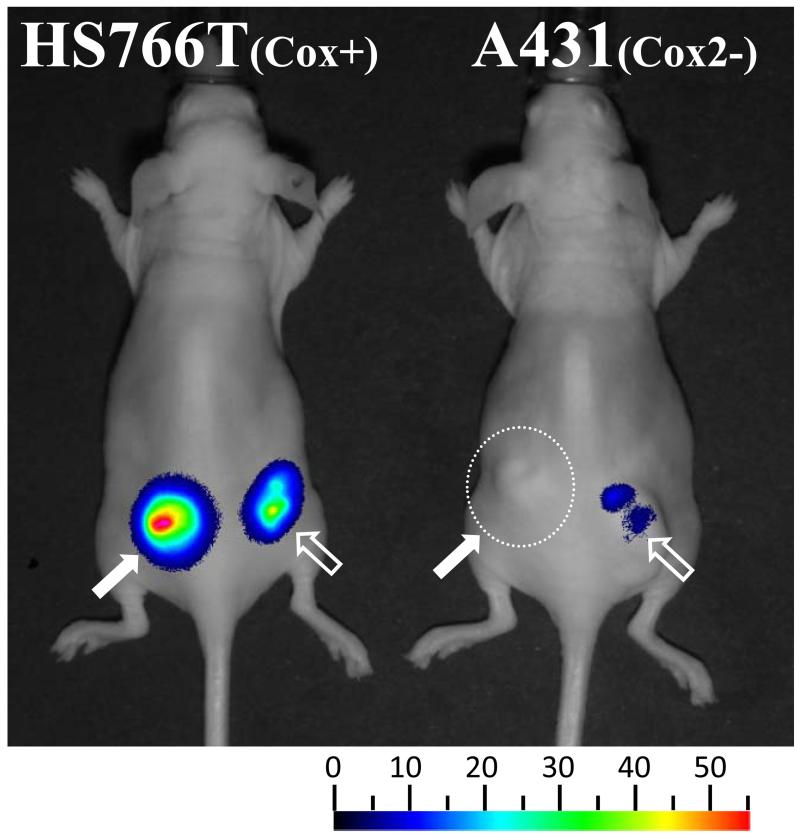
The targeted viral construct controlled by the Cox2 promoter shows selectivity and persistence in a cancer xenograft model
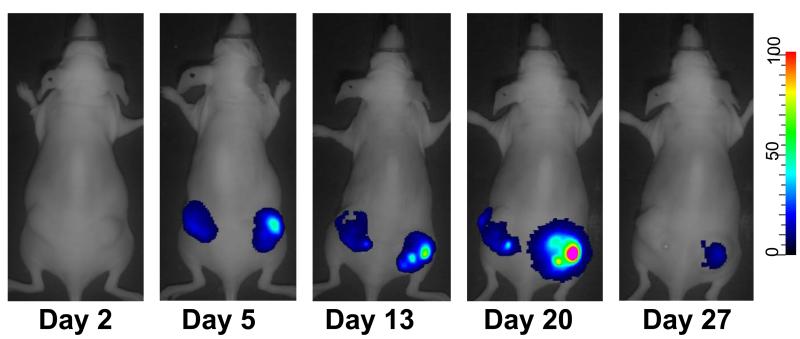
Pancreatic cancer HS766T (high Cox2 activity) and Cox2-negative A431 cells were used to form subcutaneous tumor xenografts in the flanks of nude mice (Figure 4). Mice were injected with a single intratumoral injection of either a tumor-targeted Cox2-replication-controlled (5/3Cox2CRAdΔE3ADP-Luc) or wild-type replication (5/3WtΔE3ADP-Luc) virus expressing luciferase in a replication-dependent manner. During the following 5 weeks, the mice were anesthetized, injected intraperitoneally with the luciferase substrate, D-Luciferine, and replication-dependent luciferase activity was measured using noninvasive bioluminescence imaging. Both oncolytic adenoviruses demonstrated tumor-associated bioluminescence at high levels in Cox2-positive HS766T tumors: bioluminescence peaked around day 4 (Figure 4), and persisted for up to 4 weeks. Importantly, no bioluminescence was found in mice bearing Cox2-negative A431 xenografts that were challenged with the Cox promoter-controlled Ad5/3Cox2ΔE3ADP-Luc.
Figure 4.
