Abstract
Reports of influenza A virus infections in dogs has received considerable attention from veterinarians, virologists, and epidemiologists. Interaction between influenza viral hemagglutinin and cell oligosaccharides containing sialic acid residues results in infection. Sialic acids have an α-2,3-linkage to the penultimate galactose in the avian influenza virus receptor and an α-2,6-linkage in the human receptor. To date, there are no detailed data on the tissue distribution or histological features of either type of sialic acid-linked influenza virus receptors in beagle dogs, which are common laboratory animals and pets. We conducted the current study to visualize the in situ tissue distribution of both sialic acid-linked influenza virus receptors in various organs of beagle dogs using Maackia amurensis lectin II and Sambucus nigra agglutinin. Both α-2,3- and α-2,6-sialic acid-linked receptors were detected in the endothelial cells of the respiratory tract and other organs. Endothelial cells of most gastrointestinal organs were negative for α-2,3-sialic acid-linked receptors in the dogs. Our results suggested that these canine organs may be affected by influenza virus infection. The findings from our study will also help evaluate the occurrence and development of influenza virus infections in dogs.
Keywords: dogs, influenza virus receptors, lectin, sialic acid
Introduction
Influenza A viruses have a wide host range, from birds to mammals, and exhibit varying degrees of host adaptation [1,2]. During the last decade, reports of influenza A virus infections in dogs has drawn considerable attention from veterinary practitioners and scientists specializing in virology and epidemiology [8]. Dogs have been reported to be infected by the highly pathogenic H5N1 avian influenza virus [1,3,4,7,8,21], the H3N2 avian influenza virus [8,19,20], and the H3N8 equine influenza virus [5,6,8,10,16]. It is still unknown whether other types of influenza viruses infect dogs. Infection with these viruses requires interaction between cell oligosaccharides containing sialic acid (SA) and the influenza viral hemagglutinin [13,22]. Different sialyloligosaccharides have varying roles in the entry of avian and human influenza viruses into cells. Receptors containing sialic acids with an α-2,3-linkage to the penultimate galactose (SAα-2,3-gal) are widely believed to be the receptor for the avian influenza viruses, while human viruses prefer receptors that contain an α-2,6-linkage (SAα-2,6-gal) [13,22,23]. These data suggest that the key factor for influenza infection is the prevalence of sialic acid molecules in the virus-specific receptors.
Dogs are a major risk for transmitting influenza infections to humans since these animals are commonly kept as pets. Knowledge about the distribution of sialic acid-linked receptors in canines could increase our understanding of the pathogenesis and pathogenicity of the influenza virus, and help estimate the risk of intertransmission. To date, no detailed tissue distribution data have been reported for α-2,3- or α-2,6-sialic acid-linked influenza virus receptors in beagle dogs, a standard laboratory animal and pet. The current study was performed to obtain concrete histological data of both receptors in a variety of tissues and provide fundamental data on influenza virus infections in beagle dogs.
Materials and Methods
Animals and tissues preparation
Five beagles, including three female and two male, 14~16 weeks old were obtained from the Center of Laboratory Animals of the Southern Medical University (China). The dogs were anesthetized using xylazine hydrochloride (Hanhe, China) with 0.3 mL/kg body weight and sacrificed. Samples of tissue were then taken from the trachea, lung, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, heart, liver, spleen, and kidney. All tissues were cut into 4-µm sections using routine methods [15].
Histochemical analysis with plant lectins to detect sialic acid-linked influenza virus receptors
Lectins used in this research were biotinylated Maackia (M.) amurensis lectin II (specific for α-2,3-linked sialic acid in avian influenza virus receptors) and Sambucus nigra agglutinin (SNA) (specific for α-2,6-linked sialic acid in human influenza virus receptors) from Vector Laboratories (USA). Histochemical staining with the lectins was performed as described in our previous publication [15]. Briefly, the pretreated tissue sections were incubated by SNA and M. amurensis agglutinin (Vector Laboratories, USA) and then the specific labeling was got using streptavidin-biotin complex kit (Vector Laboratories, USA) and diaminobenzidine kit (Promega, USA). Specificity of the lectin staining was confirmed by pretreatment with neuraminidase (NEB, USA) as previously described [15,25]. Briefly, after the slides were treated by neuraminidase, lectin staining was performed as described above. Negative controls were treated by phosphate buffered saline.
