Abstract
Objective
This is a retrospective review of 22 surgically treated benign and malignant tumors of brachial plexus region to describe clinical presentation, the characteristics of brachial plexus tumor and clinical outcomes with a literature review.
Methods
Twenty-one patients with consecutive 22 surgeries for primary brachial plexus tumors were enrolled between February 2002 and November 2011 were included in this study. The medical records of all patients were reviewed.
Results
Eleven male and 10 female patients were enrolled. Mean age was 39 years. Three patients had brachial plexus tumor associated with neurofibromatosis (13.6%). Presenting signs and symptoms included parenthesis and numbness (54.5%), radiating pain (22.7%), direct tenderness and pain (27.2%), palpable mass (77.3%). Twelve patients presented preoperative sensory deficit (54.5%) and 9 patients presented preoperative motor deficit (40.9%). Twenty tumors (90.9%) were benign and 2 tumors (9.1%) were malignant. Benign tumors included 15 schwannomas (68.2%), 4 neurofibromas (18.2%) and 1 granular cell tumor (4.5%). There were 1 malignant peripheral nerve sheath tumor (MPNST) and 1 malignant granular cell tumor. Gross total resection was achieved in 16 patients (72.7%), including all schwannomas, 1 neurofibroma. Subtotal resection was performed in 6 tumors (27.3%), including 3 neurofibromatosis associated with brachial plexus neurofibromas, 1 MPNST and 2 granular cell tumor in one patient.
Conclusion
Resection of tumor is the choice of tumor in the most of benign and malignant brachial plexus tumors. Postoperative outcomes are related to grade of resection at surgery and pathological features of tumor.
Keywords: Brachial plexus tumor, Nerve sheath tumor, Schwannoma, Neurofibroma, Granular cell tumor
INTRODUCTION
The brachial plexus is frequently involved by malignant tumors of the lung apex and breast, or as a result of radiotherapy. Malignant bone tumors, such as osteosarcoma, arising from the cervical spine, clavicle or first rib may less commonly invade the plexus. However, primary tumors of the brachial plexus are rare. A review of the literature over the last decades demonstrates a scarcity of publications on this type of tumor. With the exception of the large series of 226 brachial plexus region tumors from the Louisiana State University Health Sciences Center (LSUHSC), most of the publications are case reports or small series of patients7,18,19). Schwannomas and neurofibromas are the most common tumors of the brachial plexus region, and in most series in the literature neurofibroma is more prevalent6,18,21). Peripheral non-neural sheath brachial plexus tumors are even rarer than tumors of neural origin and comprise a wide variety of benign and malignant tumors and tumor-like conditions14,18). With regard to those tumors, preoperative evaluation and planning of proper treatment may be changed according to the origin, characteristics, extension and pathology.
This is a retrospective review of 22 surgically treated benign and malignant tumors of brachial plexus region to describe clinical presentation, the characteristics of brachial plexus tumor and clinical outcomes with a literature review.
MATERIALS AND METHODS
Patient population
Twenty-one patients with consecutive 22 surgeries for primary brachial plexus tumors were enrolled between February 2002 and November 2011 were included in this study. The medical records of all patients were reviewed. Relevant medical history, presence of neurofibromatosis type 1 (NF1) and positive details of the neurological examination were documented. The location, size, mobility and tenderness on palpation of the tumor were assessed. Plain X-ray films and computerized tomography (CT) scans of tumors close to the spine were examined to find evidence of intervertebral foraminal enlargement or vertebral erosion. Magnetic resonance (MR) imaging was performed to document tumor location, margins, and relationship with adjacent structures. Malignant tumors were also evaluated with lung and abdominal CT scans and bone scans. The histopathological diagnosis of all tumors based on light microscopy was reviewed. In some instances, immunohistological methods were used to confirm the differential diagnosis.