The targeted viral construct controlled by Cox2 shows selectivity and persistence in a cancer xenograft model. Subcutaneous xenografts were established in the flanks of nude mice using the PDAc cell line Hs766T and Cox2-negative control cells A431. 5/3Cox2CRAdΔE3ADP-Luc (left side) and 5/3WtΔE3ADP-Luc (right side) were injected intratumorally. Strong viral replication was seen with both viruses in PDAc xenografts but only with 5/3WtΔE3ADP-Luc in A431 tumors. Representative image at day 4 post-infection.
Furthermore, we performed an experiment to assess whether the same results would be found using intravenous delivery of virus instead of intratumoral. High Cox2-expressing A549 lung cancer cells were used to form subcutaneous flank xenografts in nude mice (Figure 5). Mice were then injected with 5/3Cox2CRAdΔE3ADP-Luc by i.v. delivery into the tail vein, and replication-dependent luciferase expression was imaged over the next several weeks. Not only did our novel 5/3Cox2CRAdΔE3ADP-Luc track subcutaneous Cox2-positive tumors with this delivery strategy, an important proof-of-concept finding for clinical application, but viral activity was again persistent for up to 30 days. Thus, this strategy is capable of in vivo cancer selectivity and sustained replication by both intravenous and direct intratumoral administration.
Figure 5.
Systemically-delivered CRAds replicate and visualize the tumor xenografts. Subcutaneous xenografts of A549 (Cox2-positive) were formed in nude mice followed by a single intravenous injection of 5/3Cox2CRAdΔE3ADP-Luc expressing luciferase in a replication-dependent manner. Tumor-associated bioluminescence was persistent for up to 30 days.
The targeted Cox2-controlled CRAd shows selective replication ex vivo in human PDAc specimens but not in normal tissues
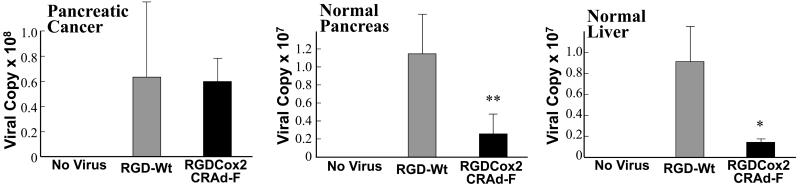
To study the selectivity of the Cox2 promoter for pancreatic cancer ex vivo, we infected resected specimens of pancreatic cancer, normal pancreas, and normal liver with RGDCox2CRAd-F, an RGD-modified CRAd requiring Cox2 activity of the infected cell for replication, and compared to RGD-Wt, the control wild type adenovirus without tumor specificity (Figure 6). In pancreatic cancer specimens, RGDCox2CRAd-F replicated as avidly as RGD-Wt indicating the strong activity of this promoter in PDAc. In sharp contrast, normal pancreatic tissue did not support CRAd replication and replication was minimal in normal liver tissue (p<0.005, p<0.05 compared to the wild type replication control RGD-Wt). When the replication of these two vectors were compared in each tissues, the percentage of compensated viral copy number RGDCox2CRAd-F in pancreatic adenocarcinoma tissue was significantly higher than those in normal pancreas and normal liver. This indicates not only the suitability of Cox2 as a tumor-specific promoter for this virus ex vivo, but also the particular advantage of using Cox2 activity as a driver of viral replication in order to avoid replication toxicity in liver, the organ of greatest concern with adenovirus.
Figure 6.
The targeted Cox2-controlled CRAd shows selective replication ex vivo in human PDAc specimens but not in normal tissues. Fresh resection specimens of pancreatic cancer, normal pancreas, and normal liver were thinly sliced and infected with RGDCox2CRAd-F or RGD-Wt. In pancreatic cancer samples, RGDCox2CRAd-F replicates as avidly as RGD-Wt, while in normal pancreas and liver minimal replication of RGDCox2CRAd-F is found indicating the high level of cancer specificity of the Cox2 promoter. *: p<0.05, **: p<0.005.