Results
Distribution of sialic acid-linked influenza virus receptors in the respiratory tract of dogs
In the trachea, goblet cells of the mucosa were strongly positive for α-2,3-sialic acid-linked receptors while only some of these cells showed weak to intermediate staining for α-2,6-sialic acid-linked influenza virus receptors. Ciliated cells were diffusely positive for α-2,3-sialic acid-linked influenza virus receptors but negative for α-2,6-sialic acid-linked influenza virus receptors. The lamina propria of the mucosa was diffusely positive for α-2,3-sialic acid-linked influenza virus receptors, but foci of α-2,6-sialic acid-linked influenza virus receptors were observed. The submucosa showed slightly diffuse staining for α-2,3-sialic acid-linked influenza virus receptors while foci of α-2,6-sialic acid-linked influenza virus receptors were detected (Fig. 1).
Fig. 1.
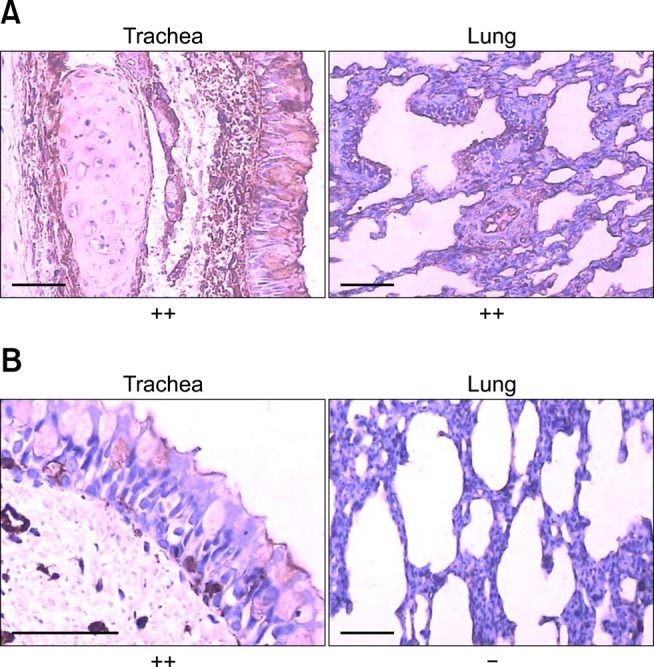
Distribution of α-2,3-sialic acid-linked (SAα-2,3-gal) influenza virus receptors (A), and α-2,6-sialic acid-linked (SAα-2,6-gal) influenza virus receptors (B) in the beagle respiratory tract. -: no staining, ++: many positive cells. Scale bars = 50 µm.
In the bronchus, distribution of both receptor types was similar to that found in the trachea. In the lamina propria of the mucosa and submucosa, diffuse staining for α-2,3-sialic acid-linked influenza virus receptors was observed and foci of α-2,6-sialic acid-linked influenza virus receptors were seen. In the respiratory zone of the lung, staining for α-2,3-sialic acid-linked influenza virus receptors was diffuse in ciliated and non-ciliated cells of the bronchi and bronchioles along with the alveolar cells of the pulmonary alveoli. In contrast, almost no positive staining for α-2,6-sialic acid-linked influenza virus receptors was detected (Fig. 1).
Distribution of sialic acid-linked influenza virus receptors in the gastrointestinal tract of dogs
In the stomach, most endothelial cells of the mucosa and glands in lamina propria did not express α-2,3-sialic acid-linked influenza virus receptors. However, a small number of endothelial cells in the mucosa, lamina propria of the mucosa, and gland connective tissues in the lamina propria, submucosa, and adventitia were positive. Staining for α-2,6-sialic acid-linked influenza virus receptors in mucosal endothelial cells was weak while the glands were strongly positive. The lamina propria of the mucosa and connective tissues of the glands were negative.