Surgical approach
All patients were admitted for surgical treatment. The surgical exposure depended on the site of the lesion and was initially performed under loupe magnification. The lesions involving roots and trunks were exposed through an anterior supraclavicular approach. The lower lesions, involving cords and terminal nerves, required an anterior infraclavicular approach and when there was a more extensive involvement of the plexus, including its retroclavicular part, a combined anterior approach, with or without section of the clavicle, was employed. After gross identification of the tumor, the surgical microscope was brought into the field. In the beginning, special attention was devoted to the identification, isolation and mobilization of all adjacent plexus elements, which must be dissected together with non-neural tissues away from the tumor. Under higher magnification, the proximal and distal elements directly involved and the margins of the tumor were defined. In the benign, solitary tumors, the thickened epineurium over the lesion was opened until the capsule and the mass were dissected away from adjacent nerve fascicles, in an extra capsular plane, usually as a single mass. Fascicles entering the substance of the tumor or its capsule were isolated at the proximal and distal poles of the mass. Intraoperative electrical stimulation and nerve action potential (NAP) recording provided information about non-functioning fascicles entering and exiting the tumor. The extent of tumor removal varied from gross total resection to sub-total resection. A more aggressive resection was substituted by a less radical level of resection whenever a significant motor impairment could result. In these cases, tumor remnants were left to preserve nerve continuity.
RESULTS
Demography and clinical presentation
In our review, we identified 21 patients with 22 brachial plexus tumors. Neurofibromatosis was present in 3 patients (13.0%). Demographic and clinical characteristics of our series are summarized in Table 1. Eleven male and 10 female patients were enrolled. Mean age was 39 years (range 16-66 years). The duration of symptoms ranged from 6 months to 10 years prior to the surgical treatment. The brachial plexus tumor was discovered incidentally in one patient. Three patients had brachial plexus tumor associated with neurofibromatosis (13.6%). Presenting signs and symptoms included paresthesis and numbness (54.5%), radiating pain (22.7%), direct tenderness and pain (27.2%), palpable mass (77.3%). Growing mass (95.4%) was the most common presenting symptoms. Twelve patients presented preoperative sensory deficit (54.5%) and 9 patients among those patients accompanied with preoperative motor deficit (40.9%).
Table 1.
Summary of clinical characteristics of tumors, localization, size, grade of resection at surgery, pathological findings and follow-up in a series of 22 patients with brachial plexus tumor
*Largest diameter (centimeters). M : male, F : female, SC : supraclavicular, IC : infraclavicular, GTR : gross total resection, STR : subtotal resection, MPNST : malignant peripheral nerve sheath tumor
MRI findings
On MRI finding, almost all brachial plexus tumors showed well-defined mass with the long axis in line with the nerve of origin and homogeneous intermediate signal intensity (SI) on T1-weighted sequences (Fig. 1). The tumors were hyperintense on T2-weighted images with inhomogeneous central low SI (the target sign) (Fig. 2) and strong enhancement following contrast administration (Fig. 3).
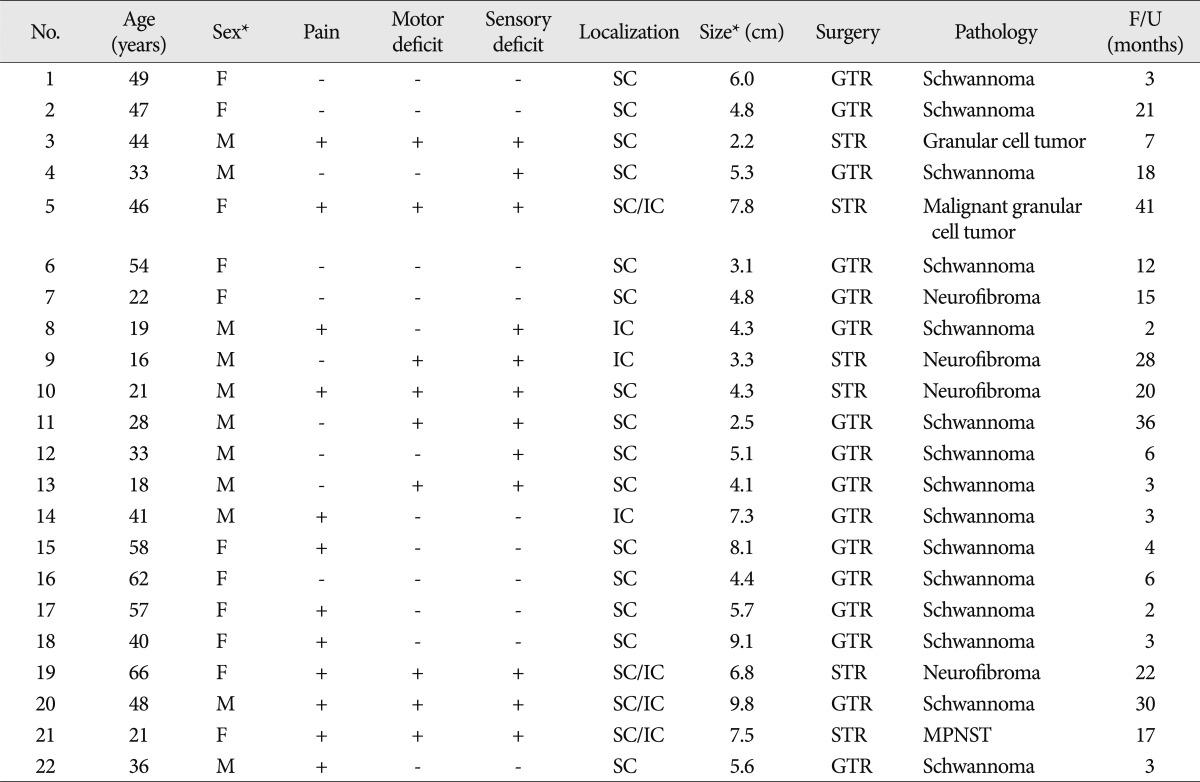
Fig. 1.