IFN expressed by Ad has a direct tumoricidal effect independent of viral replication
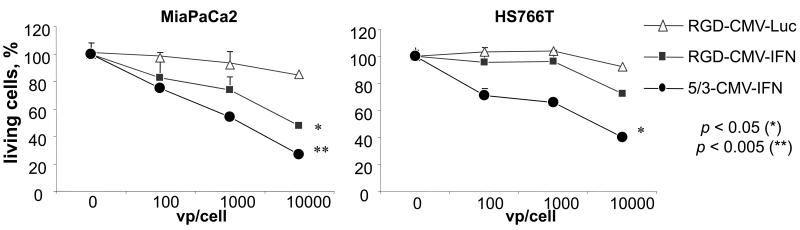
Next, we have generated RGD and 5/3 fiber infectivity-enhanced replication deficient Ads expressing the human IFN protein (RGD-CMV-IFN and 5/3-CMV-IFN) and analyzed the cytotoxic effect of adenoviral-induced IFN expression in pancreatic cancer cell lines (Figure 7). MiaPaca2 and HS766T cell lines were infected with IFN-expressing Ads at different titers (100, 1000 and 10,000 vp/cell). As a control adenovirus, the identical replication incompetent adenovirus expressing luciferase (RGD-CMV-Luc) has been used. In MiaPaCa2, increasing the viral titer from 100 to 10,000 resulted in a reduction in the number of living cells from 82% to 49% using RGD-CMV-IFN and from 75% to 27% using 5/3-CMV-IFN (p<0.05 for both), and in HS766T cells, the same increase in titer resulted in a trend toward reduction of living cells from 87% to 68% using RGD-CMV-IFN and 66% to 35% using 5/3-CMV-IFN (p<0.05). Results indicate a statistically significant increase in cell killing ability with increasing titers of virus and thus increasing amounts of virally-derived IFN.
Figure 7.
IFN expressed by adenovirus has a direct tumoricidal effect independent of viral replication. Cancer cell lines were infected with replication-incompetent RGD-CMV-IFN, 5/3- CMV-IFN, and an identical luciferase-expressing vector as control. Increasing IFNα production from increasing viral titers leads to a dose-dependent cell killing effect.
Increased oncolytic efficiency of IFN-expressing Cox2-controlled CRAds in vitro
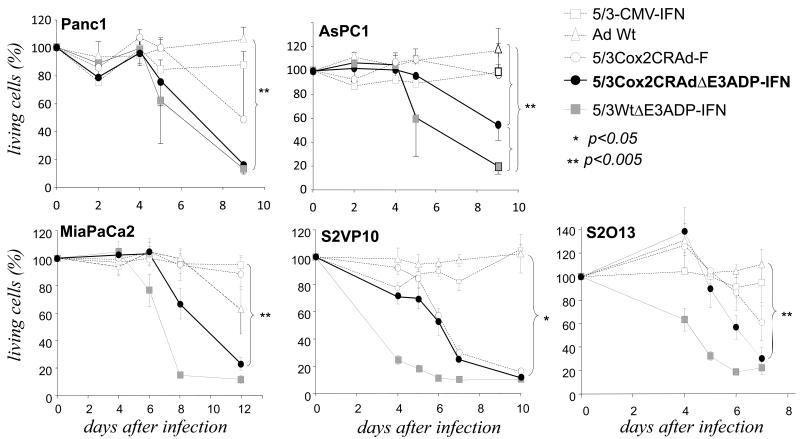
Based on these results, to further increase the oncolytic potency of our infectivity-enhanced Cox2 CRAds, we generated oncolytic adenovirus employing pancreatic cancer selectivity using the Cox2 promoter and also designed to massively express IFN from the viral E3 region, identical to Luc-expressing Ads tested earlier (Figure 8). This adenovirus (5/3Cox2CRAdΔE3ADP-IFN) represents our most advanced CRAds and possesses multiple, integrated antitumor effect through fiber modification, ADP and IFN overexpression. To analyze its improved cytocidal activity, we infected five different PDAc cell lines with a new IFN-expressing CRAd (5/3Cox2CRAdΔE3ADP-IFN) at low titers. We compared it to a nonreplicating IFN-producing virus (5/3-CMV-IFN) an IFN-producing virus without restriction on its replication (5/3WtΔE3ADP-IFN), a CRAd without IFN and ADP (5/3Cox2CRAd-F), and the gold standard adenovirus without cancer specificity and fiber modification, wild type Ad (Ad-Wt). It is evident in all cell lines that 5/3Cox2CRAdΔE3ADP-IFN significantly outperforms Ad-Wt in cell killing ability (p<0.05 for all comparisons at the final time point). Additionally, the tumor-selective IFN expressing CRAd (5/3Cox2CRAdΔE3ADP-IFN) achieves the same cell killing ability as its powerful non-selective counterpart, 5/3WtΔE3ADP-IFN, in three cell lines (S2O13, S2VP10, and Panc1) and trends toward equivalence in the remaining two. When compared with 5/3Cox2CRAd-F, the effect of the E3 region modifications for ADP and IFN expression is evident as 5/3Cox2CRAdΔE3ADP-IFN lyses pancreatic cancer cells more rapidly and completely in four out of five cell lines tested. Thus, 5/3Cox2CRAdΔE3ADP-IFN broadly displays an increased pancreatic cancer cell killing ability through fiber modification and ADP and IFN overexpression.