In the duodenum, epithelial cells of the mucosa were negative for α-2,3-sialic acid-linked influenza virus receptors but epithelial cells of the central lacteal and submucosa layer were positive. Goblet epithelial cells of the mucosa were weakly positive for α-2,6-sialic acid-linked influenza virus receptors as were epithelial cells of the central lacteal, lamina propria of the mucosa, and submucosa layer. In the jejunum, epithelial cells of the mucosa were negative for α-2,3- and α-2,6-sialic acid-linked influenza virus receptors, but positive staining for both receptors was observed in the submucosa layer, lamina propria of the mucosa, and connective tissues between the glands. In the ileum, distribution of α-2,3-and α-2,6-sialic acid-linked influenza virus receptors was similar to that found in the duodenum. In the cecum and colon, the lamina propria of the mucosa was strongly positive for α-2,3-sialic acid-linked influenza virus receptors while endothelial cells of the glands were weakly positive. Endothelial cells of the mucosa were also positive. In the rectum, goblet cells of the mucosa and endothelial cells of the glands were positive for both types of receptors. In contrast, the lamina propria of the mucosa was negative for α-2,3-sialic acid-linked influenza virus receptors but positive for α-2,6-sialic acid-linked influenza virus receptors.
Distribution of sialic acid-linked influenza virus receptors in other organs of dogs
Cerebrum neurons and ground substance of the brain were negative for both types of lectins, except for endothelial cells in blood vessels that were positive for α-2,3- and α-2,6-sialic acid-linked influenza virus receptors. In the pancreas, the endocrine portions were positive for α-2,3- and α-2,6-sialic acid-linked influenza virus receptors as were the duct epithelium and connective tissues. The exocrine portion was negative for both receptors. Kupffer cells, bile duct epithelium, and endothelial cells of blood vessels in the liver were focally positive for both receptors, but the hepatocytes were negative.
Cardiac muscle cells were weakly positive for α-2,3-sialic acid-linked influenza virus receptors but negative for α-2,6-sialic acid-linked influenza virus receptors. Connective tissues between the muscle cells and endothelial cells of blood vessels in the heart were positive for both receptors. In spleen, α-2,3-sialic acid-linked influenza virus receptors were detected primarily in the periarterial lymphatic sheath and marginal zone. Focal staining specific for this receptor was also observed in cells of the lymphoid nodules, although most of these cells were negative. In contrast, α-2,6-sialic-acid-linked influenza virus receptors were widely detected, primarily in lymphoid nodule cells, while most cells of the periarterial lymphatic sheath and marginal zone were negative. In the kidney, glomerulus endothelial cells contained diffuse staining for α-2,3- and α-2,6-sialic acid-linked influenza virus receptors. Epithelial cells of the proximal tubule were diffusely positive for both receptors although the thin segment and distal tubules were negative for both receptors.
Discussion
Both α-2,3- and α-2,6-sialic acid-linked influenza virus receptors were detected in all organs of the dogs we examined, suggesting that these organs are potential targets for influenza virus infection. The presence of α-2,3-sialic acid-linked influenza virus receptors extended from the upper to the lower respiratory tract. α-2,6-sialic acid-linked influenza virus receptors were also detected in the respiratory tract but only weak focal staining was observed. These data agree with the results of previous studies in other species [9,14,15,17,24,25]. Daly et al. [5] also reported that α-2,6-sialic acid-linked influenza virus receptors in the respiratory tract epithelium of English foxhounds were able to bind lectin. However, Song et al. [19], found that almost no α-2,6-sialic acid-linked receptors in the respiratory tract of beagle dogs bind lectin. Differences in the data from these reports may be caused by variations in lectins produced from different sources.