Coronal T1-weighted SE image showing a small schwannoma arising from a trunk of the left brachial plexus. The lesion is isointense to muscle.
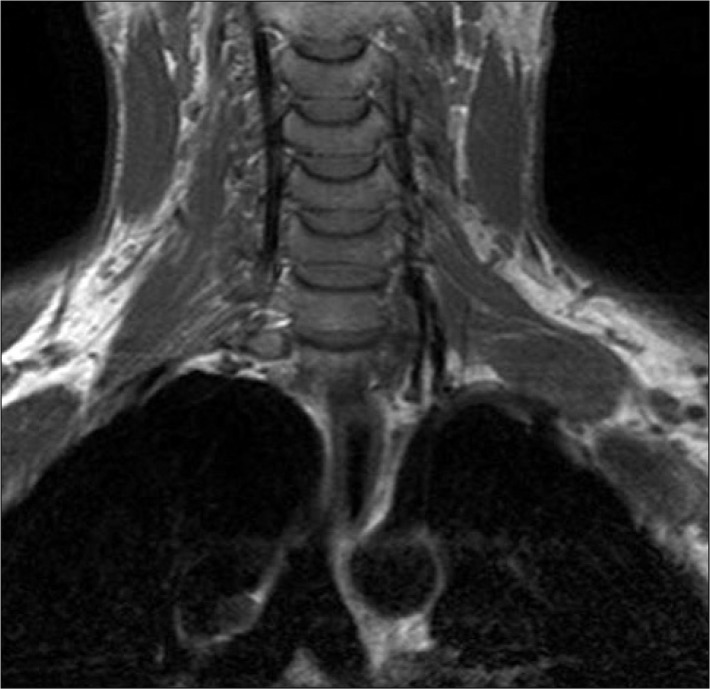
Fig. 2.
Axial T2-weighted FSE image showing a large schwannoma arising from the left brachial plexus in a patient with schwannoma. The lesion is hyperintense and displays the typical "target sign" that characterizes a benign nerve sheath tumor.
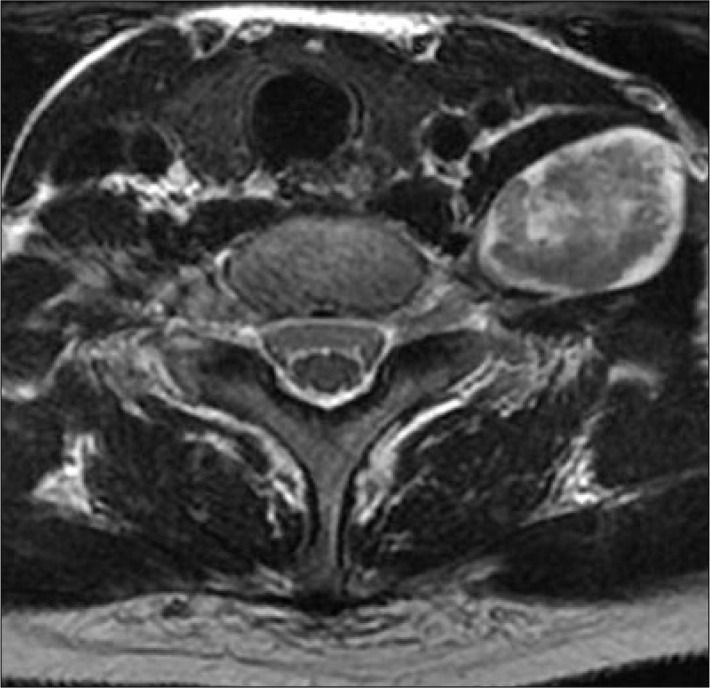
Fig. 3.