Figure 8.
Increased oncolytic efficiency of IFN-expressing Cox2-controlled CRAds in vitro. Human PDAc cell lines Panc1, ASPC1, MiaPaCa2, S2VP10 and S2O13 were infected at day 0. Cell viability was determined with a colorimetric cell proliferation assay. The results are shown as proportion of living cells remaining relative to uninfected cells. 5/3Cox2CRAdΔE3ADP-IFN shows significantly higher cell killing ability compared to Ad-Wt, the gold standard control virus, and is comparable in effect to 5/3WtΔE3ADP-IFN virus lacking cancer specificity.
DISCUSSION
Pancreatic cancer remains a highly lethal disease, which is highlighted by the closely similar numbers of new diagnoses and annual deaths from this malignancy. In the minority of patients in whom resectability is possible 2, patients still succumb after a median time of two years or less 3. Thus, a new, highly active, nonsurgical means of treatment is needed, both to improve rates of resectability as well as to improve overall survival when used in the adjuvant setting.
Oncolytic adenovirus has been studied in the setting of pancreatic cancer. Several strategies have been employed including CRAds based on frequent mutations or differences in the genetic profile of pancreatic cancer from normal cells, viruses expressing a “suicide gene” such as a metabolic enzyme to activate a chemotherapeutic prodrug, and cytokine-expressing adenoviruses 24. A conditionally replicative adenovirus requiring p53-deficient target cells for replication has been described 25, 26. This virus was quickly moved to phase I and II studies where it demonstrated a good safety profile, but disappointingly had short-lived replication and demonstrated responses in the minority of patients 5, 27. Suicide gene therapy, as with an oncolytic Ad-expressing thymidine kinase to activate ganciclovir 28, is a promising strategy; however, evidence has been conflicting on its efficacy. Recently a different gene, the sodium-iodide symporter, which can be used to deliver radioactive iodine, has been reported as a therapeutic transgene expressed by a CRAd system against prostate cancer29, and this and similar strategies hold promise for different tumor types as well. Cytokine-expressing Ads, including IL-2, IL-12, TRAIL, TNFα, as well as interferons have also been reported 30-33. However, while exciting in terms of efficacy in experimental systems and proof of concept demonstrations, these strategies generally suffer from problems such as poor adenoviral infectivity and intratumoral spread and lack of adenoviral selectivity for cancer which would make human clinical use more feasible 9. Clearly, advanced adenoviral vectors designed for tumor-selective replication in order to allow for systemic delivery as well as optimized for tumor killing ability with enhanced infectivity and virulence must all simultaneously be satisfied.
We report here an IFN-expressing CRAd which has been designed with these two critical requirements in mind: not only must it display strict selectivity for pancreatic cancer and avoid liver replication, but it must also incorporate advanced design features for enhanced potency. The most advanced virus described here, 5/3Cox2CRAdΔE3ADP-IFN, has been designed for superior cancer selectivity by Cox2-based promoter control, as well as significantly more lethal to cancer with improved infectivity by fiber modification, ADP overexpression, and massive local IFN expression.
Because infection of cancer cells requires highly efficient binding and internalization, we and others 12, 34, 35 have modified viral tropism with genetic modification of the viral capsid. We used the 5/3 and RGD fiber modification to overcome profound CAR deficiency of pancreatic cancer cells, and confirmed that across multiple pancreatic cancer cell lines, these do in fact significantly improve infectivity.