The presence of α-2,3- and α-2,6-sialic acid-linked influenza virus receptors in the gastrointestinal tract may be of interest to virologists. We hypothesize that experimentally infecting dogs with avian influenza virus through the gastrointestinal tract may be relatively easy, but infecting with human influenza virus may be difficult due to low receptor expression in digestive tract endothelial cells. The H5N1 avian influenza virus has been reported to directly infect and replicate in human gut tissues [18], so we predicted that this also occurs in dogs. Depending on the type of influenza virus infection, gastrointestinal symptoms may be minor due to differential expression of the sialic acid-linked influenza virus receptors.
Based on the distribution of influenza virus receptors in the brain, dogs may not suffer influenza virus infection of this organ. Spleen, kidney, and the endocrine portion of the pancreas express both types of receptors, so these organs may be affected by influenza virus infection. Because the connective tissues between cardiac muscle cells were positive for both receptors, influenza virus infection may damage the heart [11,12].
In conclusion, we obtained concrete histological data showing the distribution of influenza virus receptors in the tissues of beagle dogs and this will enrich the tissue distribution data of the influenza virus receptors in animals. This will also provide basic information for evaluating the occurrence and development of influenza virus infection in canines and account for the organs involvement in infection.
Acknowledgments
This study was supported by grants from the Program for the National Natural Science Foundation (No. 30972233), the Program for Guangdong Natural Science Foundation (No. 825106421000008), the Projects of Conditions in Science and Technology of Guangdong Province (Nos. 2008A060202017 and 2009B060300024), and 973 Projects (No. 2011CB504703-1), China.
References
- 1.Amonsin A, Songserm T, Chutinimitkul S, Jam-on R, Sae-Heng N, Pariyothorn N, Payungporn S, Theamboonlers A, Poovorawan Y. Genetic analysis of influenza A virus (H5N1) derived from domestic cat and dog in Thailand. Arch Virol. 2007;152:1925–1933. doi: 10.1007/s00705-007-1010-5. [DOI] [PubMed] [Google Scholar]
- 2.Cardona CJ, Xing Z, Sandrock CE, Davis CE. Avian influenza in birds and mammals. Comp Immunol Microbiol Infect Dis. 2009;32:255–273. doi: 10.1016/j.cimid.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Cleaveland S, Meslin FX, Breiman R. Dogs can play useful role as sentinel hosts for disease. Nature. 2006;440:605. doi: 10.1038/440605b. [DOI] [PubMed] [Google Scholar]
- 4.Crawford PC, Dubovi EJ, Castleman WL, Stephenson I, Gibbs EPJ, Chen L, Smith C, Hill RC, Ferro P, Pompey J, Bright RA, Medina MJ, Johnson CM, Olsen CW, Cox NJ, Klimov AI, Katz JM, Donis RO Influenza Genomics Group. Transmission of equine influenza virus to dogs. Science. 2005;310:482–485. doi: 10.1126/science.1117950. [DOI] [PubMed] [Google Scholar]
- 5.Daly JM, Blunden AS, Macrae S, Miller J, Bowman SJ, Kolodziejek J, Nowotny N, Smith KC. Transmission of equine influenza virus to English foxhounds. Emerg Infect Dis. 2008;14:461–464. doi: 10.3201/eid1403.070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshpande MS, Abdelmagid O, Tubbs A, Jayappa H, Wasmoen T. Experimental reproduction of canine influenza virus H3N8 infection in young puppies. Vet Ther. 2009;10:29–39. [PubMed] [Google Scholar]