Axial T1-weighted SE image of a patient with a schwannoma arising from the left brachial plexus. Pre-contrast image (A) shows the lesion to be homogeneous and isointense to muscle. Following gadolinium administration (B), the tumor shows inhomogeneous enhancement.
The tumors were hyperintense on T2-weighted images and showed strong enhancement following contrast administration. Schwannomas showed globoid mass with well demarcation and surrounding nerved were suspended eccentrically from the tumor. Neurofibromas showed fusiform and elongated enlargement. Grannular cell tumor showed multiple fusiform and lobulated enlargement of nerves, which were originated from several neural formamens.
Characteristic of tumor and intraoperative findings
The 22 tumors comprised a heterogeneous group of lesions in terms of their size, location and pathological characteristics. (Table 1). Third and 5th cases were developed in the same patient. The largest diameter of the smallest lesion and the largest lesion were 2.2 and 9.8 centimeters, respectively (average 6.98 cm). Most of schwannomas appeared grossly as smooth globoid masses, which did not enlarge the nerve but are suspended eccentrically from it with a discrete attachment. Complete resection of tumor could be achieved in all cases of schwannoma because of these gross findings. Most of neurofibromas produced fusiform enlargement of the involved nerve, which made it impossible to distinguish between tumor and neural tissue. Granular cell tumor appeared fusiform and multiple lobulate enlargement of the involved nerves, which was similar to gross feature of neurofibroma. But granular cell tumor was much harder consistency with poor demarcation between neural tissue and tumor, which impeded complete tumor resection.
Twenty tumors (90.9%) were benign and 2 tumors (9.1%) were malignant. Benign tumors included 15 schwannomas (68.2%), 4 neurofibromas (18.2%) and 1 GCT (4.5%). There were 1 malignant peripheral nerve sheath tumor (MPNST) and 1 malignant granular cell tumor.
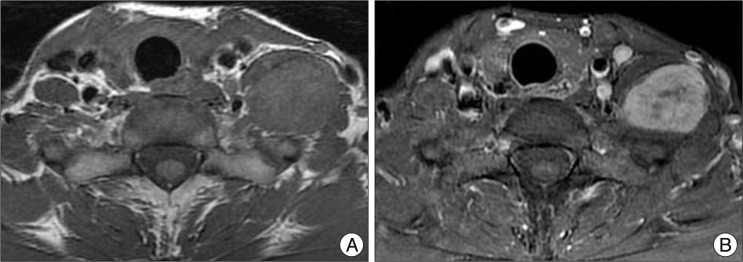
Gross total resection was achieved in 16 patients (72.7%), including all schwannomas, 1 neurofibroma. Subtotal resection was performed with a small residual lesion in 6 tumors (27.3%), including 3 neurofibromatosis associated with brachial plexus neurofibromas, 1 MPNST and 2 granular cell tumor in one patient. In case of granular cell tumor, subtotal resection was performed for the first surgery, and then small residual remnant had grown three timed size big just before the revision surgery (Fig. 4). The tumor was transformed into malignant granular cell tumor.
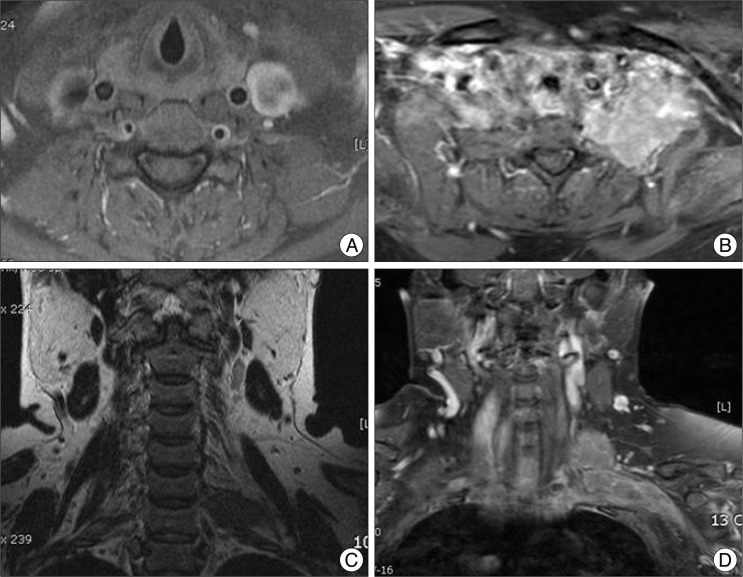
Fig. 4.