Selectivity is also achieved in our design at the transcriptional level. Most adenovirus will ultimately home to the liver and exert replication toxicity there. Therefore, in order to be clinically usable, liver replication must be avoided. Pancreatic cancer is known to be highly Cox2-positive 35, whereas the normal tissues and liver, the organ of most concern, are not 36. We tested the selectivity of our advanced virus 5/3Cox2CRAdΔE3ADP-IFN in several ways. In vitro, we showed a clear differentiation between the levels of replication in Cox2-positive cells compared to Cox2-negative cell types. Significantly, this also includes the normal human endothelial cell line HUVEC, in which replication of 5/3Cox2CRAdΔE3ADP-IFN was virtually absent. Next, we showed that Cox2 promoter control is feasible in vivo using subcutaneous xenografts of Cox2-positive pancreatic cancer cells as well as Cox2-negative tumors in nude mice. We observed both selectivity and persistence of infection, which was borne out both in direct intratumoral injection as well as with the most clinically relevant route, intravenous delivery. These observations of the selectivity for pancreatic cancer by Cox2 status were highlighted by the finding that our Cox2 CRAds also perform in a highly cancer-selective fashion when tested on actual clinical specimens of pancreatic cancer. We observed excellent replication of Cox2 CRAd in cancer samples along with minimal activity in both normal pancreas and liver. These findings underscore the promise of our strategy of selectivity and its clinical potential.
Even with high selectivity, a viral vector with low potency will not achieve its desired effects. We therefore sought to improve upon the second critical issue in vector design, low efficacy. Our virus 5/3Cox2CRAdΔE3ADP-IFN employs two strategies here: ADP overexpression for enhanced apoptosis and spread, as well as massive IFN expression. This cytokine has both direct and indirect antitumor effects. Clinically, IFN-based chemoradiotherapy for pancreatic cancer is known to greatly improve outcomes 15-17. Therefore, this is the cytokine of choice in our approach.
There have been a few attempts to study adenovirus-induced IFN expression. Dr. Aoki and his research group have made several important contributions in this area. It has been shown that IFN, expressed by a replication-incompetent Ad, can cause growth inhibition and regression of pancreatic tumors, and can also exert systemic effects in immunocompetent models 19, 37. Doronin and colleagues generated a CRAd with similar ADP-IFN expression and enhanced antitumor effect in a hepatocellular cancer xenograft model 38. We confirmed earlier observations of the therapeutic effect of IFN by using replication-deficient fiber modified Ads to infect several pancreatic cancer cell lines, and found a dose-dependent increase in killing ability which indicates the direct toxic effects of IFN expression. Taking this strategy further, we incorporated IFN expression into our most advanced conditionally replicating virus 5/3Cox2CRAdΔE3ADP-IFN, which is also fiber modified for increased infectivity, further enhanced by ADP overexpression, and cancer selective by Cox2 status. We also determined that this virus possesses excellent virulence toward pancreatic cancer, greatly outperforms the IFN-lacking counterparts and a wild type control adenovirus, and achieves comparable effects in vitro to the IFN-expressing virus with unrestricted replication. This increased complexity of our most advanced virus allows it to satisfy the fundamental requirements of both high selectivity as well as optimal virulence.
We have demonstrated a virus which builds on multiple previous achievements of both ourselves and others in the field and represents the first report of a pancreatic cancer specific, highly virulent, replication competent, IFN-expressing CRAd. We are actively working to characterize this virus in vivo in immunocompetent animal models, and ultimately hope to see it deployed for the treatment of human patients. We believe this strategy to have great promise to expand the usability of IFNα-based chemoradiotherapy for this grim disease.
Pancreatic cancer is a serious and lethal disease but gene therapy with conditionally replicative adenovirus offers promise of more effective and better tolerated adjuvant therapy. We report such a virus expressing alpha interferon as tested in vitro, in vivo, and using human tumor samples from pancreatic cancer resections. This virus demonstrates cancer specificity, increased virulence, and persistence of infection.
Acknowledgments
Supported partly by T32CA132715 from the National Cancer Institute (SV, LA), P50CA101955 from the National Cancer Institute (JD, SV, MY), R01CA094084 from the National Cancer Institute (MY, SV, JD), and NIMHD/NIH-1P60MD003422 from the National Institute for Minority Health Disparities (SV, JH).