- 7.Giese M, Harder TC, Teifke JP, Klopfleisch R, Breithaupt A, Mettenleiter TC, Vahlenkamp TW. Experimental infection and natural contact exposure of dogs with avian influenza virus (H5N1) Emerg Infect Dis. 2008;14:308–310. doi: 10.3201/eid1402.070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harder TC, Vahlenkamp TW. Influenza virus infections in dogs and cats. Vet Immunol Immunopathol. 2010;134:54–60. doi: 10.1016/j.vetimm.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Ibricevic A, Pekosz A, Walter MJ, Newby C, Battaile JT, Brown EG, Holtzman MJ, Brody SL. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J Virol. 2006;80:7469–7480. doi: 10.1128/JVI.02677-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkland PD, Finlaison DS, Crispe E, Hurt AC. Influenza virus transmission from horses to dogs, Australia. Emerg Infect Dis. 2010;16:699–702. doi: 10.3201/eid1604.091489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishimoto C, Hiraoka Y, Takada H, Kurokawa M, Ochiai H. Effects of immunoglobulin on murine myocarditis caused by influenza A virus: experimental study. J Cardiol. 2005;45:247–255. [PubMed] [Google Scholar]
- 12.Kitaura Y, Deguchi H, Terasaki F, Ukimura A, Morita H, Tatsumi T. Influenza myocarditis--pathophysiology and developmental mechanism of myocarditis. Nihon Rinsho. 2003;61:1945–1952. [PubMed] [Google Scholar]
- 13.Matrosovich MN, Gambaryan AS, Teneberg S, Piskarev VE, Yamnikova SS, Lvov DK, Robertson JS, Karlsson KA. Avian influenza A viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology. 1997;233:224–234. doi: 10.1006/viro.1997.8580. [DOI] [PubMed] [Google Scholar]
- 14.Nelli RK, Kuchipudi SV, White GA, Perez BB, Dunham SP, Chang KC. Comparative distribution of human and avian type sialic acid influenza receptors in the pig. BMC Vet Res. 2010;6:4. doi: 10.1186/1746-6148-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ning ZY, Luo MY, Qi WB, Yu B, Jiao PR, Liao M. Detection of expression of influenza virus receptors in tissues of BALB/c mice by histochemistry. Vet Res Commun. 2009;33:895–903. doi: 10.1007/s11259-009-9307-3. [DOI] [PubMed] [Google Scholar]
- 16.Payungporn S, Crawford PC, Kouo TS, Chen LM, Pompey J, Castleman WL, Dubovi EJ, Katz JM, Donis RO. Influenza A virus (H3N8) in dogs with respiratory disease, Florida. Emerg Infect Dis. 2008;14:902–908. doi: 10.3201/eid1406.071270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 18.Shu Y, Li CK, Li Z, Gao R, Liang Q, Zhang Y, Dong L, Zhou J, Dong J, Wang D, Wen L, Wang M, Bai T, Li D, Dong X, Yu H, Yang W, Wang Y, Feng Z, McMichael AJ, Xu XN. Avian influenza A (H5N1) viruses can directly infect and replicate in human gut tissues. J Infect Dis. 2010;201:1173–1177. doi: 10.1086/651457. [DOI] [PubMed] [Google Scholar]
- 19.Song D, Kang B, Lee C, Jung K, Ha G, Kang D, Park S, Park B, Oh J. Transmission of avian influenza virus (H3N2) to dogs. Emerg Infect Dis. 2008;14:741–746. doi: 10.3201/eid1405.071471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song D, Lee C, Kang B, Jung K, Oh T, Kim H, Park B, Oh J. Experimental infection of dogs with avian-origin canine influenza A virus (H3N2) Emerg Infect Dis. 2009;15:56–58. doi: 10.3201/eid1501.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Pariyothorn N, Payungporn S, Theamboonlers A, Chutinimitkul S, Thanawongnuwech R, Poovorawan Y. Fatal avian influenza A H5N1 in a dog. Emerg Infect Dis. 2006;12:1744–1747. doi: 10.3201/eid1211.060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312:404–410. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol Pharm Bull. 2005;28:399–408. doi: 10.1248/bpb.28.399. [DOI] [PubMed] [Google Scholar]
- 24.Thompson CI, Barclay WS, Zambon MC, Pickles RJ. Infection of human airway epithelium by human and avian strains of influenza A virus. J Virol. 2006;80:8060–8068. doi: 10.1128/JVI.00384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao L, Korteweg C, Hsueh W, Gu J. Avian influenza receptor expression in H5N1-infected and noninfected human tissues. FASEB J. 2008;22:733–740. doi: 10.1096/fj.06-7880com. [DOI] [PubMed] [Google Scholar]