A 44-year-old female patient presented with left shoulder pain and weakness of left arm. Initial MRI (A and B) revealed brachial plexus tumor on left side which was oval and well delineated. Pathology had been a benign granular cell tumor at the first surgery and was transformed into malignant granular cell tumor (C and D) in 17 months later after surgery.
Clinical outcomes and follow-up
Mean follow-up period was 13.7 months (range 2-41 months). Radiating pain and localized direct tenderness were improved immediately after surgery in 10 out of 11 patients who complained of pain at presentation, 1 patient developed new pain after surgery. Twelve patients with preoperative parenthesis and numbness reported improvement after surgical treatment. Motor deficit as an initial symptom was improved in 7 out of 9 patients who complained of motor weakness at presentation.
New paresthesia developed in 2 patients who had relatively small sized lesions. Tumor size was 2.2 cm and 3.1 cm, respectively. New motor deficits developed in 2 patients after surgery. Newly developed motor deficit was improved during the follow-up period in 1 patient. Profound motor weakness was developed in the other patient with granular cell tumor after the first surgery and recovered to nearly intact status (G4+) for postoperative 6 months. The patient presented severe left shoulder pain with weakness of arm (G2). Even though pain was improved, complete monoplegia was developed after revision surgery with no improvement until final follow-up. The patient received concurrent chemotherapy and rehabilitation.
DISCUSSION
Brachial plexus region may be affected by various tumors and tumor-like conditions. Many reports have been published about brachial plexus tumor since Courvoisier reported the first description of the surgical excision of a brachial plexus tumor in 1886. In 1970 Dart et al.6) reported 27 cases of plexus tumors, 22 of which were nerve sheath tumors. In that same year, Fisher and Tate10) reviewed the literature for all brachial plexus neoplasms reported earlier than 1970. Otherwise, most other publications were confined to case reports or small series of nerve sheath tumors (NSTs)2,5,7,10,11,13,14,20,21,26,27). Clinical presentation of brachial plexus tumor is various according to location, extension, involved neural elements and pathology. Symptoms are caused by direct nerve invasion, infiltration of surrounding tissues, or local mass effect22). Huang et al.14) reported their experience with 42 patients with brachial plexus tumors. The most common presenting symptom was pain (70%), followed by sensory loss (61%) and weakness (52%). About half of their patients harbored benign NSTs : of these, 55% presented with a neurofibroma whereas 45% had a schwannoma. Kehoe et al.15) reported on 104 patients who presented with a solitary benign NST, 15 of which involved the brachial plexus. The majority of their patients presented with the primary complaint of a palpable mass, less than half complained of local pain or paresthesia. More recently, Siqueira et al.27) reported on a consecutive series of 18 patients who presented with brachial plexus tumors and tumor-like conditions. Most common presenting symptom was mass (75%), pain (58%), followed by sensory change (41%). In our series, the most common presenting symptom was growing mass (95.4%), parenthesis and numbness (54.5%), sensory deficit (54.5%), motor deficit (40.9%), direct tenderness and pain (27.2%), followed by included radiating pain (22.7%).
In cases of brachial plexus lesions, MR imaging is the study of choice to delineate the margins of the tumor from surrounding tissues with greatest contrast12,23). However, MR imaging is currently unable to differentiate between schwannoma and neurofibroma4). Other imaging modalities may be useful in selected cases. Plain radiography can demonstrate apical pulmonary lesions potentially involving the brachial plexus. Computerized tomography scanning is optimal at revealing osseous erosion around the spine or changes in neural foramina8). Typical MR features of benign NSTs include a well-defined oval mass with the long axis in line with the nerve of origin and homogeneous intermediate signal intensity (SI) on T1-weighted sequences (Fig. 1). The tumors are hyperintense on T2-weighted images with inhomogeneous central low SI (the target sign) (Fig. 2) and strong enhancement following contrast administration (Fig. 3)21,24). Less commonly, schwannomas and neurofibromas may be mildly hyperintense or hypointense to muscle on T1-weighted images and show non-uniform enhancement3,29-31). Gadolinium-enhanced MRI rarely provides additional information not provided by other MRI sequences. Features, including non-homogeneous contrast enhancement, an irregular, infiltrative margin and bone destruction, may be helpful to differentiate benign from malignant NSTs. These features are associated with necrosis and hemorrhage as suggestive findings4,28,30).