Abbreviations used
- Ad
adenovirus
- Ad5
adenovirus type 5
- Ad3
adenovirus type 3
- ADP
adenoviral death protein
- CMV promoter
cytomegalovirus immediate early promoter
- Cox2
cyclooxygenase-2
- CRAd
conditionally replicative adenovirus
- CAR
coxsackie-adenovirus receptor
- FBS
fetal bovine serum
- IFN
human interferon α
- Luc
firefly luciferase
- MLP
major late promoter
- pA
polyadenylation signal
- PBS
phosphate-buffered saline
- PDAc
pancreatic ductal adenocarcinoma
- RGD
Arg-Gly-Asp
- RLU
relative light units
- vp
viral particle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancer facts & figures 2010 [homepage on the Internet] American Cancer Society; Atlanta: 2010. Available from: http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-figures-2010-rev. [Google Scholar]
- 2.Merchant NB, Parikh AA, Liu EH. Adjuvant chemoradiation therapy for pancreas cancer: Who really benefits? Adv Surg. 2010;44:149–64. doi: 10.1016/j.yasu.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009 Apr;16(4):836–47. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Navarro J, Curiel DT. Conditionally replicative adenoviral vectors for cancer gene therapy. Lancet Oncol. 2000 Nov;1:148–58. doi: 10.1016/s1470-2045(00)00030-9. [DOI] [PubMed] [Google Scholar]
- 5.Hecht JR, Bedford R, Abbruzzese JL, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003 Feb;9(2):555–61. [PubMed] [Google Scholar]
- 6.Schenk E, Essand M, Bangma CH, et al. Clinical adenoviral gene therapy for prostate cancer. Hum Gene Ther. 2010 Jul;21(7):807–13. doi: 10.1089/hum.2009.206. [DOI] [PubMed] [Google Scholar]
- 7.Young A, McNeish IA. Oncolytic adenoviral gene therapy in ovarian cancer: Why we are not wasting our time. Future Oncol. 2009 Apr;5(3):339–57. doi: 10.2217/fon.09.11. [DOI] [PubMed] [Google Scholar]
- 8.Reid TR, Freeman S, Post L, et al. Effects of onyx-015 among metastatic colorectal cancer patients that have failed prior treatment with 5-FU/leucovorin. Cancer Gene Ther. 2005 Aug;12(8):673–81. doi: 10.1038/sj.cgt.7700819. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Curiel DT. Current issues and future directions of oncolytic adenoviruses. Mol Ther. 2010 Feb;18(2):243–50. doi: 10.1038/mt.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alemany R, Cascallo M. Oncolytic viruses from the perspective of the immune system. Future Microbiol. 2009 Jun;4(5):527–36. doi: 10.2217/fmb.09.28. [DOI] [PubMed] [Google Scholar]
- 11.Davydova J, Gavrikova T, Brown EJ, et al. In vivo bioimaging tracks conditionally replicative adenoviral replication and provides an early indication of viral antitumor efficacy. Cancer Sci. 2010 Feb;101(2):474–81. doi: 10.1111/j.1349-7006.2009.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez PJ, Vickers SM, Ono HA, et al. Optimization of conditionally replicative adenovirus for pancreatic cancer and its evaluation in an orthotopic murine xenograft model. Am J Surg. 2008 Apr;195(4):481–90. doi: 10.1016/j.amjsurg.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Davydova J, Wang M, et al. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003 Oct;125(4):1203–18. doi: 10.1016/s0016-5085(03)01196-x. [DOI] [PubMed] [Google Scholar]
- 14.Doronin K, Toth K, Kuppuswamy M, et al. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003 Jan 20;305(2):378–87. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- 15.Nukui Y, Picozzi VJ, Traverso LW. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2000 May;179(5):367–71. doi: 10.1016/s0002-9610(00)00369-x. [DOI] [PubMed] [Google Scholar]
- 16.Linehan DC, Tan MC, Strasberg SM, et al. Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: A single-institution phase II study. Ann Surg. 2008 Aug;248(2):145–51. doi: 10.1097/SLA.0b013e318181e4e9. [DOI] [PubMed] [Google Scholar]