Schwannomas and neurofibromas are the most common tumors of the brachial plexus region, and in most series in the literature neurofibroma is more prevalent14,21). Huang et al.14) reported that about half of their patients harbored benign NSTs : of these, 55% presented with a neurofibroma whereas 45% had a schwannoma. On the contrary, Siqueira et al.27) reported that 9 presented with a schwannoma and 1 had a neurofibroma. In our series, the most common benign tumors of the brachial plexus region were schwannoma and neurofibroma as same as mentioned prior, but benign tumors included 15 schwannomas (68.2%), 4 neurofibromas (18.2%) and 1 granular cell tumor (4.5%). Schwannoma was more prevalent than neurofibroma. There were 1 malignant peripheral nerve sheath tumor (MPNST) and 1 malignant granular cell tumor. Twenty tumors (90.9%) were benign and 2 tumors (9.1%) were malignant.
Artico reported on cases involving 119 peripheral nerve sheath tumors (PNSTs), 11 of which involved the brachial plexus. In 93% of their patients with schwannomas and 81% of their patients with neurofibromas, motor function stabilized or improved postoperatively. At the 6-year follow-up, the best surgical results were observed in the patients with schwannoma, whereas the worst results were demonstrated in those with plexiform neurofibroma2). The interest in pre-therapeutic biopsy on benign lesions is limited because the sensitivity of this procedure is moderate and the procedure could damage intact fascicles or cause haemorrhage1,14,25). On the review of literature, postoperative outcomes were not influenced by surgical approach, but were related to grade of resection at surgery and pathological features of tumor. Gross total resection (GTR) can be expected for relatively small and medium size tumors, benign schwannoma, some of neurofibroma and other benign tumors, including desmoids, ganglions, cysts, epidermoids, and lipomas, and relatively good postoperative outcomes and prognosis can be expected after GTR18,19). In case of large size schwannoma, GTR can be expected. Postoperative neurologic dysfunction may occur after GTR. In our series, postoperative paresthesia and numbness was developed in three patients who had relatively small size tumors. Tumor capsule may be encountered after skin retraction in case of large size tumor, and then further procedure can be proceeded in side of the tumor capsule. On the contrary, manipulation of nerve tissues may be needed after skin retraction in case of small size tumor. Whereas, GTR can not be expected in case of some neurofibromas, MPNST and other malignant tumors because those tumors have diffuse infiltration to surrounding nerves and connective tissue. Subtotal resection (STR) may be recommended for those tumors to preserve neurological function. Even when benign tumors are carefully dissected from the nerve involved under magnification, transient incomplete nerve paralysis may occur27).
Hosoi calculated that MPNST develops in approximately 13% of patients with von Recklinghausen disease27). Based on longer-term observation of a large series of patients, Brasfield and Das Gupta reported an overall incidence of 29%7). Although a small number of cases of MPNSTs have been reported in patients without NF, it has long been recognized that NF (especially NF Type 1, von Recklinghausen disease) is associated with a high incidence of MPNST. MPNST is generally considered chemotherapy resistant tumors. The role of radiation therapy is not completely defined in the management of MPNSTs. However, post-operative radiotherapy is currently recommended by oncology consensus as part of standard treatment guidelines for these tumors, despite clear surgical margins32). Particularly in patients with small-sized sarcomas, surgical treatment may promote cure, but in bigger lesions (the majority of the cases), the patients should undergo adjuvant treatment after surgery. External radiation therapy prevents local recurrence more effectively than surgery alone. Despite aggressive surgery with adjuvant therapies, both morbidity and mortality rates are high.
One case of our series was granular cell tumor at the first surgery and was transformed into malignant granular cell tumor at 17 months later after surgery. Granular cell tumor is benign or malignant depends histologically on evidence of increased cellularity, nuclear atypia and mitotic activity9). However, on rare occasions, a benign-appearing granular cell tumor may exhibit signs of malignancy, including invasion, local recurrence and distant metastasis before or after surgery. The tumor occurs unifocally (93%) and multifocally (7%)9,16,17). Granular cell tumor usually has a poor prognosis despite surgery, radiation and chemotherapy. Local recurrence rate is 69% from 3 months to 3 years or more after initial surgery, and distant metastasis rate is 80% of patients before or after surgery9,16,17).