- 17.Picozzi VJ, Abrams RA, Decker PA, et al. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b-based chemoradiation: ACOSOG trial Z05031. Ann Oncol. 2010 Aug 9; doi: 10.1093/annonc/mdq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003 May;185(5):476–80. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 19.Hara H, Kobayashi A, Yoshida K, et al. Local interferon-alpha gene therapy elicits systemic immunity in a syngeneic pancreatic cancer model in hamster. Cancer Sci. 2007 Mar;98(3):455–63. doi: 10.1111/j.1349-7006.2007.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallaux FJ, Kranenburg O, Cramer SJ, et al. Characterization of 911: A new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther. 1996 Jan 20;7(2):215–22. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 21.Davydova J, Le LP, Gavrikova T, et al. Infectivity-enhanced cyclooxygenase-2-based conditionally replicative adenoviruses for esophageal adenocarcinoma treatment. Cancer Res. 2004 Jun 15;64(12):4319–27. doi: 10.1158/0008-5472.CAN-04-0064. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Alemany R, Adachi Y, et al. Characterization of the cyclooxygenase-2 promoter in an adenoviral vector and its application for the mitigation of toxicity in suicide gene therapy of gastrointestinal cancers. Mol Ther. 2001 Mar;3(3):385–94. doi: 10.1006/mthe.2001.0275. [DOI] [PubMed] [Google Scholar]
- 23.Ono HA, Le LP, Davydova JG, et al. Noninvasive visualization of adenovirus replication with a fluorescent reporter in the E3 region. Cancer Res. 2005 Nov 15;65(22):10154–8. doi: 10.1158/0008-5472.CAN-05-1871. [DOI] [PubMed] [Google Scholar]
- 24.Sunamura M, Hamada H, Motoi F, et al. Oncolytic virotherapy as a novel strategy for pancreatic cancer. Pancreas. 2004 Apr;28(3):326–9. doi: 10.1097/00006676-200404000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Alemany R. Cancer selective adenoviruses. Mol Aspects Med. 2007 Feb;28(1):42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Garber K. China approves world’s first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006 Mar 1;98(5):298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 27.Yan BM, Van Dam J. Endoscopic ultrasound-guided intratumoural therapy for pancreatic cancer. Can J Gastroenterol. 2008 Apr;22(4):405–10. doi: 10.1155/2008/104398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cascante A, Abate-Daga D, Garcia-Rodriguez L, et al. GCV modulates the antitumoural efficacy of a replicative adenovirus expressing the Tat8-TK as a late gene in a pancreatic tumour model. Gene Ther. 2007 Oct;14(20):1471–80. doi: 10.1038/sj.gt.3303008. [DOI] [PubMed] [Google Scholar]
- 29.Trujillo MA, Oneal MJ, McDonough S, et al. A probasin promoter, conditionally replicating adenovirus that expresses the sodium iodide symporter (NIS) for radiovirotherapy of prostate cancer. Gene Ther. 2010 Nov;17(11):1325–32. doi: 10.1038/gt.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bortolanza S, Bunuales M, Otano I, et al. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in syrian hamsters. Mol Ther. 2009 Apr;17(4):614–22. doi: 10.1038/mt.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furukawa K, Ohashi T, Haruki K, et al. Combination treatment using adenovirus vector-mediated tumor necrosis factor-alpha gene transfer and a NF-kappaB inhibitor for pancreatic cancer in mice. Cancer Lett. 2011 Jul 1;306(1):92–8. doi: 10.1016/j.canlet.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 32.Motoi F, Sunamura M, Ding L, et al. Effective gene therapy for pancreatic cancer by cytokines mediated by restricted replication-competent adenovirus. Hum Gene Ther. 2000 Jan 20;11(2):223–35. doi: 10.1089/10430340050015978. [DOI] [PubMed] [Google Scholar]
- 33.Sova P, Ren XW, Ni S, et al. A tumor-targeted and conditionally replicating oncolytic adenovirus vector expressing TRAIL for treatment of liver metastases. Mol Ther. 2004 Apr;9(4):496–509. doi: 10.1016/j.ymthe.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996 Oct;70(10):6839–46. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesseling JG, Yamamoto M, Adachi Y, et al. Midkine and cyclooxygenase-2 promoters are promising for adenoviral vector gene delivery of pancreatic carcinoma. Cancer Gene Ther. 2001 Dec;8(12):990–6. doi: 10.1038/sj.cgt.7700403. [DOI] [PubMed] [Google Scholar]
- 36.Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J. 1998 Sep;12(12):1063–73. [PubMed] [Google Scholar]
- 37.Ohashi M, Yoshida K, Kushida M, et al. Adenovirus-mediated interferon alpha gene transfer induces regional direct cytotoxicity and possible systemic immunity against pancreatic cancer. Br J Cancer. 2005 Aug 22;93(4):441–9. doi: 10.1038/sj.bjc.6602713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shashkova EV, Kuppuswamy MN, Wold WS, Doronin K. Anticancer activity of oncolytic adenovirus vector armed with IFN-alpha and ADP is enhanced by pharmacologically controlled expression of TRAIL. Cancer Gene Ther. 2008 Feb;15(2):61–72. doi: 10.1038/sj.cgt.7701107. [DOI] [PMC free article] [PubMed] [Google Scholar]