CONCLUSION
Various tumors and tumor-like conditions may affect brachial plexus region. Resection of tumor is the choice of treatment in the most of benign and malignant brachial plexus tumors. The appropriate surgical strategy is necessary in every brachial plexus tumor because postoperative outcome is influenced by grade of resection at surgery and pathological features of tumor. Appropriate intraoperative microsurgical dissection technique and electrophysiologic monitoring are essential for favorable clinical outcome. In malignant tumors, surgical treatment should be planed and followed by adjuvant chemotherapy or radiation therapy to alleviate presenting symptom and reduction of tumor volume.
References
- 1.Amoretti N, Grimaud A, Hovorka E, Chevallier P, Roux C, Bruneton JN. Peripheral neurogenic tumors : is the use of different types of imaging diagnostically useful? Clin Imaging. 2006;30:201–205. doi: 10.1016/j.clinimag.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Artico M, Cervoni L, Wierzbicki V, D'Andrea V, Nucci F. Benign neural sheath tumours of major nerves : characteristics in 119 surgical cases. Acta Neurochir (Wien) 1997;139:1108–1116. doi: 10.1007/BF01410969. [DOI] [PubMed] [Google Scholar]
- 3.Beggs I. Pictorial review : imaging of peripheral nerve tumours. Clin Radiol. 1997;52:8–17. doi: 10.1016/s0009-9260(97)80299-1. [DOI] [PubMed] [Google Scholar]
- 4.Cerofolini E, Landi A, DeSantis G, Maiorana A, Canossi G, Romagnoli R. MR of benign peripheral nerve sheath tumors. J Comput Assist Tomogr. 1991;15:593–597. doi: 10.1097/00004728-199107000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Miyahara R, Matsunaga Y, Koyama T. Schwannoma of the brachial plexus presenting as an enlarging cystic mass : report of a case. Ann Thorac Cardiovasc Surg. 2008;14:311–313. [PubMed] [Google Scholar]
- 6.Dart LH, Jr, MacCarty CS, Love JG, Dockerty MB. Neoplasms of the brachial plexus. Minn Med. 1970;53:959–964. [PubMed] [Google Scholar]
- 7.Das S, Ganju A, Tiel RL, Kline DG. Tumors of the brachial plexus. Neurosurg Focus. 2007;22:E26. doi: 10.3171/foc.2007.22.6.27. [DOI] [PubMed] [Google Scholar]
- 8.Filler AG, Kliot M, Howe FA, Hayes CE, Saunders DE, Goodkin R, et al. Application of magnetic resonance neurography in the evaluation of patients with peripheral nerve pathology. J Neurosurg. 1996;85:299–309. doi: 10.3171/jns.1996.85.2.0299. [DOI] [PubMed] [Google Scholar]
- 9.Finkel G, Lane B. Granular cell variant of neurofibromatosis : ultrastructure of benign and malignant tumors. Hum Pathol. 1982;13:959–963. doi: 10.1016/s0046-8177(82)80064-6. [DOI] [PubMed] [Google Scholar]
- 10.Fisher RG, Tate HB. Isolated neurilemomas of the brachial plexus. J Neurosurg. 1970;32:463–467. doi: 10.3171/jns.1970.32.4.0463. [DOI] [PubMed] [Google Scholar]
- 11.Forte A, Gallinaro LS, Bertagni A, Montesano G, Prece V, Illuminati G. Neurinomas of the brachial plexus : case report. Eur Rev Med Pharmacol Sci. 1999;3:19–21. [PubMed] [Google Scholar]
- 12.Grant GA, Goodkin R, Maravilla KR, Kliot M. MR neurography : diagnostic utility in the surgical treatment of peripheral nerve disorders. Neuroimaging Clin N Am. 2004;14:115–133. doi: 10.1016/j.nic.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Gyhra A, Israel J, Santander C, Acuña D. Schwannoma of the brachial plexus with intrathoracic extension. Thorax. 1980;35:703–704. doi: 10.1136/thx.35.9.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang JH, Zaghloul K, Zager EL. Surgical management of brachial plexus region tumors. Surg Neurol. 2004;61:372–378. doi: 10.1016/j.surneu.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Kehoe NJ, Reid RP, Semple JC. Solitary benign peripheral-nerve tumours. Review of 32 years' experience. J Bone Joint Surg Br. 1995;77:497–500. [PubMed] [Google Scholar]
- 16.Khansur T, Balducci L, Tavassoli M. Identification of desmosomes in the granular cell tumor. Implications in histologic diagnosis and histogenesis. Am J Surg Pathol. 1985;9:898–904. doi: 10.1097/00000478-198512000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Khansur T, Balducci L, Tavassoli M. Granular cell tumor. Clinical spectrum of the benign and malignant entity. Cancer. 1987;60:220–222. doi: 10.1002/1097-0142(19870715)60:2<220::aid-cncr2820600217>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 146 peripheral non-neural sheath nerve tumors : 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:256–266. doi: 10.3171/jns.2005.102.2.0256. [DOI] [PubMed] [Google Scholar]
- 19.Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors : 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246–255. doi: 10.3171/jns.2005.102.2.0246. [DOI] [PubMed] [Google Scholar]
- 20.Kori SH, Foley KM, Posner JB. Brachial plexus lesions in patients with cancer : 100 cases. Neurology. 1981;31:45–50. doi: 10.1212/wnl.31.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Lusk MD, Kline DG, Garcia CA. Tumors of the brachial plexus. Neurosurgery. 1987;21:439–453. doi: 10.1227/00006123-198710000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Mrugala MM, Batchelor TT, Plotkin SR. Peripheral and cranial nerve sheath tumors. Curr Opin Neurol. 2005;18:604–610. doi: 10.1097/01.wco.0000179507.51647.02. [DOI] [PubMed] [Google Scholar]
- 23.Posniak HV, Olson MC, Dudiak CM, Wisniewski R, O'Malley C. MR imaging of the brachial plexus. AJR Am J Roentgenol. 1993;161:373–379. doi: 10.2214/ajr.161.2.8392788. [DOI] [PubMed] [Google Scholar]
- 24.Richardson RR, Siqueira EB, Oi S, Nunez C. Neurogenic tumors of the brachial plexus : report of two cases. Neurosurgery. 1979;4:66–70. doi: 10.1227/00006123-197901000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Russell SM, Kline DG. Complication avoidance in peripheral nerve surgery : injuries, entrapments, and tumors of the extremities--part 2. Neurosurgery. 2006;59:ONS449–ONS456. doi: 10.1227/01.NEU.0000235143.60461.E7. discussion ONS456-ONS457. [DOI] [PubMed] [Google Scholar]
- 26.Saini S, Dhasmana JP. Apical mass presentation of brachial plexus Schwannoma. Indian J Chest Dis Allied Sci. 2006;48:129–131. [PubMed] [Google Scholar]
- 27.Siqueira MG, Martins RS, Teixeira MJ. Management of brachial plexus region tumours and tumour-like conditions : relevant diagnostic and surgical features in a consecutive series of eighteen patients. Acta Neurochir (Wien) 2009;151:1089–1098. doi: 10.1007/s00701-009-0380-8. [DOI] [PubMed] [Google Scholar]
- 28.Stull MA, Moser RP, Jr, Kransdorf MJ, Bogumill GP, Nelson MC. Magnetic resonance appearance of peripheral nerve sheath tumors. Skeletal Radiol. 1991;20:9–14. doi: 10.1007/BF00243714. [DOI] [PubMed] [Google Scholar]
- 29.Suh JS, Abenoza P, Galloway HR, Everson LI, Griffiths HJ. Peripheral (extracranial) nerve tumors : correlation of MR imaging and histologic findings. Radiology. 1992;183:341–346. doi: 10.1148/radiology.183.2.1561333. [DOI] [PubMed] [Google Scholar]
- 30.Varma DG, Moulopoulos A, Sara AS, Leeds N, Kumar R, Kim EE, et al. MR imaging of extracranial nerve sheath tumors. J Comput Assist Tomogr. 1992;16:448–453. doi: 10.1097/00004728-199205000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Verstraete KL, Achten E, De Schepper A, Ramon F, Parizel P, Degryse H, et al. Nerve sheath tumors : evaluation with CT and MR imaging. J Belge Radiol. 1992;75:311–320. [PubMed] [Google Scholar]
- 32.Wanebo JE, Malik JM, VandenBerg SR, Wanebo HJ, Driesen N, Persing JA. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 28 cases. Cancer. 1993;71:1247–1253. doi: 10.1002/1097-0142(19930215)71:4<1247::aid-cncr2820710413>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]